ABSTRACT
Whooping cough, or pertussis, is resurgent in numerous countries worldwide. This has renewed interest in Bordetella pertussis biology and vaccinology. The in vitro growth of B. pertussis has been a source of difficulty, both for the study of the organism and the production of pertussis vaccines. It is inhibited by fatty acids and other hydrophobic molecules. The AcrAB efflux system is present in many different bacteria and in combination with an outer membrane factor exports acriflavine and other small hydrophobic molecules from the cell. Here, we identify that the speciation of B. pertussis has selected for an Acr system that is naturally mutated and displays reduced activity compared to B. bronchiseptica, in which the system appears intact. Replacement of the B. pertussis locus with that of B. bronchiseptica conferred higher levels of resistance to growth inhibition by acriflavine and fatty acids. In addition, we identified that the transcription of the locus is repressed by a LysR-type transcriptional regulator. Palmitate de-represses the expression of the acr locus, dependent on the LysR regulator, strongly suggesting that it is a transcriptional repressor that is regulated by palmitate. It is intriguing that the speciation of B. pertussis has selected for a reduction in activity of the Acr efflux system that typically is regarded as protective to bacteria.
Introduction
The genus Bordetella consists of nine characterized species (recently other novel species have been reported), including those that cause respiratory infections. B. pertussis is a fastidious, Gram-negative coccobacillus that is a strict pathogen of humans that causes whooping cough or pertussis. B. pertussis has evolved recently from B. bronchiseptica or a B. bronchiseptica-like ancestor [Citation1]. This speciation involved considerable genome reduction and rearrangement that resulted in an organism restricted to the human respiratory tract. In comparison, B. bronchiseptica has a broad host range and grows freely in the environment [Citation2,Citation3]. Pertussis is considered resurgent in many parts of the world, despite high levels of vaccination. Possible reasons for this have been well discussed (reviewed in [Citation4Citation5 Citation6–Citation7]). Resurgence has emphasized sizeable gaps in the understanding of the mechanisms of B. pertussis pathogenesis and of B. pertussis physiology. In vitro, growth of B. pertussis has proved challenging, especially in liquid culture. B. pertussis is unable to metabolize sugars, appearing reliant on the metabolism of amino acids [Citation8,Citation9]. Glutamate and proline are the most readily oxidized and glutamate has been used as the primary carbon and nitrogen source in most defined B. pertussis growth media, for example Stainer–Scholte (SS) broth [Citation9]. B. pertussis is unable to grow in some media, even though they contain sources of glutamate, for example on Luria–Bertani agar, due to the presence of compounds inhibitory to growth. B. pertussis is sensitive to a number of compounds including peptone, fatty acids, sulphur, peroxide, and manganese [Citation10]. Often, charcoal, blood, or cyclodextrins are added to B. pertussis growth media to sequester hydrophobic inhibitory compounds. In particular, growth of B. pertussis is inhibited by a number of fatty acids including palmitic acid (16:0) [Citation10]. However, in the commonly used laboratory media B. pertussis releases fatty acids, particularly palmitic acid, into the culture supernatant to concentrations that inhibit growth [Citation11]. This contributes to a poor yield from in vitro B. pertussis cultures and to issues with reproducibility of the quality of the biomass produced, creating major problems for vaccine manufacturers. This will be particularly challenging for increasing vaccine production as part of any intervention to combat resurgence.
Many bacteria possess mechanisms to resist the inhibitory effects of small hydrophobic molecules, for example the AcrAB-TolC efflux system of Escherichia coli that is responsible for resistance to a wide range of hydrophobic inhibitors [Citation12]. This system consists of an inner membrane transporter AcrA, a periplasmic coupling protein, AcrB, that couples AcrA to TolC, the outer membrane channel [Citation13,Citation14]. In addition, E. coli AcrZ associates with AcrB to enhance the export of some substrates [Citation15]. Here, we report that the B. pertussis acrAB-cusC (acrABC) locus contains two small deletions, resulting in low activity of B. pertussis AcrABC compared to the B. bronchiseptica system. We demonstrate that Bordetella AcrABC confers resistance to inhibition of growth by acriflavine and fatty acids and that a LysR-type transcriptional regulator represses transcription of acrABC, repression that is alleviated by the substrate palmitate.
