ABSTRACT
Candida auris is an emerging worldwide concern, but comparative data about the virulence of different C. auris lineages in mammalian hosts is lacking. Different isolates of the four prevalent C. auris clades (South Asian n = 5, East Asian n = 4, South African n = 5, and South American n = 5) were compared to assess their virulence in a neutropenic murine bloodstream infection model with C. albicans as reference. C. auris, regardless of clade, proved to be less virulent than C. albicans. Highest overall mortality at day 21 was observed for the South American clade (96%), followed by the South Asian (80%), South African (45%) and East Asian (44%) clades. Fungal burden results showed close correlation with lethality. Histopathological examination revealed large aggregates of blastoconidia and budding yeast cells in the hearts, kidneys and livers but not in the spleens. The myocardium of apparently healthy sacrificed mice as well as of mice found moribund showed contraction band necrosis in case of all lineages. Regardless of clade, the heart and kidneys were the most heavily affected organs. Isolates of the same clade showed differences in virulence in mice, but a markedly higher virulence of the South American clade was clearly demonstrated.
Introduction
Candida auris was first identified in 2009 at a geriatric hospital in Japan and then in a nosocomial fungemia in Korea in 2011 [Citation1,Citation2]. Retrospective identification by DNA sequencing revealed the earliest known strain of C. auris was a bloodstream isolate from 1996 in a paediatric surgery patient in Korea, and the next earliest was found in 2008 in Pakistan [Citation3]. In the last decade C. auris became an emerging organism with a public health significance causing asymptomatic colonization (skin, urine, indwelling devices) as well as sporadic hospital-acquired invasive infections (i.e. candidemia, intra-abdominal infections) worldwide [Citation4]. Clinical studies showed that while C. auris affects both children and adults, it has been identified predominantly in critically ill patients in intensive care units [Citation4–6]. Moreover, this pathogen is commonly multidrug resistant, posing a therapeutic challenge, however, susceptibility profiles may vary widely across isolates [Citation4,Citation7].
Five phylogenetically distinct clades (South Asian, East Asian, South African, South American and Iranian) of C. auris were identified based on whole genome sequence data of global clinical isolates [Citation3,Citation8]. Each clade differs from the other clades by >200,000 single-nucleotide polymorphisms, but isolates from the same clade are highly clonal, suggesting emergence of C. auris on all continents almost simultaneously.
The growing body of data suggests that significant differences exist between the five clades regarding their phenotypic characteristics and virulence [Citation4,Citation9,Citation10]. In previous studies we have demonstrated that isolates belonging to South African and East Asian clades grew better in the presence of actidione, but do not produce even rudimentary pseudohyphae in Dalmau cultures as compared to South Asian clade [Citation9]. The vast majority (84%) of South African isolates produce large aggregates in aqueous suspension on microscope slides and these isolates proved to be significantly less virulent in Galleria mellonella than the non-aggregating isolates of the South Asian lineage [Citation10]. Moreover, isolates belonging to the South Asian, South African and South American clades but not the East Asian clade frequently cause outbreaks of invasive infections [Citation4,Citation11]. A single isolate representing a novel Iranian clade has been recently reported, but data on its pathogenicity and virulence is lacking [Citation8].
Data about in vivo virulence of different C. auris clades in mammalian hosts is scant, the available studies focus mainly on the South Asian clade [Citation12-17], moreover, the South African clade has never been examined. Fakhim et al. infected ICR outbred immunocompetent mice with inocula of 105-, 106 and 107 CFU/mouse using two C. auris (both from the South Asian clade) and one C. albicans strains. They did not find statistically significant differences regarding lethality (60-80%) or fungal tissue burdens between C. auris and C. albicans [Citation12]. In contrast, a single C. auris isolate from the South Asian clade proved to be significantly less virulent (0% lethality) than C. albicans (100% lethality) despite the 10-fold lower intravenous inocula of C. albicans in an immunocompetent BALB/c mouse model [Citation13]. In another study using a temporarily neutropenic BALB/c murine model, 7 × 107 CFU/mouse inoculated intravenously produced significantly shorter survival of mice with C. albicans than with the one C. auris isolate from the South Asian clade tested [Citation14]. In a further study with two South Asian isolates even 108 CFU/mouse did not cause death in immunocompetent BALB/c mice, but using immunosuppression with neutrophil-depleting antibodies (RB6-8C5 and 1A8) led to 100% lethality with the same dose [Citation15]. Similar lethality (100%) was obtained by Singh et al. with a single South Asian isolate, using 5 × 107 CFU/mouse inoculated intravenously in a deeply neutropenic CD-1 murine model [Citation16].
