ABSTRACT
MgO is an excellent candidate for thermal conductive fillers due to its high thermal conductivity, low electric conductivity, low cost, and appropriate hardness, though the reaction with water to form Mg(OH)2 causes a decrease in the weatherability of MgO. In order to improve the humidity resistance of MgO, the core (MgO)-shell (MgCO3) structure was designed using hydrothermal reactions under-compressed CO2 gas. The reactions proceeded rapidly to produce Mg(OH)2, 4MgCO3-Mg(OH)2-4H2O, and MgCO3 depending on reaction conditions. It was found that the reactions at high temperature under high CO2 pressure with a high heating rate suppressed the reaction of MgO and increased the amount of MgCO3 in the reaction products. The addition of ethanol enhanced this tendency, which resulted in the successful formation of reformed MgO particles covered by thin MgCO3 layers consisting of fine crystals. Humidity resistance tests of the PPS plates including the reformed MgO fillers confirmed that coating of MgO particles by MgCO3 layers improved weatherability.
1. Introduction
The development of electric devices causes accumulation of heat which will reduce the accuracy and service life of the devices. The heat dissipation of electronic devices is important to evaluate their comprehensive performances. In the development of electric devices, much attention has been paid to heat dissipation [Citation1–7]. Polymeric materials are widely used for electronic packaging due to their excellent properties such as lightness, moldability, electrical insulation performance, mechanical strength, and chemical/moisture resistance [Citation1,Citation8,Citation9]. However, polymer resins have a rather low thermal conductivity in the range from 0.1 to 0.5 W/(m·K) [Citation4,Citation5,Citation10–12], because they are formed by covalent bonding. The addition of fillers with high thermal conductivity can improve the thermal conductive property of plastics [Citation1,Citation3,Citation4,Citation7,Citation10–14].
Metals and carbonaceous materials such as carbon black, carbon fiber, graphene, and carbon nanotubes, have excellent thermal transport properties [Citation15] but at the same time high electrical conductivity, which might lead to electron leakage [Citation3]. Thus, various ceramic fillers with low electric conductivity such as AlN [Citation16–18], BN [Citation19–22], Al2O3 [Citation23–28], ZnO [Citation29], and MgO [Citation30,Citation31] have been incorporated into resins to provide the high thermal conductivity. Though the nitrides have high thermal conductivity (100–300 W/(m·K)) in comparison with the oxides (10–60 W/(m·K)) except BeO [Citation12,Citation14], their price is much higher than that of the oxides. Al2O3 has widely used as a filler but its high hardness causes equipment consumption. On the other hand, MgO is soft and cheap with relatively high thermal conductivity (60 W/(m·K)) [Citation30]. However, the weatherability of MgO is low due to the reaction with moisture to produce Mg(OH)2.
In this study, we designed the core-shell structure produced by a coating of MgO particles with MgCO3 (magnesite) surface layer to improve humidity resistance of MgO. It is expected that magnesite has high humidity resistance because it is a stable compound with low solubility in water. The core of a MgO particle should be completely covered by the shell of the magnesite reaction layer to avoid the reaction of MgO with water. However, the thermal conductivity of magnesite is 15 W/(m·K) [Citation32], lower than that of MgO. The reaction to produce magnesite should be controlled to leave as much MgO as possible. The shell consisting of magnesite should be as thin as possible but dense enough for water not to get through the shell.
We used hydrothermal reactions under-compressed CO2 gas to produce the core-shell structure. MgO particles were hydrothermally treated by changing reaction conditions such as reaction temperature, CO2 gas pressure, and heating rate. Hydrothermal reactions of MgO under-compressed CO2 gas proceeded fast to reduce the amount of unreacted MgO. We found that the addition of ethanol to water depressed the reaction to produce thin reaction layers consisting of fine magnesite crystals. It was confirmed that reformed MgO particles with the core-shell structure had high weatherability.
2. Experimental
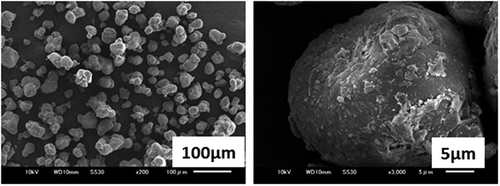
The starting MgO (RF-98) was purchased from Ube Ind. LTD., Japan. According to the supplier, it was produced by calcination at a high temperature beyond 1500°C. It consisted of monodispersed-rounded particles with smooth surfaces () and its average particle size was 55μm. The crystallite size of the starting MgO was determined to be 97 nm by the X-ray diffraction (2 0 0) of MgO.
