ABSTRACT
Yellow Fever (YF) remains a major public health issue in Sub-Saharan Africa and South America, despite the availability of an effective vaccine. In Africa, most YF outbreaks are reported in West Africa. However, urban outbreaks occurred in 2016 in both Angola and the Democratic Republic of Congo (DRC), and imported cases were reported in Chinese workers coming back from Africa. In Central Africa, Cameroon and the Republic of Congo host a high proportion of non-vaccinated populations increasing the risk of urban outbreaks. The main vector is Aedes aegypti and possibly, Aedes albopictus, both being anthropophilic and domestic mosquitoes. Here, we provide evidence that both Ae. aegypti and Ae. albopictus in Cameroon and the Republic of Congo are able to transmit Yellow fever virus (YFV) with higher rates of infection, dissemination, and transmission for Ae. aegypti. We conclude that the potential of both Aedes species to transmit YFV could increase the risk of urban YF transmission and urge public health authorities to intensify their efforts to control domestic vectors, and extend vaccine coverage to prevent major YFV outbreak.
Background
Yellow fever (YF) is a mosquito borne viral disease endemic in South America and Sub-Saharan African countries. Clinical signs vary from a fever with aches and pains to severe liver disease with bleeding and yellowing skin (jaundice), for which there is no specific treatment. Despite the availability of an effective vaccine, which can offer a lifelong immunity, numerous cases of YF are still being reported. Indeed, a modelling study based on African data sources estimated that the burden of YF during 2013 was 84 000–170 000 severe cases and 29 000–60 000 deaths [Citation1]. Yellow fever virus (YFV, Flavivirus, Flaviviridae) is transmitted to humans and non-human primates mainly by bites of infected mosquitoes belonging to Aedes and Haemagogus genera. This virus primarily circulates in the forest between non-human primates and sylvatic Aedes spp. mosquitoes (e.g. Ae. africanus) in Africa and Haemagogus spp. in South America [Citation2]. Nevertheless, YF can spread widely in urban environments when transmitted from human to human by the anthropophilic mosquitoes, Aedes aegypti [Citation3] or potentially, Aedes albopictus [Citation4,Citation5]. Indeed, between 2015 and 2016 in Central Africa, major urban YF outbreaks occurred in Angola and Democratic Republic of Congo with 7,334 suspected cases, of which 962 have been confirmed, and 393 deaths [Citation6]. Aedes aegypti was suspected as the main YFV vector involved during the Angola outbreak due to its high densities reported across the country [Citation7]. On the other hand, recent studies on entomological surveillance in Central Africa particularly in Cameroon [Citation8] and the Republic of Congo [Citation9], where sporadic cases of YF were frequently reported, showed that Ae. aegypti is present in all urban environments while Ae. albopictus introduced in 2000s has a distribution limited under 6°N latitude. In sympatric areas, Ae. albopictus tends to be the most prevalent species by replacing the resident species Ae. aegypti [Citation8–10]. In Cameroon, the first isolation of YFV was in 1990 during an outbreak with 180 cases, of which 125 fatalities [Citation11]. The suspected mosquito vectors were Ae. aegypti, Ae. furcifer, and Ae. luteocephalus. From 2010 to 2016, 13,837 suspected cases of YF were reported of which 109 cases were confirmed with 66% mostly in rural areas [Citation7]. The epidemiological importance of both vectors in urban YFV transmission in Central Africa has not been assessed precisely up to now. As the vector competence is one of the key parameters to assess the pathogen transmission, we undertook a study aimed at establishing the ability of Ae. aegypti and Ae. albopictus populations collected in different urban settings in Central Africa to transmit YFV strain isolated in West Africa.
Material and methods
Ethics statement
This study was approved by the Cameroonian national ethics committee for human health research N˚2017/05/911/CE/CNERSH/SP. Oral consent to inspect the potential breeding sites was obtained in the field in household or garage owners. The Institut Pasteur animal facility received accreditation from the French Ministry of Agriculture to perform experiments on live animals in compliance with the French and European regulations on care and protection of laboratory animals (EC Directive 2010/63, French Law 2013-118, 6 February 2013). All experiments were approved by the Ethics Committee and registered under the reference APAFIS6573-201606l412077987 v2.