Xin et al., intravenously infected four different mouse strains with single C. auris isolates from the East Asian and South American lineages. They did not find lethality in BALB/c, neutrophil elastase-deficient and C57BL/6 non-neutropenic mice and only a few deaths in neutropenic models even using an infectious dose as high as 2 × 108 CFU/mouse. In contrast, the same dose produced 100% mortality within 2 days in A/J mice with C5 complement factor deficiency [Citation17].
In this study we compared the behaviour of 17 clinical isolates derived from sterile and non-sterile body sites belonging to four C. auris lineages (South Asian, East Asian, South African, and South American) using a neutropenic murine bloodstream infection model. We also tested two additional C. auris isolates of environmental origin from the South American clade. As previous data from the G. mellonella model have suggested that at least some C. auris isolates showed virulence similar to C. albicans, we included two C. albicans bloodstream isolates in the same model for comparison [Citation10].
Materials and methods
Isolates
Isolates of the four prevalent C. auris clades (South Asian n = 5, East Asian n = 4, South African n = 5, South American n = 5) tested, their origin and the origin of the C. albicans isolates are listed in . C. auris isolates were identified by a combination of ribosomal DNA gene sequencing targeting the 28S rRNA and/or ITS1 regions, which was also used for clade delineation [Citation9,Citation10]. C. albicans isolates were derived from a previous study [Citation18]. Strains were stored at –70°C. Two days before the in vivo experiments, isolates were subcultured using Sabouraud agar and screened on CHROMagar Candida (Becton Dickinson) to ensure purity of Candida isolates.
Table 1. Characteristics of Candida auris and Candida albicans isolates used in the study. Bold indicates the isolates used in the fungal burden experiments.
Preparation of inocula
Yeast inocula were initially prepared in sterile saline, but in case of the South African isolates large, visible and highly stable aggregates were observed in the tubes or in the syringes at 106 and 107 CFU/mL; homogeneous suspensions were never obtained in saline. Aggregate formation was never observed with the other three C. auris clades nor with C. albicans isolates. Thus, to avoid aggregate formation, inocula were prepared in phosphate-buffered saline (PBS) and washed three times.
Mice and immunosuppression
In our preliminary experiments with immunocompetent BALB/c mice using inoculum densities of 106 and 107 CFU C. auris per mouse prepared in PBS all mice survived. With the isolates of the South African clade aggregating in saline (but not with the three aggregating isolates from the East Asian lineage, ), infectious doses >107 CFU/mice produced early mortality (within one day) in 10-40% of mice, most probably due to embolization by fungal aggregates, which were either too small to be visible to the naked eye or produced in vivo. As early mortality was never observed at lower cell densities, we abandoned the immunocompetent model and used an immunocompromised murine model with the lower 107 CFU/mouse inoculum for all four lineages to allow robust comparisons.
BALB/c male mice (23–25 g) were given cyclophosphamide 4 days before infection (150 mg/kg), 1 day before infection (100 mg/kg), 2 and 5 days post-infection (100 mg/kg) in the fungal tissue burden experiments; in the lethality experiments, immunosuppression was continued by administration of 100 mg/kg cyclophosphamide every third day until the end of the experiment at the 21st day [Citation18]. The Guidelines for the Care and Use of Laboratory Animals were strictly followed during maintenance of the animals; experiments were approved by the Animal Care Committee of the University of Debrecen (permission no. 12/2014).