In typical experiments, the staring MgO of 5 g and water of 15 g were put in a stainless-steel autoclave with an inner volume of 50 mL, equipped with a stirring system. Then, pressurized CO2 gas was injected into the autoclave at 5 MPa. During the hydrothermal treatments, CO2 pressure was kept constant. The autoclave was heated to 175°C with a heating rate of 5°C/min, and the temperature was kept constant for 1 h. Then, the autoclave was cooled to 60°C in 80 min, and the gas was released. The content of the autoclave was taken out and dried at 115°C. Separate experiments were conducted to observe the effects of the following reaction factors, reaction temperature, heating rate, CO2 gas pressure, and period of CO2 gas supply.
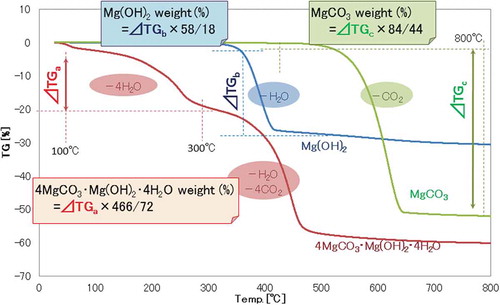
X-ray powder diffraction (XRD) analysis of the samples was carried out under a Rigaku Ultima IV X-ray diffractometer with Cu Kα radiation to identify the crystalline products. The identified phases in this study were MgO, Mg(OH)2 (brucite), 4MgCO3-Mg(OH)2-4H2O (hydromagnesite), and MgCO3 (magnesite). The quantitative analysis of these phases was carried out by the thermogravimetric analysis with Seiko SII TG/DTA 6300 at a heating rate of 10°C/min. shows the TG curves of brucite, hydromagnesite, and magnesite. The amount (wt%) of each component in the samples was determined from the weight loss at different temperatures. The weight loss at low temperatures from 100°C to 300°C was attributed to dehydration of 4H2O from hydromagnesite, that at temperatures from 350°C to 450°C to dehydration of H2O from brucite, and that at high temperatures beyond 500°C to decarbonisation of CO2 from magnesite. The size and surface morphology of the reaction products were observed by scanning electron microscopy (JEOL, FE-SEM). The cross-sections of the coated MgO particles were observed by FE-SEM. The particles were embedded in thermosetting resin and then the cross-sections were produced by ion beam milling (Hitachi, Ion Milling System IM4000).
Figure 2. TG curves of Mg(OH)2 (brucite), 4MgCO3-Mg(OH)2-4H2O (hydromagnesite), and MgCO3 (magnesite)
A humidity resistance test was carried out for the reformed samples prepared in a large autoclave with an inner volume of 2 L. PPS (polyphenylene sulfide) resin is a high-performance thermoplastic with a melting point of 280°C. PPS compounds were produced at 300°C by mixing PPS resin (60.4 vol%), glass fiber (16.8 vol%), and the sample (22.8 vol%) with the use of a twin-screw extruder, and shaped into a plate (110 × 80 × 1.5 mm3) by injection molding. After leaving the PPS plates in water vapor at 121°C and 2 atm for 1000 h, their surface roughness was observed by an optical microscope.
3. Results and discussion
3.1. Reactions in water
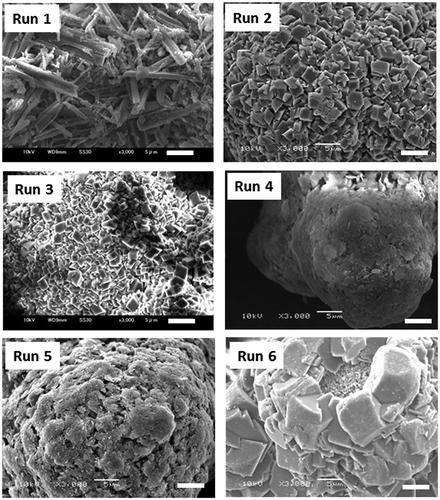
The representative results obtained by the reactions in water are summarized in , and SEM photographs of each product in are shown in .