Mosquito sampling
Larvae and pupae were collected from August 2017 to April 2018 in several locations in Central Africa including the Republic of Congo (Brazzaville) and Cameroon (Yaoundé, Douala, Tibati and Bénoué National Park). Each of these locations has been previously described [Citation8,Citation9] except Bénoué National Park (8°20′N, 13°50′E); it is a biosphere reserve located in Northern part of Cameroon on the Bénoué River plain, at the foot of the Adamawa plateau. In Benoué park, Aedes larvae were collected across the park in tree holes (1), tin cans (15), used tires (2) and discarded chair (1). For other locations, mosquitoes were collected in peri-urban and downtown in a minimum of 20 containers per environment. Immature stages of Aedes were transported in the insectary and pooled together according to the city and raised until adults before morphological identification using criteria established by Jupp (1996) [Citation12]. Adult mosquitoes were pooled together according to the location, species and reared at 28°±1°C under 12 h dark:12 h light cycle and 80% relative humidity. Eggs obtained () were transported to the Institut Pasteur in Paris, reared to adult stage and used to challenge with YFV.
Table 1. Origin of Ae. aegypti and Ae. albopictus used for vector competence.
Virus strain
YFV was isolated from a human case in Senegal in 1979 (YFV S79; accession number: MK060080) [Citation13]. YFV S79 was passaged twice on newborn mice and two times on C6/36 cells. Viral stocks were produced on Aedes albopictus C6/36 cells.
Challenge mosquitoes with YFV
For each population, six batches of 60 7–10 day-old females were challenged with an infectious blood meal containing 1.4 mL of washed rabbit erythrocytes and 700 μL of viral suspension. The blood meal was supplemented with adenosine 5’-triphosphate (ATP) as a phagostimulant at a final concentration of 1 mM and provided to mosquitoes at a titer of 107 focus-forming unit (FFU)/mL using a Hemotek membrane feeding system (Hemotek Ltd, Blackburn, UK). Mosquitoes were allowed to feed for 20 min through a piece of pork intestine covering the base of a Hemotek feeder maintained at 37°C. Fully engorged females were transferred in cardboard containers and maintained with 10% sucrose under controlled conditions (28±1°C, relative humidity of 80%, light:dark cycle of 12h:12 h) for up to 21 days with mosquito analysed at 14 and 21 days post-infection (dpi). 21–32 mosquitoes were examined at each dpi.
Infection, dissemination and transmission assays
For each mosquito examined, body (abdomen and thorax) and head were tested respectively for infection and dissemination rates at 14 and 21 dpi per population when the number permitted. For this, each part was ground individually in 300 μL of L15 medium (Invitrogen, CA, USA) supplemented with 2% fetal serum bovine (FBS), and centrifuged at 10,000×g for 5 min at +4°C. The supernatant was processed for viral titration. Saliva was collected from individual mosquitoes at 21 dpi using the forced salivation technique as described previously [Citation14]. Briefly, mosquitoes were cool anesthetized, wings and legs of each mosquito were removed and the proboscis inserted into a tip of 20 µL containing 5 µL of FBS. After 30 min, FBS containing saliva was added to 45 µL of L15 medium for titration. Transmission rates were assessed only at 21 dpi based on previous studies demonstrated that higher transmission rates were reported at this time point [Citation15].
Infection rate (IR) refers to the proportion of mosquitoes with infected body (i.e. abdomen and thorax) among tested mosquitoes. Disseminated infection rate (DIR) corresponds to the proportion of mosquitoes with infected head among the previously detected infected mosquitoes (i.e. virus positive abdomen/thorax). Transmission rate (TR) represents the proportion of mosquitoes with infectious saliva among mosquitoes with disseminated infection. Vector competence can be summarized by the transmission efficiency (TE) which was calculated as the proportion of mosquitoes with infectious saliva among all mosquitoes tested [Citation16].
Viral titration by focus forming assay
Samples were titrated by focus fluorescent assay on C6/36 Ae. albopictus cells [Citation17]. Body, head and saliva suspensions were serially diluted in L15 medium supplemented with 2% of FBS and inoculated onto cells in 96-well plates. After an incubation of 5 days at 28°C, samples were fixed with 0.1 mL/well of formaldehyde 3.6% in phosphate buffer saline (PBS) during 20 min at room temperature. Then, plates were stained using antibodies specific to YFV (Bio-techne, Minneapolis, Minnesota, USA) as the primary antibody and conjugated Alexa Fluor 488 goat anti-mouse IgG as the second antibody (Life Technologies, California, USA). Titers were expressed as FFU/mL.
Statistical analysis
All statistical analyses were performed with R software v 3.5.2 (R Core Team, Vienna, Austria). Qualitative variables were expressed as proportion and compared using Fisher’s exact test the RVAideMemoire package and quantitative variables by mean and compared using non-parametric test of Kruskal–Wallis because of non-normal distribution. P-value <0.05 was considered as statistically different.