Lethality experiments
Mice (groups of 10 mice/isolate) were infected intravenously through the lateral tail vein (day 0). The infectious dose for C. auris and C. albicans were 107 and 105 CFU/mouse, respectively, in volumes of 0.2 mL. Inoculum densities were confirmed by plating serial dilutions on Sabouraud agar plates.
Mice were monitored twice daily for lethality for 21 days. Animals that became immobile or showed signs of severe illness were terminated and recorded as dying on the following day. Dead animals (1–2 from each clade) were dissected for histopathology. Survival rates were compared within the same clade using the Kaplan–Meier log rank test; then data of isolates of the same clade were aggregated and survival was also analysed between different fungal clades [Citation18]. Statistical tests were performed in Graph Pad 6.0.3.
Fungal kidney tissue burden experiments
The infectious doses for C. auris and C. albicans were 107 and 5 × 104 CFU/mouse, respectively. Two C. auris isolates of each clade and one C. albicans isolate were chosen based on the lethality experiments (). One group included 11 mice. On day 2 and 6, 5 mice were sacrificed; both kidneys, the heart, the liver and the spleen were removed from each animal, weighed and homogenized aseptically in 1 ml of saline; the resulting tissue suspension was serially diluted. Fungal tissue burden was determined by quantitative culturing. The lower limit of detection was 100 CFU/g of tissue [Citation18].
Mean fungal tissue burdens produced by different isolates/clades in the same organs on day 2 or 6 were compared using the Kruskal–Wallis test with Dunn’s post-test. T-test (with Welch’s correction, where appropriate) was used to compare the mean fungal tissue burdens at day 2 with day 6 for the same organ with each isolate [Citation18].
Histopathology
Two mice from two different groups representing each clade (eight isolates altogether) derived from the fungal tissue burden experiments were used for histopathological examination on day 6. Seven mice freshly found dead in the lethality experiment (infected with C. auris type strain (NCPF 13029=CBS 10913), isolates CBS 12372, 2 (NCPF 8977), 27 (NCPF 89891), I-24, I-172, and CDC B-13108) were dissected and analysed similarly. Moreover, on day 1 a single mouse infected with isolates 196, CBS 12373, 204 and I-24 (representing each clade) were used to determine the early heart involvement. Organs (heart, both kidneys, liver and spleen) were fixed in formalin and embedded in paraffin. Tissue sections (4 µm) were stained with haematoxylin-eosin and Periodic Acid Schiff (PAS). Hearts were stained with Mallory’s phosphotungstic acid haematoxylin (PTAH) as well.
Results
Lethality
Candida auris
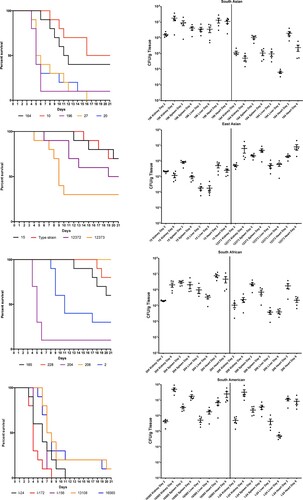
Mice infected with different C. auris isolates, with the exception of the isolates from Israel (South American clade), did not show clinical signs of systemic infection. Death occurred unexpectedly; one day before their death mice appeared clinically normal. Torticollis or other signs of the central nervous system involvement were never observed. Highest overall mortality at day 21 was observed for the South American clade (96%, range 90%-100%), followed by South Asian isolates (80%, range 50–100%), while infection with South African and East Asian isolates resulted in markedly lower cumulative lethality (45% and 44%, ranges 0–90% and 30–70%, respectively) ( and S1). Survival differed significantly within clades (P = 0.0005, P = 0.0010, P < 0.0001 and P = 0.0255 for South American, South Asian, South African and East Asian clades, respectively). In cases of the South American and South Asian clades mortality was first observed at days 3 and 4, respectively. All mice inoculated with isolates from Israel (South American clade) died by day 11. In contrast, late onset of mortality (later than day 8) was typical for South African and East Asian clades (). Notably, the two bloodstream isolates from the East Asian clade caused higher mortality (50–70% vs. 30%) than isolates from external ear samples. However, isolate 206 (a bloodstream isolate from the South African clade) did not kill any mice during the 21-day experiment.