Materials and methods
Bacterial strains and culture conditions
Bacterial strains used in this study are listed in . Plasmids used in this study are listed in . BP536 is a single passage, streptomycin resistant derivative of Tohama I and used as WT in this study. B. pertussis was cultured on charcoal agar (CA) (Fisher Scientific, Loughborough, UK) for 3 days at 37°C or in Stainer–Scholte broth (SS) or in SSH (SS with 1 g/L heptakis [Citation9] (Sigma-Aldrich, Gillingham, UK)) at 37°C with shaking at 180 rpm. E. coli were grown on LB agar or in LB broth at 37°C. Antibiotics were used where appropriate at the following concentrations: kanamycin 50 µg/ml and gentamycin 30 µg/ml. For growth of E. coli ST18 [Citation16] media was supplemented with amino-levulinic acid at 50 µg/ml.
Table 1. Strains used in this study.
Table 2. Plasmids used in this study.
PCR
PCR was conducted using OneTaq 2x master mix (NEB, Hitchin, UK) following the manufacturer's instructions modified to include 0.75 µl of DMSO per reaction. The cycle conditions were as follows: 94°C for 4 min followed by 30 cycles of 94°C for 30 s, annealing for 1 min, 68°C for 1 min/kb and 5 min at 68°C. Details for the primers used can be found in .
Table 3. Primers used in this study.
Mutagenesis
Deletions of acrABC and BP0983 were created by amplifying by PCR approximately 500 bp of the regions flanking the deletions. For acrABC, these flanking regions were cloned either side of a kanamycin resistance cassette to produce acrDELKanABpSS4940. For deletion of BP0983, the flanking regions were joined together to create an in-frame deletion of BP0983. PCR fragments were cloned into pSS4940GG by Golden Gate cloning [Citation17]. Constructs were transformed into chemically competent E. coli ST18, which was subsequently used as the donor for conjugation. Conjugations were carried out as previously described [Citation18]. Conjugants were selected on charcoal agar supplemented with gentamycin and 50 mM MgSO4. Following counter-selection against merodiploids, deletion mutants were distinguished from WT by PCR. For replacement of the B. pertussis acr locus with that of B. bronchiseptica, B. bronchiseptica locus was amplified by PCR as two sections and ligated together using Gibson Assembly. The resulting locus fragment was cloned into pSS4940GG as above and conjugated into BPΔacrABC.
Inhibition assays
Plate grown B. pertussis strains were resuspended in SS to an OD600 of 0.1 and grown overnight. Cultures were diluted in fresh SS supplemented with different concentrations of the inhibitory compound: acriflavine, palmitic acid, myristic acid, oleic acid, and decanoic acid (Sigma-Aldrich, BioXtra grade). Cultures (200 μl) were incubated at 37°C with shaking at 200 rpm in microtitre plates. After 3 days, the OD600 was measured in a Fluostar Omega plate reader (BMG LabTech, Aylesbury, UK). The OD600 of cultures grown in the presence of inhibitor was divided by the OD600 of the same strain grown without inhibitor to give relative growth as a percentage. Resistance to ampicillin was determined using E-test strips in accordance with manufacturer's instructions (Biomerieux, Basingstoke, UK). Plates were inoculated with 100 µl of a suspension of bacteria at OD600 = 0.8 (∼1.5 × 108 cfu/ml).
RT-qPCR
Bacteria were grown overnight in SS or SSH and these cultures were used to inoculate fresh media at an OD600 of 0.05. Cultures were harvested at OD600 = 0.9 ± 0.1 by centrifugation (4000g for 10 min) and resuspended in 700 µl of Tri-reagent (Invitrogen; ThermoFisher, Loughborough, UK), vortexed vigorously, and frozen at −80°C. Nucleic acids were precipitated with ethanol, DNA was removed using 4U of Turbo DNase (Ambion, ThermoFisher) for 1 h, and RNA was purified using the RNeasy kit (Qiagen, Manchester, UK) in accordance with the manufacturer's instructions. The concentration of RNA was determined using Qubit broad range RNA quantification kit (Fisher Scientific). RNA integrity was determined by agarose gel electrophoresis. Finally, RNA was confirmed as being DNA free by PCR using 50 ng of RNA as template in PCR with recAF and recAR primers. First strand cDNA was synthesized using ProtoScript II (NEB) with 1 µg of total RNA as template and 6 µM random primers and incubated for 5 min at 25°C, 1 h at 42°C. The reaction was stopped by incubating at 65°C for 20 min. cDNA was diluted 1/30 in H2O for use in qPCR.
