ABSTRACT
Human babesiosis is an important tick-borne infectious disease. We investigated human babesiosis in the Gansu province and found that it is prevalent in this area with a prevalence of 1.3%. Results of gene sequencings indicate that 1.3% of patients were positive for Babesia divergens. This initial report of human B. divergens infections in Gansu Province should raise awareness of human babesiosis.
Human babesiosis, caused by parasites of the genus Babesia, is an important tick-borne infectious disease that is predominantly transmitted by tick bites and by infected blood transfusions [Citation1]. The first case of human babesiosis was reported in Zagreb, Croatia, in 1957 and subsequently in Asia, Africa, South and North America, and Europe [Citation1–3]. At least nine Babesia spp. have been identified so far from humans: Babesia crassa, Babesia sp. TW1, and Babesia sp. XXB/HangZhou (China), Babesia microti (China, Europe, Bolivia and America) [Citation3–5], Babesia divergens and Babesia sp. EU1/Babesia venatorum (China and Europe), Babesia microti-like (Japan), Babesia duncani (America) and Babesia sp. KO1 (Korea) [Citation6–10].
Recently, increasing numbers of cases of human babesiosis have been reported in China, caused by two new emerging Babesia species, named B. crassa and Babesia sp. XXB/HangZhou. However, few surveys of the prevalence of human babesiosis have been conducted in northeastern China. Available results revealed the higher prevalence of B. crassa and B. venatorum [Citation11]. However, despite the wide distribution of tick species in Gansu province, northwestern China, including 7 Ixodes spp., 13 Haemaphysalis spp., 5 Hyalomma spp. and 3 Dermacentor spp. which are all possible vectors of Babesia spp. that are infective to humans, no cases or relevant epidemiological data have been published.
This study was conducted in a total of 754 patients who lived in the Gannan Tibetan Autonomous Prefecture, Gansu province and visited the Second Hospital of Lanzhou University for a tick bite in the past few months between April 2016 and March 2018. A standardized questionnaire was applied to record the patient's basic information, including sex, age, career, and history of tick bites and blood transfusion. Two blood samples were taken from each patient: one was sent to the medical laboratory for clinical examination, whereas the second was analysed using nested PCR (nPCR) assays to detect the presence of any Babesia spp. All participants agreed to participate in this study and signed an informed consent form. The study was approved by the Ethics Committee of The Second Hospital of Lanzhou University (reference 2018A-046). All the procedures conducted were according to the Ethical Procedures and Guidelines of the People's Republic of China.
The collected blood specimens were used to extract genomic DNA by using a commercially available DNA extraction kit (QIAamp DNA Blood Mini-Kit, USA) according to the manufacturer's instructions. nPCR assays targeting the 18S ribosomal RNA (18S rRNA) gene were applied to detect piroplasm by using two pairs of primers (Piro1-S: 5′-CTTGACGGTAGGGTATTGGC-3′, Piro3-AS: 5′-CCTTCCTTTAAGTGATAAGGTTCAC-3′ and Piro-A: 5′-ATTACCCAATMCBGACACVGKG-3′ and Piro-B: 5′-TTAAATACGAATGCCCCCAAC-3′), as previously described [Citation12,Citation13]. The positive amplicons (∼400 bp) were purified by using a gel DNA purification kit (ZYMO, USA) according to the manufacturer's instructions. The purified amplicons were cloned into pGEM-Teasy vectors (Promega, USA) and recombinant plasmids were sent for sequencing to Genscript (Nanjing, China). The obtained sequences were run through a Blast analysis on the National Center for Biotechnology Information (NCBI) website using the BLASTn program. A set of primers, PIRO-F (5′-GGATAACCGTGSTAATTSTAGGGC-3′) and PIRO-R (5′-GTGTGTACAAAGGGCAGGGACG-3′), was used to amplify the long fragment of 18S rRNA for further confirmation of the Babesia species detection [Citation13]. To avoid a risk of contamination, genomic DNA isolation from blood samples, PCR amplification and agarose gel electrophoresis were performed by different operators and in separated rooms. On the other hand, there was no human samples infected Babesia spp. including B. divergens in our laboratory based on our record.
Of the 754 blood samples, 10 (1.3%) were positive for piroplasms upon molecular amplification and gene sequencing. BLASTn results indicated that 10 sequences are identical to each other. A representative sequence was submitted to GenBank with accession number MK256977. These isolates could be classified as B. divergens group and shared 99.9% identity with that of B. divergens derived from Europe (Accession No. AY046576) (). The age of the 10 patients ranged from 22 to 60 years (median 41.4 years old). All patients were immunocompetent and only two of them showed clinical symptoms at the time of sampling, such as fever, asthenia and headache. Clinical and laboratory data for the 10 patients are presented in .
Figure 1. Phylogenetic tree of Babesia spp. sequences based on the 18S rRNA genes obtained in this study (bold triangles) and those previously registered in GenBank. The tree was constructed using the neighbour joining method of MEGA7, and values are given at the nodes. Numbers above the branch demonstrate bootstrap support from 1000 replications.