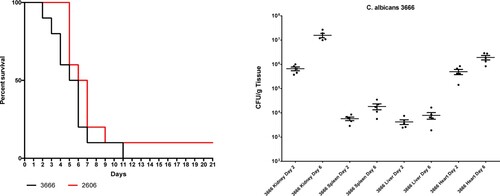
Figure 1. Survival (left) and fungal tissue burden (right) of neutropenic BALB/c mice infected with the four Candida auris clades. In survival studies mice were infected with 19 isolates (South Asian n = 5, East Asian n = 4, South African n = 5 and South American n = 5) representing the four clades. In the graph representing the East Asian clade the “Type strain” represents NCPF 13029 (CBS 10913) type strain isolated from external ear. Fungal kidney, spleen, liver and heart burdens were determined with isolates 196 and 164 from the South Asian, isolates 15 (NCPF 8984) and 12373 (CBS 12373) from the East Asian, isolates 206 (NCPF 13042) and 204 from the South African and isolates I-24 and 16565 (CDC B-16565) from the South American clades at days 2 and 6. The bars represent the medians.
Candida albicans
The two C. albicans isolates using a 100-fold lower inoculum than C. auris produced 90–100% mortality rates with typical signs (lethargy, loss of appetite, hunched back, and ruffled fur) of systemic infection (P > 0.05) ().
Fungal organ tissue burden
Candida auris
Regardless of clade, the heart and kidneys were the most heavily affected organs. Fungal burden results showed close correlation with lethality, i.e. infection with isolates causing high mortality in the lethality experiments within each clade produced higher fungal tissue burdens (). Six-day heart and kidney burdens with the isolates causing high lethality (≥90%) were at least one order of magnitude higher than burdens caused by isolates associated with intermediate or low lethality (30–60%). This was apparent even at day 2 in case of heart burdens. Kidney and heart burdens tended to increase from day 2 to day 6 in case of highly lethal isolates, but not for isolates causing intermediate-low lethality. In case of the South Asian, East Asian and South African clades, the lower-lethality isolates produced lower fungal burdens, highly lethal isolates produced higher burdens, while in case of the South American clade both tested isolates produced high lethality and high burdens ().
Spleen and liver burdens were more variable. Mean fungal spleen burdens at day 2 did not differ significantly between isolates, but at day 6 CFU number of the isolate 16,565 (South American clade) was significantly higher than those for isolates 15 (NCPF 8984) and 164 (East Asian and South Asian clades, respectively) (P < 0.001) (). Mean fungal liver burdens were the lowest on both tested days compared to other examined organs; the highest (3.6 × 106 CFU/g) and lowest (6.5 × 103 CFU/g) mean fungal burdens were noticed in cases of high-lethality isolates 196 and intermediate-lethality 164, respectively, (both belonging to South Asian lineage) at day 2.
Candida albicans
Fungal tissues burden increases with isolate 3666 were statistically significant in cases of heart, kidneys and spleen (P = 0.016, P = 0.008 and P = 0.008, respectively) from day 2 to day 6. Mean fungal liver burdens at days 2 and 6 were lower than 104 CFU/g ().
Mean fungal heart and kidney burdens were comparable to those with high-lethality C. auris isolates at day 6, but were higher than in case of C. auris isolates causing intermediate or low lethality. However, mean liver fungal burden was significantly lower than isolates 16,565 and 196 of C. auris (both being high-lethality isolates) ( and ).
Histopathology
Candida auris
Only single yeast cells and numerous budding yeast cells were produced in the organs, pseudohyphae were never observed. However, all isolates produced numerous, large aggregates in all examined organs with the exception of the spleen, both in mice dissected during the fungal burden experiments and in mice found dead in the lethality experiments ().