qPCR was run on an a StepOne Real-time PCR System (Applied Biosystems, ThermoFisher) using SyberGreen Turbo Master mix (Applied Biosystems), in a total reaction volume of 25 µl with primers at 300 nM. Triplicate reactions were run for each sample. Reactions conditions were: 95°C for 10 min and 40 cycles of 95°C for 15 s and 1 min at 60°C. The housekeeping gene recA was used to calculate ΔCT and ΔΔCT was determined by determining the difference between the reference condition and experimental condition. Relative expression was represented as fold change (fold change = 2−ΔΔCT). Three biological repeats were used for each experiment. Significance was determined using Students t-test using a hypothetical value of 1 (no relative expression difference).
Ethidium bromide accumulation assay [Citation19,Citation20]
B. pertussis strains were grown in SS until OD600 = 1.00 ± 0.1. Ethidium bromide was added at 16 µM. Samples were vortexed and transferred to a 96-well plate, stored in the dark for 30 min before fluorescence was measured in a FLOUstar Optima microplate reader (BMG Labtech). Variation in OD600 was accounted for by dividing the fluorescence value by the OD600 and expressed as relative fluorescence.
Modelling the structure of BP0983
This was conducted using I-TASSER under default settings [Citation21]. The C-score of the model produced by I-TASSER was determined by comparison to a number of similar protein sequences the most similar to BP0983 was CrgA Protein Data Bank number 3hhgE [Citation22]. Model images were made using CHIMERA 1.13.1 [Citation23].
Results
Deletions in acrA and cusC in B. pertussis results in inhibition of growth on LB agar
B. pertussis is sensitive to free fatty acids and other hydrophobic molecules, and its growth is limited on rich media including Luria–Bertani media [Citation24], whereas B. bronchiseptica is less sensitive. During comparisons of genome sequences of B. pertussis Tohama I and B. bronchiseptica RB50, it was noticed that while both species contain homologues of acrAB-tolC genes, the B. pertussis system contains two mutations. Compared to B. bronchiseptica, B. pertussis acrA (BP0984) contains a 646 bp deletion of the 3’ region of the gene, predicted to result in a truncated AcrA that due to a frameshift created by the deletion contains different carboxyl-terminal amino acids to that of B. bronchiseptica AcrA. Also, there is an 84 bp in-frame deletion in BP0986, originally annotated as cusC, compared to the homologue in B. bronchiseptica, which we predict to encode the outer membrane factor of the efflux system, and refer to here as acrC (a). Aside from these two deletions, the two loci are highly homologous. There are just five amino acid differences between the two AcrB proteins (1059 amino acids) and (outside of the 28 amino acid deletion) one amino acid difference between the two AcrC proteins. Furthermore, these deletions are conserved across all currently available B. pertussis genome sequences, indicating that these deletions were likely acquired early in the evolution of B. pertussis. We hypothesized that B. pertussis contains a non-functional Acr efflux system whereas it is functional in B. bronchiseptica. To test this, the B. bronchiseptica locus (BB2527-9), including the adjacent gene encoding a putative LysR-type transcriptional regulator (BB2526) carried on pBBRK, a plasmid that replicates in Bordetella, was introduced into B. pertussis to produce BPpBBRKacrABCBB. Also, acrA-C were deleted from B. pertussis and replaced with a kanamycin resistance cassette to produce BPΔacrABC. This strain was used as a background to clone the B. bronchiseptica locus onto the chromosome in place of the kanamycin cassette, effectively replacing the B. pertussis acr locus with that of B. bronchiseptica, producing BPacrABCBB.
The ability of each strain to grow on LB agar was assessed. WT B. pertussis, WT containing pBBRK plasmid alone, and BPΔacrABC were unable to grow on LB agar, in contrast to both BPpBBRKacrABCBB and BPacrABCBB (b), suggesting that the inability of B. pertussis to grow on LB is caused by the deletions in BPacrA and BPacrC.