Table 1. Summary of the reaction results in the water
Even at 30°C (Run 1), MgO reacted with water under 5 MPa of CO2 to produce MgCO3 (magnesite), 4MgCO3-Mg(OH)2-4H2O (hydromagnesite), and Mg(OH)2 (brucite). The main product was hydromagnesite. MgO easily reacted with carbonated water at low temperature to produce hydromagnesite which crystallized into rod-shaped crystals with a length beyond 5 µm. With the increase in the reaction temperature to 120°C (Run 2), the amount of the reaction products was increased to 59.4 wt% and the main reaction product was magnesite. Polyhedral magnesite crystals with a size of less than 3.5 µm were homogeneously formed on the MgO particles. When the reaction temperature was further increased to 175°C (Run 3) from 120°C, the amount of the reaction products was decreased to 38.2 wt% from 59.4 wt% but the content of magnesite in the reaction products was increased to 87% from 78%. The size of magnesite crystals was also decreased to less than 2.8 µm. The higher reaction temperature is favorable for the production of MgO fillers coated by magnesite layers by enhancing the formation of magnesite and increasing the amount of unreacted MgO.
When MgO particles were treated at 120°C under 1 MPa of CO2 (Run 4), the main reaction product was hydromagnesite. The comparison with the result of Run 2 shows that high CO2 pressure increases the formation region of magnesite. Run 5 at 175°C under 1 MPa of CO2 had more reaction products and less content of magnesite in the reaction products than Run 3 at 175°C under 5 MPa of CO2. The high pressure of CO2 suppressed the reaction of MgO and enhanced the formation of magnesite.
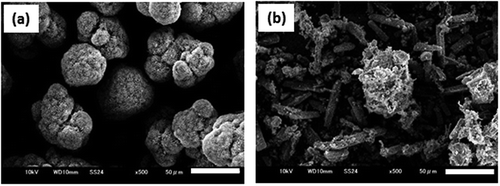
When the heating rate was reduced from 5°C/min to 1.25°C/min for the reaction of MgO at 175°C for 1 h with CO2 pressure of 5 MPa, the main product in both cases was likewise magnesite but the appearance of the reaction products changed from granular to powdery. The reaction was stopped at 80°C to observe the reaction process. The product obtained at a heating rate of 5°C/min consisted of particles mainly composed of MgO and hydromagnesite ()). The slow heating enhanced the reaction of MgO and MgO particles were completely converted into long rod-shaped hydromagnesite crystals ()). When the reaction of MgO with water proceeds at a slow heating rate, the granular shape of the starting material cannot be maintained, and the magnesite powder is formed instead of the core (MgO)-shell (MgCO3) structure.
Figure 4. SEM photographs of the reaction products obtained by stopping the reaction at 80°C. (bar = 50 µm). (b). heating rate at 5°C/min, (b) heating rate at 2.5°C/min
We used the following different ways to supply CO2 gas at 5MPa (Case 1) the valve of the CO2 gas cylinder was opened before heating and closed when the temperature reached to 175°C (Case 2) the valve was opened before heating and closed after heating at 175°C for 1 h (Case 3 for typical experiments) the valve was opened before heating and closed after cooling to 60°C (Run 3), and (Case 4) the valve was opened when the temperature reached to 175°C and closed after heating for 1 h (Run 6). Similar results were obtained for both quantity and morphology of magnesite crystals by the reactions in which CO2 gas was supplied at room temperature (Case 1–3), which suggested that the reaction of MgO with water pressurized by CO2 gas proceeded very rapidly and was completed when the reaction temperature reached to 175°C. When carbonation started at 175°C, a larger amount of MgO reacted and magnesite crystals grew larger (Run 6).
Thus, it can be concluded that the preferable conditions to get magnesite with a large amount of unreacted MgO are as follows: the reactants (MgO and water) should be heated to high temperature (175°C) with a high heating rate (5°C/min) after high-pressure injection of CO2 gas (5 MPa) at room temperature.