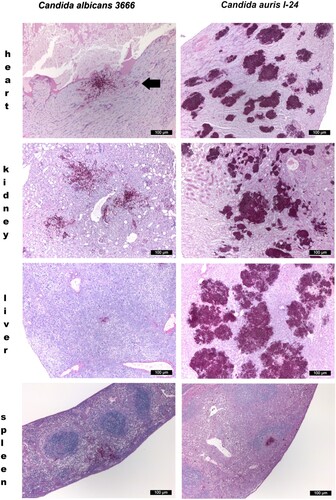
Figure 3. Histopathological examination of the heart, kidney, liver and spleen using Periodic Acid Schiff staining from neutropenic mice intravenously challenged with Candida albicans isolate 3666 (left panels) and C. auris isolate I-24 (right panels), respectively. Histopathological examination was performed six days post infection. In case of C. albicans in the heart, in the kidney and in the spleen blastoconidia and budding yeast cells, pseudohyphae and hyphae were seen. In the liver hyphae were not detected. In the heart both endo- and myocardial involvement is noticeable, the subendocardial myocardium is affected most markedly. Signs of beginning blood vessel invasion in the myocardium is detectable (black arrow). In the kidney C. albicans was detected within the parenchyma, tubuli and glomeruli. In the spleen pseudohyphae and hyphae were seen in the red pulp. C. auris produced large aggregates in the heart, kidney and liver with numerous blastoconidia and budding yeast cells were detected. In the heart coagulative necrosis of myocytes was noticed. In the kidney C. auris cells were detected in the parenchyma, tubuli but not in the glomeruli. In the liver dilated liver sinusoids were filled with yeast cells with central necrosis of lobuli and vacuolar degeneration of hepatocytes. In the spleen yeast cells were seen at the border of the red and white pulp with focal destruction of the white pulp. Small fungal lesions were detectable under the capsule of the spleen and in the sinuses. Magnification × 100.
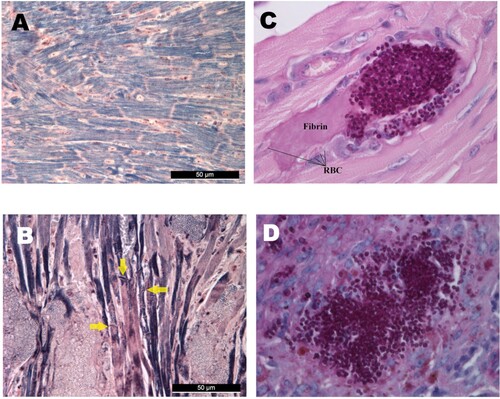
All examined hearts (regardless of clade) exhibited multiple foci of abundant yeast cells between myofibres with coagulative necrosis of myocytes (). Myofibres were frequently distorted by the fungal lesions. Mallory’s PTAH staining revealed that myocardial fibres lost their cross-striations, confirming the contraction band necrosis or myofibrillar degeneration in all mice freshly found dead in the lethality experiments ((A, B)) [Citation19]. Contraction band necrosis was found in infected but apparently healthy mice dissected on day 6 as well. Early heart involvement on day 1 was confirmed by detecting clumps of blastoconidia and budding yeast cells filling the arterioles of the myocardium ((C)). Foci appeared in the sub-endocardial myocardium and the pericardium only in the late stages of the infection.
Figure 4. Histological findings from neutropenic mice intravenously challenged with Candida auris. Normal (A) and damaged heart (B) with Mallory’s PTAH staining. PTAH staining performed on the heart of a moribund dissected mouse on day 5 infected with isolate 196 (B) revealed contraction band necrosis or myofibrillar degeneration (yellow arrows). Morphology of C. auris with Periodic Acid Schiff (PAS) staining in the heart’s arterioles on day 1 post infection (C), and in the spleen (D). PAS staining showed blastoconidia and budding yeast cells but never pseudohyphae and hyphae (C–D). Magnification, A-B × 400, C × 1000, D × 400, RBC; red blood cells.