B. pertussis AcrABC retains residual function
The relative activities of the B. pertussis and B. bronchiseptica Acr systems were investigated by assaying the accumulation of ethidium bromide within strains, that in other bacteria is effluxed by Acr [Citation19,Citation20]. High levels of bacterial fluorescence were indictive of a low level of efflux of ethidium bromide, and this was represented relative to WT to control for variation in fluorescence between experiments (c). BPΔacrABC accumulated more ethidium bromide than WT (22% greater than WT, p = 0.026), indicating that the deletion of acrABC decreased efflux of ethidium bromide. This suggested that B. pertussis AcrABC retains some function, despite the two mutations. The presence of the B. bronchiseptica locus resulted in significantly less accumulation of ethidium bromide (24% less than WT, p = 0.021) demonstrating that the B. bronchiseptica system has greater activity for efflux of ethidium bromide than that of B. pertussis.
BP0983 is a transcriptional repressor of acrABC
Adjacent to, but divergent from, acrABC in both B. pertussis and B. bronchiseptica is a gene encoding a putative LysR family transcriptional regulator (BP0983 and BB2526 respectively, ). Genes encoding this class of regulator are often situated upstream of, and divergent from, the genes that they regulate [Citation25]. The predicted structure of BP0983 contains two conserved domains, an N-terminal helix-turn-helix (HTH) domain found in LysR family proteins (pfam00126) and a LysR substrate binding domain (pfam03466) (a). An N-terminal HTH is the characteristic of LysR-type transcriptional regulators that act as transcriptional repressors.
Figure 1. B. pertussis acr is mutated and has lower activity than B. bronchiseptica acr. (a) Diagram showing the arrangement of the B. pertussis and B. bronchiseptica acrABC loci. BP0983/BB2526 encodes a LysR-type transcriptional regulator. B. pertussis acrA and acrC (cusC) have 646 and 84 bp (in-frame) deletions respectively, compared to B. bronchiseptica. (b) B. pertussis strains were plated on charcoal agar (left) and LB agar (right). All strains grew on charcoal agar. WT, WT containing pBBRK (BPpBBRK) and BPΔacrABC were unable to grow on LB agar whereas the presence of acrABCBB conferred growth on LB. (c) An ethidium bromide accumulation assay was used to measure AcrABC activity. acrABCBB conferred greater efflux activity compared to that attributable to the B. pertussis locus. Deletion of acrABC from B. pertussis resulted in decreased activity suggesting B. pertussis AcrABC has residual function. The data is based on six independent experiments. Error bars represent standard deviation and significance was determined by a one-sample t-test comparing each strain to WT. *: p < 0.05; **: p < 0.01.
Figure 2. BP0983 is a transcriptional repressor of acrABC. (a) Diagram of the conserved domains of the LysR-family transcriptional regulator encoded by BP0983. A predicted helix-turn-helix motif (green) at the N-terminus is indicative of repressor activity. It also contains a LysR substrate domain (blue). (b) Expression of acrA determined by RT-qPCR. Growth of WT in SS broth with heptakis (SSH) was the reference condition for comparison on the left of the dotted line, and BPΔ0983 in SSH on the right. There was a significant increase in expression of acrA in WT in SS broth without heptakis (WT-SS) and BPΔ0983 SSH (BPΔ0983-SSH) with a fold change of 4.1 and 2.5 respectively. There was no difference between acrA expression in BPΔ0983 in SS compared to SSH. The data is based on three biological repeats. Error bars represent standard deviation and significance was determined by one-sample t-test comparing each condition to the reference.
To test the involvement of BP0983 in regulating acr expression, BP0983 was deleted from B. pertussis in both the WT and BPacrABCBB backgrounds, producing BPΔ0983 and BPacrABCBBΔ0983 respectively. The relative level of transcription of acrA in WT and BPΔ0983 was measured using RT-qPCR. Strains were grown in SS broth either with or without heptakis. Heptakis is a cyclodextrin used to supplement B. pertussis growth media by sequestering small hydrophobic molecules that are inhibitory to B. pertussis growth. WT grown with heptakis was used as the reference condition. Compared to this, there was a 4.1-fold increase in the expression of acrA in WT when grown in SS without heptakis (b). B. pertussis growing in SS broth produces free fatty acids that are sequestered by heptakis when present in the medium [Citation11]. The increased transcription of acrA in the absence of heptakis, and thus in the presence of free fatty acids in the culture medium, is consistent with the notion that free fatty acids signal to increase transcription of acr. In BPΔ0983, transcription of acrA was increased, and this was insensitive to the presence or absence of heptakis (2.5-fold with heptakis and 2.8-fold without heptakis, relative to WT).