Results
Infection and disseminated infection rates in Ae. albopictus and Ae. aegypti
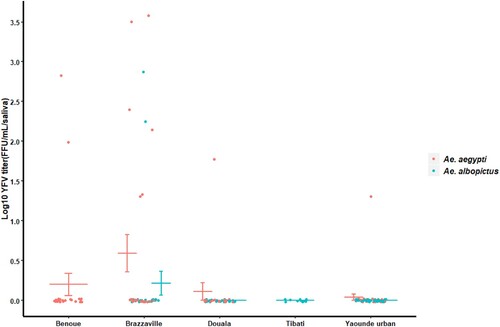
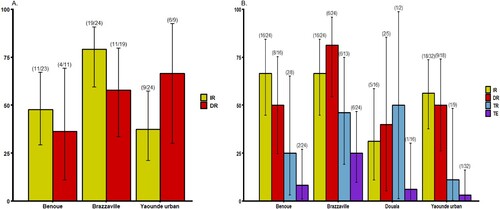
Mosquitoes were analysed at two time points following the infectious blood meal when the number of mosquitoes was sufficient: 14 and 21 days post infection (dpi) ( and ). At 14 dpi, no significant difference of infection rate (IR) and disseminated infection rate (DIR) was found between Ae. albopictus populations, respectively P = 0.89 and P = 0.18 (Fisher’s exact test) (A). Besides, in Ae. aegypti, IRs were significantly different (Fisher’s exact test: P=0.01) ranging from 37.5% in Yaoundé to 79.2% in Brazzaville (A) while DIRs were not significantly different (Fisher’s exact test: P = 0.49). When considering all populations of same species, IRs for Ae. aegypti (mean = 54.9%) was significantly higher than Ae. albopictus (mean = 30.7%) (Fisher’s exact test: P = 0.02) while DIRs were not significantly different (Ae. aegypti: mean = 56.8% and Ae. albopictus: mean = 54.5%). At 21 dpi, IRs for Ae. albopictus ranged from 16.8% in Douala to 54.5% in Tibati and were not statistically different (B; Fisher’s exact test: P=0.08). In Ae. albopictus, DIRs ranged from 0 for Douala population suggesting no dissemination to 55% in Brazzaville population. Meanwhile, for three populations where viral dissemination was reported, no statistical difference was found (B; Fisher’s exact test: P = 0.34). In Ae. aegypti, IRs varied between 31.2% (Douala) and 66.7% (Bénoué, Brazzaville) and were not statistically different (Fisher’s exact test: P = 0.11). DIRs were not significantly different (Fisher’s exact test: P = 0.14) ranging from 40% (Douala) to 81.2% (Brazzaville) (B). Overall, at 21 dpi, IRs were higher for Ae. aegypti (54.5%) than for Ae. albopictus (16.7%) (Fisher’s exact test: P = 0.005) while for DIRs, no significant difference was reported between both species (Fisher’s exact test: P > 0.5). When considering the two mosquito species from a same location, IRs and DIRs were not significantly different (Fisher’s exact test: P > 0.05) except for IRs of Ae. aegypti and Ae. albopictus from Brazzaville at 14 dpi (P = 0.013).
Figure 1. Infection, disseminated infection, transmission rates and transmission efficiency of Ae. albopictus from Central Africa to yellow fever virus. (A) Infection and disseminated infection rates at 14 days post-infection (dpi). (B) Infection, disseminated infection, transmission rates and transmission efficiency at 21 dpi. Error bars show the 95% confidence interval. In brackets, the number of mosquitoes examined. IR: the proportion of mosquitoes with infected body among engorged mosquitoes; DIR: the proportion of mosquitoes with infected head among mosquitoes with infected body; TR: the proportion of mosquitoes with infectious saliva among mosquitoes with infected head. TE: the proportion of mosquitoes with infectious saliva among all analysed ones.