3.2. Reactions with the addition of ethanol
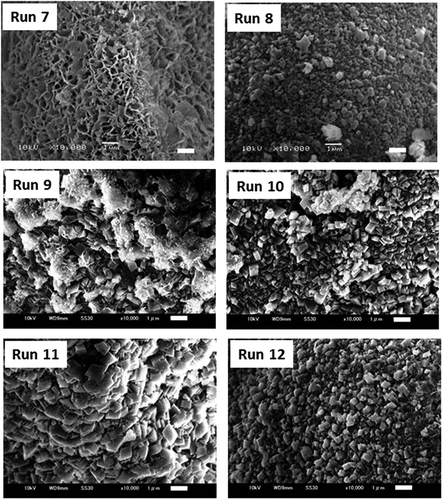
We added ethanol to water to depress the reaction of MgO with water to obtain thin layers of magnesite on the unreacted MgO particles. The results are summarized in and SEM photographs of each product in are shown in .
Table 2. Summary of the reaction results with the addition of ethanol
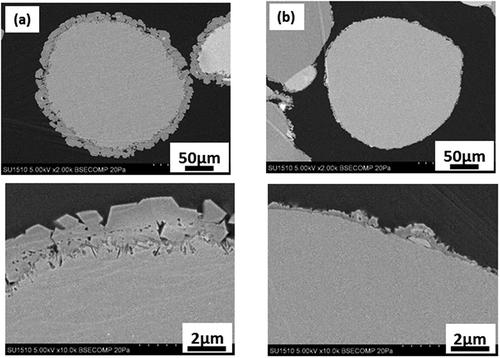
When ethanol was added to water up to 80 wt%, the reaction amounts obtained by the reactions at 30°C, 120°C, and 175°C (Run 7, 8, and 10, respectively) markedly decreased in comparison with those by Run 1, 2, and 3 without the addition of ethanol. At 30°C (Run 7), thin scale-like crystals covered the MgO particles. They must be hydromagensite based on their morphology. At 120 (Run 8) and 175°C (Run 10), the main reaction product was magnesite. At high temperature, magnesite crystals consisted of well-faceted fine rhombohedrons. The reactions at high temperatures reduced the reaction amount, similar to the results obtained without adding ethanol. The reaction amount was 30.2 wt% by the reaction at 175°C in water (Run 3), 14.6 wt% in 50 wt% ethanol (Run 11), and 6.7% in 80 wt% ethanol (Run 10). The reaction products were granular particles covered with faceted magnesite crystals, but the size of magnesite crystals decreased from 1–3 µm to 0.2–0.5 µm with the increase in ethanol content. The addition of ethanol resulted in a decrease in the reaction amount and size of magnesite crystals. The cross-sections of the MgO particles reformed at 175°C in 50 wt% ethanol (Run 11), and 80 wt% ethanol (Run 10) are shown in ), respectively. It is clearly observed that each MgO particle is covered with the reaction layer consisting of magnesite crystals. With the increase in ethanol content from 50 to 80 wt%, the size of magnesite crystals decreased, and the thickness of the reaction layer decreased from 3 to less than 1 µm.
Figure 6. Cross-sections of the MgO particles reformed at 175°C in 50 wt% ethanol (a), and 80 wt% ethanol (b)
The reaction of MgO does not start without the dissolution of MgO into water. Organic solvents have a lower solubility of ceramic powders than water so that they are used to prepare fine ceramic powders [Citation33]. The addition of ethanol to water must suppress the dissolution of MgO. In fact, the total reaction product was 29.5 wt% by the reaction in the water at 30°C (Run 1), but it was reduced to 1.4 wt% by the addition of 80 wt% ethanol (Run 7). The decrease of the solubility also resulted in a decrease in the crystal size of the reaction products.
Instead of ethanol, we added NMP (N- methyl-2-pyrrolidone) which is used as a solvent for polymerization reaction to produce PPS and is completely mixed with water. NMP had the same effect as ethanol to inhibit the reaction of MgO with water (Run 12). It is interesting that NMP, an aprotic solvent, has the same effect as ethanol, aprotic solvent, and suppresses the reaction of MgO with water under-pressurized CO2.
3.3. Humidity resistance
PPS plates were produced from three kinds of PPS compounds including unreformed MgO and reformed MgO with 10 and 30 wt% magnesite. Their thermal conductivity is 0.78, 0.72, and 0.68 W/(m·K), and their bending strength 144, 148, and 148 MPa, respectively. The thermal conductivity decreases as the content of magnesite in the PPS plate increases because magnesite has lower thermal conductivity than MgO. The type of MgO filler did not affect the mechanical strength, which suggests that the adhesion strength of the magnesite reaction layers on MgO particles is strong enough for the processing to produce PPS compounds.