Deletion of BP0983 resulted in increased levels of transcription of acrA, but regardless of the presence or absence of heptakis, and thus of the presence of fatty acids in the culture medium. This supports the idea that BP0983 is a transcriptional repressor of acrABC and that fatty acids derepress transcription. However, deletion of BP0983 would be expected to completely relieve repression of acrA transcription and it is not clear why the level of expression of acrA in BPΔ0983 was not as high as in WT grown in SSH, suggesting that there may be a synergistic repressor activity by something that is sequestered by heptakis.
The role of Acr in resistance to acriflavine, ampicillin and fatty acids
E. coli AcrAB-TolC expels acriflavine and other small hydrophobic molecules from the cell. To test the activity of Bordetella Acr against such compounds, the sensitivity of strains to acriflavine, ampicillin and fatty acids was tested. BPΔacrABC proved difficult to grow in broth in the absence of heptakis. The inability to grow this strain in the absence of heptakis, that might sequester the test compounds, precluded it from being included in these assays.
The growth of strains over 48 h in the presence of different concentrations of acriflavine was measured and normalized to growth without acriflavine. There was no significant difference between growth of any of the strains at ≤4 µg/ml acriflavine. However, at 8 µg/ml the growth of BPacrABCBBΔ0983 was significantly greater than any of the other strains (a). These data suggest that B. pertussis AcrABC could efflux acriflavine, but with low activity as growth of BPΔ0983, in which the expression of the system was higher, was no different to WT. The increased growth of BPacrABCBB suggests that the B. bronchiseptica locus conferred increased resistance to acriflavine but only when repression of its transcription was relieved by deletion of BP0983. In turn, this suggested that although acriflavine was a substrate of Acr, it was unable to induce relief of BP0983-mediated repression.
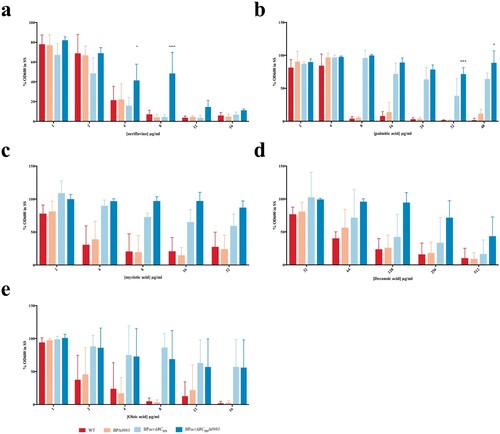
Figure 3. The role of Acr in resistance to inhibition of growth by small hydrophobic molecules. B. pertussis WT (red), BPΔ0983 (orange), BPacrABCBB (light blue), and BPacrABCBBΔ0983 (dark blue) were grown in SS broth overnight and seeded into 96-well plates with various concentrations of acriflavine (a), palmitate (b), myristate (c), dodecanoate (d), and oleate (e). Plates were incubated at 37°C for 48 h after which OD600 was measured. The OD600 for each strain was represented as a percent of the OD600 of untreated samples. Only BPacrABCBBΔ0983 was resistant to acriflavine at ≥8 µg/ml. BPacrABCBB and BPacrABCBBΔ0983 were resistant to higher levels of fatty acids than either WT or BPΔ0983. The data is based on three independent experiments. Error bars represent standard deviation and significance was determined by 2-way ANOVA.
The role of Acr in resistance to ampicillin was tested using E-test strips. The MIC for WT was 0.094 µg/ml. The MIC for BPΔacrABC (0.19 µg/ml) and for BPΔ0983 (0.25 µg/ml) was not largely different to that of WT. The MICs of BPacrABCBB (16 µg/ml) and BPacrABCBBΔ0983 (>256 µg/ml) suggested that the B. bronchiseptica locus conferred significantly increased resistance to ampicillin, and that the sensitivity of B. pertussis to ampicillin may, at least in part, be due to the low activity of B. pertussis Acr.
Palmitate is the main fatty acid released by B. pertussis during growth [Citation11]. The role of Acr in the sensitivity of B. pertussis to palmitate was investigated (b). There was no difference between the growth of strains in the presence of palmitate up to 4 µg/ml. Above this level, the growth of strains containing acrABCBP was inhibited, even if transcription of it was derepressed (BPΔ0983). There was a significant difference in the growth of BPacrABCBB in the presence of palmitate compared to both WT and BPΔ0983, demonstrating that AcrABCBB conferred much higher levels of resistance to growth inhibition by palmitate than AcrABCBP (B). There was a significant difference in growth between BPacrABCBB and BPacrABCBBΔ0983 at both 32 and 48 µg/ml of palmitate (b), consistent with the deletion of BP0983 increasing the level of expression of acrABCBB.