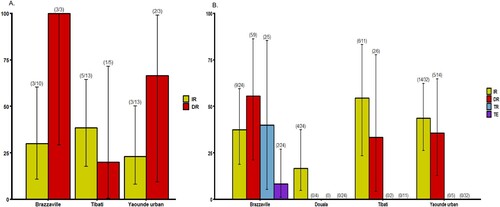
Figure 2. Infection, disseminated infection, transmission rates and transmission efficiency of Ae. aegypti from Central Africa to yellow fever virus. (A) Infection and disseminated infection rates at 14 days post-infection (dpi). (B) Infection, disseminated infection, transmission rates and transmission efficiency at 21 dpi. Error bars show the 95% confidence interval. In brackets, the number of mosquitoes examined. IR: the proportion of mosquitoes with infected body among engorged mosquitoes; DIR: the proportion of mosquitoes with infected head among mosquitoes with infected body; TR: the proportion of mosquitoes with infectious saliva among mosquitoes with infected head. TE: the proportion of mosquitoes with infectious saliva among all analysed ones.
Transmission rate and transmission efficiency
Our analysis showed that YFV was able to replicate, disseminate and be excreted in saliva of both Ae. albopictus and Ae. aegypti (B and 2B). However, in Ae. albopictus, YFV was detected only in saliva of Brazzaville population. In contrast, in Ae. aegypti, YFV was found in saliva of all tested populations with transmission rate (TR) and transmission efficiency (TE) ranging from 11.1% (Yaoundé) to 50% (Douala) and 3.2% (Yaoundé) to 25% (Brazzaville) respectively. Collectively, Ae. aegypti exhibited a higher TE (10.4%) than Ae. albopictus populations (2.2%) (Fisher’s exact test: P = 0.03). In Ae. aegypti, viral titers varied significantly from Yaoundé population to Brazzaville population (; Chi-squared = 7.91; df = 3; P = 0.04). In Ae. albopictus Brazzaville population, viral load in saliva was higher than in some Ae. aegypti populations ().
Discussion
Yellow fever virus is circulating in Central Africa where massive outbreaks have been reported recently in Angola and the Democratic Republic of Congo [Citation6] in spite of the availability of an effective vaccine. In this study, we assessed the ability of Ae. aegypti and Ae. albopictus collected in different ecological settings in Cameroon and the Republic of Congo to transmit YFV isolated from a human case in Senegal. Our analysis showed that YFV was able to replicate, disseminate and be excreted in saliva of both Ae. aegypti and Ae. albopictus from Central Africa at 21 dpi. High levels of infection and disseminated infection rates were reported in both species from different locations. YFV was only detected in saliva of a single population of Ae. albopictus from Brazzaville (Congo) at 21 dpi with a transmission rate comparable to that found for Ae. albopictus populations from South France and Morocco [Citation4,Citation18], suggesting a low potential of this species to sustain an active viral transmission. Furthermore, YFV was found at 21 dpi in saliva of all populations of Ae. aegypti from different ecological settings, indicating a higher epidemiological risk related to this mosquito in urban areas. Interestingly, transmission rate reported in Ae. aegypti populations was similar to those reported in previous studies undertaken in Carbo Verde [Citation19], Brazil [Citation20] and Guadeloupe [Citation15] using the same YFV strain (Senegal 1979). The unique Ae. albopictus population in which virus was detected has a higher viral load than many other Ae. aegypti populations tested suggesting that in some areas, Ae. albopictus could intervene in YFV transmission. This result is quite alarming since Ae. albopictus has been found most prevalent in some rural [Citation21], urban and peri-urban environments [Citation8–10] in Central Africa. Interestingly, Ae. albopictus has been found naturally infected by YFV in Brazil and could serve as bridge vector for transferring enzootic YFV at the urban-forest/rural interface in Central Africa into cities as suggested previously in Brazil [Citation22]. However, other factors should be considered to determine if a mosquito species can act as a vector under natural conditions: mosquito lifespan, trophic preferences or vector abundance [Citation23]. Likewise, bacterial symbionts of mosquitoes have been shown to alter the vector competence to arboviruses [Citation24]; Ae. albopictus from Brazzaville might have undergone changes in bacteria composition as the 5th generation in the laboratory was used for experimental infections.
Our experiment is the first one establishing the vector competence of Ae. aegypti and Ae. albopictus towards YFV in Central Africa. We showed that Ae. albopictus in a populated city like Brazzaville, can experimentally transmit YFV at 21 dpi suggesting a potential of this species to participate in YFV transmission but perhaps too late to pose an immediate threat for the region. However, YFV can evolve by becoming more adapted for a higher transmission by an unusual vector species [Citation5]. Further studies using a local strain of YFV circulating in Central Africa are needed to validate these results. Our findings support the efforts needed in vector surveillance and control, and vaccine coverage to prevent major YFV outbreak as reported recently in Angola.