Surface photographs of the PPS plates after the humidity resistance test is shown in . The small white dots in the photographs were formed by the hydration of MgO particles during the humidity resistance tests. The surface of the PPS plate including unreformed MgO was rough and covered by many white particles ()), which suggested that the exposed MgO filler particles on the surface of the PPS plate were easily hydrated by the humidity resistance test. On the other hand, the surface of the PPS plate including reformed MgO with 10 wt% magnesite was smooth, and even polishing scars formed by the humidity resistance test were observed on the surface. This result suggests that humidity resistance is remarkably improved by the reformation of MgO with only 10 wt% magnesite. The number of white dots on the PPS plate including reformed MgO with 30 wt% magnesite was smaller than that with 10 wt% magnesite. It is concluded that the plate including reformed MgO with 30 wt% magnesite had higher humidity resistance than that with 10 wt% magnesite. Based on the assumption that the reaction layer of MgO particles with a diameter of 55 µm reformed with 10 wt% magnesite is composed of well-packed magnesite fine crystals, the reaction layer thickness is estimated to be 1.1 µm. In the same way, the reaction layer thickness is calculated to be 3.6 µm for the reformed MgO particles with 30 wt% magnesite. It is considered that reformed MgO particles containing a large amount of magnesite had a thick shell of magnesite, which prevented the penetration of water to the MgO core.
4. Conclusions
MgO particles with an average particle size of 55 µm were hydrothermally treated under CO2 pressurized conditions to produce reaction layers of magnesite on MgO particles to improve the humidity resistance of MgO. Brucite, hydromagnesite and magnesite were formed by the hydrothermal reactions. The reactions at high temperatures under high CO2 pressure with a high heating rate suppressed the reaction of MgO and increased the amount of magnesite in the reaction products, which resulted in the successful formation of reformed MgO particles with a core (MgO)-shell (magnesite) structure. The addition of ethanol to water inhibited the reaction of MgO, increased the amount of unreacted MgO, and promoted the formation of a thin but dense reaction layer consisting of fine magnesite crystals. It was confirmed that the PPS plates including MgO particles reformed in this way had properties not only excellent in thermal conductivity but also in water resistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Meng M, Lin X, Lele Q, et al. Nanofibrillated Cellulose/MgO@rGO composite films with highly anisotropic thermal conductivity and electrical insulation. Chem Eng J. 2020;392:123714.
- Ohgoshi A, Takahashi K, Nakane K. Polymer/magnesia nanofiber composite sheets with anisotropic high thermal conductivity. J Mater Sci Mater Electron. 2019;30:20566–20573.
- Zhang J, Du Z, Zou W, et al. MgO nanoparticles-decorated carbon fibers hybrid for improving thermal conductive and electrical insulating properties of Nylon 6 composite. Compos Sci Technol. 2017;148:1–8.
- Yu W, Xie H, Chen L, et al. Synergistic Thermal conductivity enhancement of PC/ABS composites containing Alumina/Magnesia/Graphene nanoplatelets. Polym Compos. 2017;38:2221–2227.
- Yoshihara S, Tokita M, Ezaki T, et al. Main-chain smectic liquid crystalline polymer exhibiting unusually high thermal conductivity in an isotropic composite. J Appl Polym Sci. 2014;131:39896.
- Yoon YS, Oh MH, Kim AY, et al. The development of thermal conductive polymer composites for heat sink. J Chem Chem Eng. 2012;6:515–519.
- Kim K, Kim J. Core-shell structured BN/PPS composite film for high thermal conductivity with low filler concentration. Compos Sci Technol. 2016;134:209–216.
- Chen C, Xue Y, Li X, et al. High performance epoxy/binary spherical alumina composite as underfill material for electronic packaging. Compos Part A Appl S. 2019;118:67–74.
- Lee D, Lee S, Byun S, et al. Novel dielectric BN/epoxy nanocomposites with enhanced heat dissipation performance for electronic packaging. Compos Part A Appl S. 2018;107:217–223.
- Drozdov AD, Christiansen JD. Modeling thermal conductivity of highly filled polymer. Polym Eng Sci. 2019;59:2174–2179.
- Du F-P, Yang W, Zhang F, et al. Enhancing the heat transfer efficiency in graphene–epoxy nanocomposites using a magnesium oxide–graphene hybrid structure. Appl Mater Interfaces. 2015;7(26):14397–14403.