In the kidneys, there was evidence of multifocal infiltration with C. auris within the parenchyma, with focal destruction of tubuli, but glomeruli were not affected. Necrotizing areas were frequently seen (). In the livers C. auris produced large, multifocal lesions; the dilated liver sinusoids were filled with abundant yeast cells, fungi spread radially in the liver parenchyma with central necrosis of lobuli. Vacuolar degeneration of hepatocytes was commonly seen (). In the spleens, in contrast, only small clumps of fungal cells were seen ( and (D)).
Candida albicans
In the heart, kidneys and spleen abundant single and budding yeast cells, pseudohyphae and hyphae were seen (). In the liver only a small number of blastoconidia and budding yeast cells and pseudohyphae were noticed (). In the heart large necrotic areas were seen involving the endo- and myocardium but without contraction band necrosis ().
Discussion
The most common clinical presentation of C. auris infection is candidemia in intensive care units among critically ill patients. Major risk factors are diabetes mellitus, surgical cardiovascular and gastrointestinal diseases, haematological malignancies and corticosteroid therapy [Citation3–6,Citation20–23]. Interestingly, neutropenia is not considered as an important risk factor for invasive C. auris infection. This clinical experience may be related with the in vitro finding that C. auris triggers neutrophil engulfment and production of neutrophil extracellular traps less effectively compared to C. albicans [Citation24]. However, Chowdhary et al. have reported 6 of 12 patients with C. auris candidemia with neutropenia [Citation25]. Candidemia is frequently persistent in spite of apparently adequate targeted antifungal therapy; septic metastatic complications (i.e. spondylodiscitis, endo- and pericarditis) are also often encountered [2,4,22,23]. Kidneys are often involved in the pathogenesis as well, both in cases with or without candidemia [Citation4–7,Citation20-23,Citation25,Citation26]. The crude in-hospital mortality rate for C. auris candidemia may be as high as 68–80% (3,22). Attributable mortality due to C. auris is hard to determine due to the severe underlying conditions of the patients; limited available estimates fall between 22.2 and 66.6% [Citation2,Citation6,Citation23,Citation26,Citation27].
Data on clade-specific mortality are scant. In the first reported cases of C. auris candidemia two of three patients infected with the East Asian clade died in Korea and showed persistent candidemia in spite of fluconazole and amphotericin B treatment [Citation2]. Lockhart et al. reported 47–72%, 60% and 33% mortality rate with South Asian, South American and South African clades, respectively [Citation3].
Our lethality results in a deeply neutropenic murine model were consistent with other groups showing that C. auris, regardless of clade, is less virulent than C. albicans [Citation13,Citation14,Citation17]. In this study, the ranking in the virulence of the four C. auris clades was South American > South Asian > South African = East Asian (, and S1). Similar high virulence was reported by Xin et al. in A/J (100% lethality) but not in BALB/c neutropenic mice (40% lethality) using a single South American isolate [Citation17]. Our lethality results with the South Asian clade (50–100% lethality) correlate well to those previously reported for neutropenic BALB/c or CD-1 mice (40–100% lethality) with the same or higher (from 107 to 108 CFU/mouse) infectious doses [Citation15,Citation16]. The lack of similar studies with the South African clade precludes comparison to our results. However, we confirmed the previous observation in a G. mellonella model that aggregating South-African C. auris isolates are less virulent than non-aggregating South-African isolates [Citation10]. Moreover, we found significant differences in virulence among isolates of the same clade in case of virtually all clades. These results are concordant with the previous in vitro findings that C. auris isolates may produce several virulence factors in a strain-dependent manner but to a lower extent than C. albicans [Citation4,Citation28]. Notably, the C. auris isolates from the South American clade originating from bloodstream infection and the hospital environment produced very similar mortality rates at day 21 (100% and 90%, respectively). This highlights that virulent C. auris persists for long periods in the hospital environment and these isolates are potential sources of colonization and infection for high-risk patients [Citation3–5,Citation23,Citation27].