Acknowledgments
We would like to thank the populations of different collection sites for their collaboration during the field works. BK, CSW and ABF designed the experiments. BK performed the research. MV and LM provided a technical help. ANT, APY, TAWB helped in mosquito collections; BK, CSW and ABF wrote the paper with contribution from all other authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Garske T, Van Kerkhove MD, Yactayo S, et al. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014 May;11(5):e1001638. doi: 10.1371/journal.pmed.1001638
- Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454
- WHO. A global strategy to eliminate yellow fever epidemics 2017–2026. Geneva: World Health Organization; 2018 Jan.
- Amraoui F, Vazeille M, Failloux AB. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveillance. 2016 Sep 29;21(39).
- Amraoui F, Pain A, Piorkowski G, et al. Experimental Adaptation of the yellow fever virus to the mosquito Aedes albopictus and potential risk of urban epidemics in Brazil, South America. Sci Rep. 2018 Sep 25;8(1):14337. doi: 10.1038/s41598-018-32198-4
- Kraemer MUG, Faria NR, Reiner Jr. RC, et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect Dis. 2017 Mar;17(3):330–338. doi: 10.1016/S1473-3099(16)30513-8
- Marquetti Fernández MC, Hidalgo Flores Y, Lamothe Nuviola D. Longitudinal spatial distribution of Aedes aegypti (Diptera: Culicidae) during the yellow fever epidemic in Angola, 2016. Glob J Zool. 2019;4(1):0001–0006. doi: 10.17352/gjz.000011
- Tedjou AN, Kamgang B, Yougang AP, et al. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2019 Mar 18;13(3):e0007137. doi: 10.1371/journal.pntd.0007137
- Kamgang B, Wilson-Bahun TA, Irving H, et al. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018;3:79. doi: 10.12688/wellcomeopenres.14659.3
- Kamgang B, Ngoagouni C, Manirakiza A, et al. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis. 2013;7(12):e2590. doi: 10.1371/journal.pntd.0002590
- Vicens R, Robert V, Pignon D, et al. [Yellow fever epidemic in the extreme North of Cameroon in 1990: first yellow fever virus isolation in Cameroon]. Bull World Health Organ. 1993;71(2):173–176.
- Jupp PG. Mosquitoes of Southern Africa. Culicinae and Toxorhynchitinae. Hartebeespoort: Ekogilde; 1996 Mar - Apr.
- Rodhain F, Hannoun C, Jousset FX, et al. [Isolation of the yellow fever virus in Paris from 2 imported human cases]. Bull Soc Pathol Exot Filiales. 1979 Sep-Dec;72(5-6):411–415.
- Dubrulle M, Mousson L, Moutailler S, et al. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4(6):e5895. doi: 10.1371/journal.pone.0005895
- Yen PS, Amraoui F, Vega Rua A, et al. Aedes aegypti mosquitoes from Guadeloupe (French West Indies) are able to transmit yellow fever virus. PloS one. 2018;13(9):e0204710. doi: 10.1371/journal.pone.0204710
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016 Mar;10(3):e0004543. doi: 10.1371/journal.pntd.0004543
- Payne AF, Binduga-Gajewska I, Kauffman EB, et al. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006 Jun;134(1-2):183–189. doi: 10.1016/j.jviromet.2006.01.003
- Amraoui F, Ben Ayed W, Madec Y, et al. Potential of Aedes albopictus to cause the emergence of arboviruses in Morocco. PLoS Negl Trop Dis. 2019 Feb;13(2):e0006997. doi: 10.1371/journal.pntd.0006997
- Vazeille M, Yebakima A, Lourenco-de-Oliveira R, et al. Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vector Borne Zoonotic Dis. 2013 Jan;13(1):37–40. doi: 10.1089/vbz.2012.0982
- Couto-Lima D, Madec Y, Bersot MI, et al. Potential risk of re-emergence of urban transmission of yellow fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017 Jul 7;7(1):4848. doi: 10.1038/s41598-017-05186-3
- Paupy C, Kassa Kassa F, Caron M, et al. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector Borne Zoonotic Dis. 2012 Feb;12(2):167–169. doi: 10.1089/vbz.2011.0736
- Pereira Dos Santos T, Roiz D, Santos de Abreu FV, et al. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg Microbes Infect. 2018 Nov 28;7(1):191. doi: 10.1038/s41426-018-0194-y
- Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/S0065-3527(03)60006-0
- Jupatanakul N, Sim S, Dimopoulos G. The insect microbiome modulates vector competence for arboviruses. Viruses. 2014 Nov 11;6(11):4294–4313. doi: 10.3390/v6114294