- Hong J, Park DW, Shim SE. A review on thermal conductivity of polymer composites using carbon-based fillers: carbon nanotubes and carbon fibers. Carbon Lett. 2010;11:347–356.
- Pan W, He M, Zhang L, et al. Interfacial engineering of graphene nanosheets at MgO particles for thermal conductivity enhancement of polymer composites. Nanomater. 2019;9:798.
- Chen H, Ginzburg VV, Yang J, et al. Thermal conductivity of polymer-based composites: fundamentals and applications. Prog Polym Sci. 2016;59:41–85.
- Jin J, Lin Y, Song M, et al. Enhancing the electrical conductivity of polymer composites. Eur Polym J. 2013;49:1066–1072.
- Huang X, Iizuka T, Jiang P, et al. Role of interface on the thermal conductivity of highly filled dielectric epoxy/AlN Composites. J Phys Chem C. 2012;116:13629–13639.
- Zhou W. Thermal and dielectric properties of the AlN particles reinforced linear low-density polyethylene composites. Thermochim Acta. 2011;512:183–188.
- Chiu HT, Sukachonmakul T, Kuo MT, et al. Surface modification of aluminum nitride by polysilazane and its polymer-derived amorphous silicon oxycarbide ceramic for the enhancement of thermal conductivity in silicone rubber composite. Appl Surf Sci. 2014;292:928–936.
- Kemaloglu S, Ozkoc G, Aytac A. Properties of thermally conductive micro and nano size boron nitride reinforced silicon rubber composites. Thermochim Acta. 2010;499:40–47.
- Zhou WY, Qi SH, Zhao HZ, et al. Thermally conductive silicone rubber reinforced with boron nitride particle. Polym Compos. 2007;28:23–28.
- Wattanakul K, Manuspiya H, Yanumet N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J Appl Polym Sci. 2011;119:3234–3243.
- Zhou WY, Qi SH, Li HD, et al. Study on insulating thermal conductive BN/HDPE composites. Thermochim Acta. 2007;452:36–42.
- Heo Y, Im H, Kim J. The influence of Al(OH)3-coated graphene oxide on improved thermal conductivity and maintained electrical resistivity of Al2O3/epoxy composites. J Nanopart Res. 2012;14:1–10.
- Fu JF, Shi LY, Zhong QD, et al. Thermally conductive and electrically insulative nanocomposites based on hyperbranched epoxy and nano-Al2O3 particles modified epoxy resin. Polym Adv Technol. 2011;22:1032–1041.
- Zhang S, Ke Y, Cao X, et al. Effect of Al2O3 fibers on the thermal conductivity and mechanical properties of high density polyethylene with the absence and presence of compatibilizer. Appl Polym Sci. 2012;124:4874–4881.
- Choi S, Kim J. Thermal conductivity of epoxy composites with a binary-particle system of aluminum oxide and aluminum nitride fillers. Compos B. 2013;51:140–147.
- Zhou WY, Qi SH, Tu CC, et al. Effect of the particle size of Al2O3 on the properties of filled heat-conductive silicone rubber. J Appl Polym Sci. 2007;104:1312–1318.
- Ju HS, Hyeok ID, Park SD, et al. Thermal conductivity of Al2O3/Poly(vinyl butyral) composites. J Appl Phys. 2012;5:539–545.
- Sim LC, Ramanan SR, Ismail H, et al. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim acta. 2005;420:155–165.
- Murakami K, Yamada K, Deguchi K, et al. Preparation of soluble polyimide/MgO nanohybrid films by in situ hybridization method and evaluation of their thermal conductivity. J Photopolym Sci Technol. 2010;23:501–506.
- Machrafi H, Lebon G, Iorio CS. Effect of volume-fraction dependent agglomeration of nanoparticles on the thermal conductivity of nanocomposites: applications to epoxy resins, filled by SiO2, AlN and MgO nanoparticles. Compos Sci Technol. 2016;130:78–87.
- Magnesium compounds for thermal conductive fillers. Kagawa, Japan: Konoshima Chemical Co.; 2020. Available from: http://www.konoshima.co.jp/eng/resdev/resdev04.html
- Sato T. Synthesis of environmentally friendly ceramic materials via solvothermal reactions. J Ceram Soc Jpn. 2010;118:1105–1114.