To determine the range of fatty acids that are substrates for AcrABC, growth in the presence of decanoic acid (C10:0), myristic acid (C14:0), and oleic acid (C18:1) was tested (c–e). There was a similar pattern of resistance to that observed with palmitate in that AcrABCBB conferred higher levels of resistance than AcrABCBP and deletion of BP0983 increased further the resistance conferred by AcrABCBB, although this increase was not always significant. The data suggested that short chain fatty acids inhibited the growth of B. pertussis less than longer chain fatty acids.
BP0983 contains a potential fatty acid binding site
Bordetella AcrABC was active against a range of fatty acids, and B. pertussis releases palmitate during its growth in vitro. We hypothesized that fatty acids signalled to increase expression of acrABC by binding to BP0983 to relieve its repression of acrABC. A structural model of BP0983 was produced using I-TASSER [Citation21] (). The model had a high C-score (0.42) and a TM-score (0.77 ± 0.10), which was indicative of a realistic prediction of the structure of BP0983. This structure was consistent with that of LysR transcriptional regulators, comprising a DNA binding domain, a hinge domain that allows for a conformational change following the binding of a co-factor which interacts with a binding pocket that is formed between the two lobes of the co-factor binding domain. This putative binding pocket forms a channel lined with hydrophobic amino acids and this would appear to be compatible with the binding of fatty acids.
Figure 4. Modelling the structure of BP0983 identifies a putative hydrophobic channel. (a) A model of the structure of the LysR transcriptional regulator, BP0983, using I-Tasser (default settings), based on a number of similar proteins including CrgA (PDB: 3hhgE) [Citation22], with a C-score of 0.65. The structure comprises a helix-turn-helix DNA binding domain (blue), a hinge domain (purple), and two co-factor binding domains (green and red). The LysR co-factor binding site is found between the two co-factor binding domains. (b) Mapping of hydrophobic amino acids (red) reveals that the co-factor binding region contains a channel lined by hydrophobic amino acids that could be compatible with binding fatty acids such as palmitate.
![Figure 4. Modelling the structure of BP0983 identifies a putative hydrophobic channel. (a) A model of the structure of the LysR transcriptional regulator, BP0983, using I-Tasser (default settings), based on a number of similar proteins including CrgA (PDB: 3hhgE) [Citation22], with a C-score of 0.65. The structure comprises a helix-turn-helix DNA binding domain (blue), a hinge domain (purple), and two co-factor binding domains (green and red). The LysR co-factor binding site is found between the two co-factor binding domains. (b) Mapping of hydrophobic amino acids (red) reveals that the co-factor binding region contains a channel lined by hydrophobic amino acids that could be compatible with binding fatty acids such as palmitate.](/cms/asset/21db8cb7-c596-41ce-a61e-f948e5c3c789/temi_a_1601502_f0004_oc.jpg)
Palmitate induces the expression of acrA
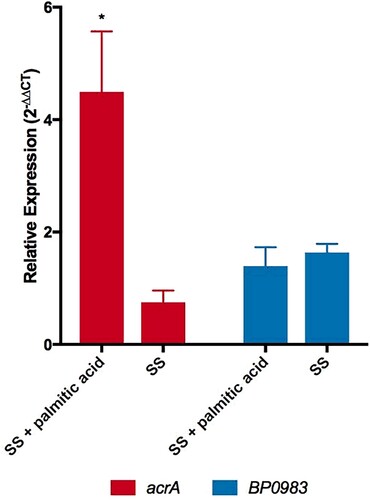
It was reasoned that if palmitate interacts with BP0983 to relieve repression of acrABC then adding palmitate to B. pertussis would be expected to increase the expression of the locus. To test this, the transcription of acrA was determined by RT-qPCR. Cultures of WT were grown in the presence of heptakis to sequester palmitate produced during growth that might derepress transcription of acrA in all samples. Bacteria were diluted into fresh SS broth or SS broth supplemented with palmitic acid (16 µg/ml) and after incubation for 1 h RNA was extracted for RT-qPCR analyses (). Relative to the level of expression of the housekeeping gene, recA, the expression of acrA increased 4.5-fold in the presence of palmitate (p = 0.0302) (). This is consistent with palmitate interacting with BP0983 to relieve repression of acrABC transcription. In the control culture without palmitate, the expression of acrA decreased slightly during the hour of incubation, suggesting that even in the presence of heptakis, in the original culture some free palmitate caused a slight derepression of transcription, but that diluting the bacteria into fresh SS broth in which there was no palmitate led to full repression and thus a decrease in transcription of acrA. There was no significant change in the expression of BP0983 either in the presence or absence of palmitate (), demonstrating that the change in transcription of acrA was not due to a change in the level of the repressor itself.