High fungal tissue burdens supported our histopathological findings regardless of clade; at day six multifocal, large aggregates of single and budding yeast cells were always found in the hearts, kidneys and livers but not in the spleens (). Interestingly, these lesions were detected both with aggregating and non-aggregating isolates, suggesting that both phenotypes behave similarly in vivo. These large yeast cell aggregates in tissues may shield the fungus from the effective immune response and promote fungal persistence and replication [Citation4,Citation10,Citation11]. Moreover, it cannot be ruled out that homogenization of these large tissue aggregates was not perfectly successful and despite homogenization, some cells might have remained aggregated, thus the CFU numbers may have been underestimated. This may explain why some isolates (i.e. isolate 164 from the South Asian clade) showed unexpectedly less CFU on day 6 than day 2.
Large, multifocal lesions in the myocardium without the involvement of the endocardium suggest hematogenous seeding; on day 1 abundant single and budding yeast cells were seen in the coronary arteriolae in mice infected with all C. auris lineages ((C)). Our results are in accordance with others who observed high fungal burdens (≥×105 CFU/g) in the myocardium with BALB/c, A/J, neutrophil elastase-deficient and C57BL/6 neutropenic and non-neutropenic mice infected with C. auris isolates from the South American and South Asian clades [Citation15,Citation17]. In contrast, with the more virulent C. albicans endocardial involvement was found and myocardial involvement was primarily subendocardial, suggesting a penetration of the heart muscle by yeasts starting from the endocardium.
In spite of the high fungal burden in the heart even at day 2 (≥5.2 × 105 CFU/g), increased early mortality was not observed with the less virulent isolates from the South African, East Asian and South Asian lineages. However, the heavy myocardial fungal burden is associated with irreversible damage of the myocardial cells as detected by the contraction band necrosis ((B)) found in apparently healthy dissected mice as well as in mice freshly found dead. Translating our results to clinical situations, delay in the diagnosis and treatment of disseminated C. auris candidiasis may increase the risk for fungus-induced myocardial failure and sudden death.
High fungal kidney and/or heart burdens are frequently associated with heavy fungal burden in the brain and lungs as reported both in immunocompetent and neutropenic mice. Singh et al., noticed simultaneously heavy fungal kidney (2 × 108 CFU/g) and brain (2 × 106 CFU/g) burdens. Interestingly, C. auris cells found in the brain were localized mainly in the capillaries rather than in the cerebral tissue itself [Citation16]. Candida pneumonia is a rare manifestation of invasive Candida infections in humans, but in immunocompetent ICR and BALB/c mice higher infectious doses (≥107 CFU/g) produced 103–>105 CFU/g lungs tissue burdens [Citation12,Citation13]. Although in this study we did not examine the fungal burdens in the brain and lungs, based on the high fungal burdens in the hearts and kidneys, central nervous system and lung invasion is also feasible. However, we did not observe signs of meningeal involvement [Citation15].
To our best knowledge this is the first study to compare the in vivo virulence of the four C. auris lineages distributed worldwide in a mammalian host. We observed high fungal burdens in organs, especially in the myocardium, confirming that the heart is a major target in cases of disseminated C. auris infection. However, failure of other organs especially the kidney may contribute to mortality as well. Isolates of the same clade showed differences in murine virulence; a markedly higher virulence of the South American clade was clearly demonstrated both in case of bloodstream and hospital environmental isolates.
Supplemental Material
Download ()Acknowledgements
The authors thank Katalin Orosz-Tóth for inoculation of mice, and Drs Shawn R. Lockhart and Ronen Ben-Ami for their generous gifts of Candida auris strains from South America and Israel, respectively. Z. T. and F. N. were supported by the EFOP-3.6.3-VEKOP-16–2017-00009 Program. R. K. was supported by the TA ´MOP 4.2.4.A/2–11-1–2012-0001 National Excellence Program (Elaborating and operating an inland student and researcher personal support system). The project was subsidized by the European Union and co-financed by the European Social Fund. Z. T. and F. N. were supported by the U ´NKP-19–3 New National Excellence Program of the Ministry of Human Capacities.