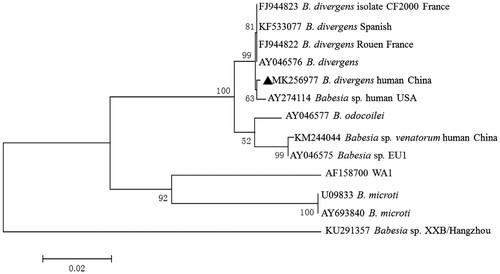
Table 1. Clinical information of 10 patients with Babesia species infections in Gansu province, China.
In this study, our findings have several unusual characteristics. First, several cases of Babesia infections in human were identified in Gansu province located in northwestern rather than eastern or northeastern China, where almost all of these cases were already reported. Our study is the first to report the presence of Babesia infection in humans in Gansu province, northwestern China. The infected parasites were identified as B. divergens that is an etiological agent of bovine babesiosis but has not been identified in cattle in China. By contrast, B. divergens is associated with poor patient outcomes, particularly in aged, asplenic and/or immunocompromised patients.
To our knowledge, only one case of human babesiosis, caused by B. venatorum, has been reported in northwestern China, in Xinjiang, with most occurrences (more than 100 cases) of babesiosis reported in eastern or northeastern China [Citation11]. One case and eleven cases caused by B. microti were reported in Yunnan province (southwestern China) and Zhejiang province (eastern China), respectively. Two cases caused by B. divergens were reported in Shandong province in eastern China, whereas 48 cases caused by B. venatorum and 31 caused by B. crassa were reported in forested areas of northeastern China [Citation9,Citation13]. Babesia sp. XXB/HangZhou infection in an immunocompetent patient was reported in Zhejiang province in eastern China [Citation8]. Additionally, the etiological agents of several human babesiosis cases, reported in Chongqing and Yunnan provinces, in China, have not been identified.
Most of the patients enrolled in this study visited hospital either for physical examination or as outpatients; therefore, it was not possible to collate data concerning the outcomes of infection for these patients. However, our results suggest that human babesiosis is prevalent in this region and, thus, residents should take steps to protect themselves against exposure to ticks. Asymptomatic patients carrying etiological agents of human babesiosis in their blood might be responsible for increasing the number of cases of transfusion-transmitted babesiosis [Citation14,Citation15]. Thus physicians should pay increased attention to this life-threating disease and be aware of the differential diagnoses and treatments available for babesiosis. Given the threat to public health, systematic epidemiological surveys should also be carried out to determine the real prevalence of this disease.
Acknowledgements
We thank numerous volunteers and their families for participating in this study. We are grateful to Xiaoxing Wang, Zhi Li, Jianlin Xu and Xuan Li for carrying out the genomic DNA extraction.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi:10.1056/NEJMra1202018.
- Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–16.
- Meer-Scherrer L, Adelson M, Mordechai E, et al. Babesia microti infection in Europe. Curr Microbiol. 2004;48:435–437. doi:10.1007/s00284-003-4238-7.
- Kogut SJ, et al. Babesia microti, upstate New York. Emerg Infect Dis. 2005;11:476–478. doi:10.3201/eid1103.040599.
- Hunfeld KP, et al. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J Clin Microbiol. 2002;40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002
- Kim JY, et al. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol. 2007;45:2084–2087. doi:10.1128/JCM.01334-06.
- Shih CM, Liu LP, Chung WC, et al. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454.
- Man SQ, et al. A case of human infection with a novel Babesia species in China. Infect Dis Poverty. 2016;5:28, doi:10.1186/s40249-016-0121-1.
- Jia N, et al. Human babesiosis caused by a Babesia crassa-like pathogen: a case series. Clin Infect Dis. 2018;67:1110–1119. doi:10.1093/cid/ciy212.
- Gonzalez LM, et al. First report of Babesia divergens infection in an HIV patient. Int J Infect Dis. 2015;33:202–204. doi:10.1016/j.ijid.2015.02.005.
- Jiang JF, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203. doi:10.1016/S1473-3099(14)71046-1.
- Olmeda AS, et al. A subtropical case of human babesiosis. Acta Trop. 1997;67:229–234. doi: 10.1016/S0001-706X(97)00045-4
- Yang JF, et al. Molecular evidence for piroplasms in wild Reeves’ muntjac (Muntiacus reevesi) in China. Parasitol Int. 2014;63:713–716. doi:10.1016/j.parint.2014.06.002.
- Castro E, et al. The efficacy of the ultraviolet C pathogen inactivation system in the reduction of Babesia divergens in pooled buffy coat platelets. Transfusion. 2014;54:2207–2216. doi:10.1111/trf.12598.
- Cursino-Santos JR, Alhassan A, Singh M, et al. Babesia: impact of cold storage on the survival and the viability of parasites in blood bags. Transfusion. 2014;54:585–591. doi:10.1111/trf.12357.