Figure 5. Palmitate induces the expression of acrA. Expression of acrA (red) and BP0983 (blue) was measured by RT-qPCR. B. pertussis was grown overnight in SS broth with heptakis and then inoculated into fresh SS with or without 16 µg/ml palmitate. A sample was taken (t = 0) (reference condition) after 1 hour and RNA was extracted from these. The presence of palmitate induced a 4.5-fold increase in transcription of acrA (p = 0.0302). There was no increase in expression of acrA when incubated in SS alone. There was no increase in expression of BP0983 in either SS with palmitic acid or SS. The data is based on three biological repeats. Error bars represent standard deviation and significance was determined by one-sample t-test.
Discussion
The role of AcrAB-TolC as an efflux pump of hydrophobic molecules with an emphasis on its role in antimicrobial resistance [Citation26Citation27–Citation28] has been described. This study reveals that Bordetella AcrABC is as a non-specific efflux pump of small hydrophobic molecules. Intriguingly, B. pertussis has suffered two deletions, in acrA and cusC (that we have referred to as acrC) that results in a reduction in activity of B. pertussis AcrABC leading to an increase in sensitivity to ampicillin, acriflavine and fatty acids. However, B. pertussis AcrABC retains low level function rather than being non-functional, as a strain from which the locus was deleted accumulated more ethidium bromide than WT, and in SS in the absence of heptakis this strain grew less than WT, consistent with increased sensitivity to inhibitory compounds present in the growth media. B. bronchiseptica-derived AcrABC demonstrated significantly greater activity than the B. pertussis system, revealing that it is active against acriflavine, ampicillin but particularly fatty acids. A transcriptional repressor controls the expression of Bordetella acrABC and we show data consistent with palmitate interacting with the repressor to control its activity, such that B. pertussis senses palmitate to increase the expression of an efflux pump that protects the cell from the inhibitory effect of the fatty acid. It is logical for the Bordetella to possess an efflux system that protects them from the effects of free fatty acids that they secrete during their growth, the reason for which is unknown. However, it is unclear why B. pertussis has evolved to possess a poorly functional AcrABC efflux system that appears to render the organism highly susceptible to the palmitate that it secretes. It is possible that palmitate secretion is an artefact of growth in vitro. Alternatively, B. pertussis may secrete palmitate but in its natural niche, the human respiratory tract, the palmitate does not build to levels that are inhibitory. In other bacteria, high levels of Acr expression can result in excretion of metabolites, resulting in growth inhibition of the organism [Citation29]. Thus the low activity AcrABCBP may provide sufficient protection from hydrophobic molecules within its niche, but prevent excretion of important metabolites. B. parapertussis is thought to have evolved, like B. pertussis, from a B. bronchiseptica-like ancestor to become a cause of whooping cough in people and restricted to the human respiratory tract as its sole niche, although there appears to be an ovine-associated lineage of B. parapertussis distinct to the human-associated strains. Examination of B. parapertussis genome sequences reveals that these bacteria contain the full-length acrABC locus that is over 99% identical to acrABCBB at the nucleotide level and thus, like B. bronchiseptica, contains a high-activity efflux system. Thus the evolution of a low-activity AcrABC appears to be specific to B. pertussis and its unique pathogenicity and understanding will contribute to deciphering this important host–pathogen interaction.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Linz B, Ivanov YV, Preston A, et al. Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of Bordetella species. BMC Genomics. 2016;17:767. doi: 10.1186/s12864-016-3112-5
- Parkhill J, Sebaihia M, Preston A, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227
- Cherry JD. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174(Suppl. 3):S259–S263. doi: 10.1093/infdis/174.Supplement_3.S259
- Preston A. The role of B. pertussis vaccine antigen gene variants in pertussis resurgence and possible consequences for vaccine development. Hum Vaccin Immunother. 2016;12:1274–1276. doi: 10.1080/21645515.2015.1137402
- Sealey KL, Belcher T, Preston A. Bordetella pertussis epidemiology and evolution in the light of pertussis resurgence. Infect Genet Evol. 2016;40:136–143. doi: 10.1016/j.meegid.2016.02.032
- Chiappini E, Stival A, Galli L, et al. Pertussis re-emergence in the post-vaccination era. BMC Infect Dis. 2013;13:151. doi: 10.1186/1471-2334-13-151
- Burns DL, Meade BD, Messionnier NE. Pertussis resurgence: perspectives from the Working Group Meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis. 2014;209(Suppl. 1):S32–S35. doi: 10.1093/infdis/jit491
- Abe T. Studies on the metabolism of Hemophilus pertussis. I. On the metabolic cycle. Jpn J Exp Med. 1953;23:197–203.
- Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211
- Imaizumi A, Suzuki Y, Ono S, et al. Effect of heptakis (2,6-O-dimethyl) beta-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect Immun. 1983;41:1138–1143.
- Frohlich BT, d'Alarcao M, Feldberg RS, et al. Formation and cell-medium partitioning of autoinhibitory free fatty acids and cyclodextrin's effect in the cultivation of Bordetella pertussis. J Biotechnol. 1996;45:137–148. doi: 10.1016/0168-1656(95)00155-7
- Ma D, Cook DN, Alberti M, et al. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x
- Thanabalu T, Koronakis E, Hughes C, et al. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487
- Tikhonova EB, Zgurskaya HI. Acra, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J Biol Chem. 2004;279:32116–32124. doi: 10.1074/jbc.M402230200
- Hobbs EC, Yin X, Paul BJ, et al. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc Natl Acad Sci USA. 2012;109:16696–16701. doi: 10.1073/pnas.1210093109
- Thoma S, Schobert M. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol Lett. 2009;294:127–132. doi: 10.1111/j.1574-6968.2009.01556.x
- Engler C, Marillonnet S. Golden Gate cloning. Methods Mol Biol. 2014;1116:119–131. doi: 10.1007/978-1-62703-764-8_9
- Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x
- Markham PN, Westhaus E, Klyachko K, et al. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–2408. doi: 10.1128/AAC.43.10.2404
- Bailey AM, Paulsen IT, Piddock LJ. Rama confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob Agents Chemother. 2008;52:3604–3611. doi: 10.1128/AAC.00661-08
- Yang J, Yan R, Roy A, et al. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213
- Sainsbury S, Lane LA, Ren J, et al. The structure of CrgA from Neisseria meningitidis reveals a new octameric assembly state for LysR transcriptional regulators. Nucleic Acids Res. 2009;37:4545–4558. doi: 10.1093/nar/gkp445
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF chimera – a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084
- Field LH, Parker CD. Effects of fatty acids on growth of Bordetella pertussis in defined medium. J Clin Microbiol. 1979;9:651–653.
- Perez-Rueda E, Collado-Vides J. The repertoire of DNA-binding transcriptional regulators in Escherichia coli K-12. Nucleic Acids Res. 2000;28:1838–1847. doi: 10.1093/nar/28.8.1838
- Li B, Yao Q, Pan XC, et al. Artesunate enhances the antibacterial effect of {beta}-lactam antibiotics against Escherichia coli by increasing antibiotic accumulation via inhibition of the multidrug efflux pump system AcrAB-TolC. J Antimicrob Chemother. 2011;66:769–777. doi: 10.1093/jac/dkr017
- Perez A, Poza M, Aranda J, et al. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56:6256–6266. doi: 10.1128/AAC.01085-12
- Bialek-Davenet S, Lavigne JP, Guyot K, et al. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70:81–88. doi: 10.1093/jac/dku340
- Helling RB, Janes BK, Kimball H, et al. Toxic waste disposal in Escherichia coli. J Bacteriol. 2002;184:3699–3703. doi: 10.1128/JB.184.13.3699-3703.2002
- Mishra M, Parise G, Jackson KD, et al. The BvgAS signal transduction system regulates biofilm development in Bordetella. J Bacteriol. 2005;187:1474–1484. doi: 10.1128/JB.187.4.1474-1484.2005
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390.
- Inatsuka CS, Xu Q, Vujkovic-Cvijin I, et al. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect Immun. 2010;78:2901–2909. doi: 10.1128/IAI.00188-10
- Antoine R, Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from gram-positive organisms. Mol Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x