Disclosure statement
L. Majoros received conference travel grants fromMSD, Astellas, Pfizer and Cidara.
Additional information
Funding
References
- Satoh K, Makimura K, Hasumi Y, et al. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x
- Lee WG, Shin JH, Uh J, et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 2011;49:3139–3142. doi: 10.1128/JCM.00319-11
- Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2016;64:134–140. doi: 10.1093/cid/ciw691
- Sekyere J O. Candida auris: a systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen. 2018;7:e578, doi:10.1002/mbo3.578.
- Armstrong PA, Rivera SM, Escandon P, et al. Hospital-associated multicenter outbreak of emerging fungus Candida auris, Colombia, 2016. Emerg Infect Dis. 2019;25:1339–1346. doi: 10.3201/eid2507.180491
- Rudramurthy SM, Chakrabarti A, Paul RA, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother. 2017;72:1794–1801. doi: 10.1093/jac/dkx034
- Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–899. doi: 10.1093/jac/dkx480
- Chow NA, de Groot T, Badali H, et al. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019;25:1780–1781. doi: 10.3201/eid2509.190686
- Szekely A, Borman AM, Johnson EM. Candida auris isolates of the Southern Asian and South African lineages exhibit different phenotypic and antifungal susceptibility profiles in vitro. J Clin Microbiol. 2019;57(5). pii: e02055-18. doi: 10.1128/JCM.02055-18
- Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen, Candida auris and other key pathogenic Candida species. mSphere. 2016;18:e00189–16.
- Alfouzan W, Dhar R, Albarrag A, et al. The emerging pathogen Candida auris: A focus on the Middle-Eastern countries. J Infect Public Health. 2019;12:451–459. doi: 10.1016/j.jiph.2019.03.009
- Fakhim H, Vaezi A, Dannaoui E, et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61:377–382. doi: 10.1111/myc.12754
- Wang X, Bing J, Zheng Q, et al. The first isolate of Candida auris in China: clinical and biological aspects. Emerg Microbes Infect. 2018;7:93.
- Ben-Ami R, Berman J, Novikov A, et al. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis. 2017;23:195–203. doi: 10.3201/eid2302.161486
- Torres SR, Pichowicz A, Torres-Velez F, et al. Impact of Candida auris infection in a neutropenic murine model. Antimicrob Agents Chemother. 2020;64(3). pii: e01625-19. doi: 10.1128/AAC.37.3.491
- Singh S, Uppuluri P, Mamouei Z, et al. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019;15(8):e1007460. doi: 10.1371/journal.ppat.1007460
- Xin H, Mohiuddin F, Tran J, et al. Experimental mouse models of disseminated Candida auris infection. mSphere. 2019;4(5). pii: e00339-19. doi: 10.1128/mSphere.00339-19
- Prépost E, Tóth Z, Perlin DS, et al. Efficacy of humanized single large doses of caspofungin on the lethality and fungal tissue burden in a deeply neutropenic murine model against Candida albicans and Candida dubliniensis. Infect Drug Resist. 2019;12:1805–1814. doi: 10.2147/IDR.S198764
- Hopster DJ, Milroy CM, Burns J, et al. Necropsy study of the association between sudden cardiac death, cardiac isoenzymes and contraction band necrosis. J Clin Pathol. 1996;49:403–406. doi: 10.1136/jcp.49.5.403
- Morales-López SE, Parra-Giraldo CM, Ceballos-Garzón A, et al. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis. 2017;23:162–164. doi: 10.3201/eid2301.161497
- Adams E, Quinn M, Tsay S, et al. Candida auris in Healthcare Facilities, New York, USA, 2013-2017. Emerg Infect Dis. 2018;24:1816–1824. doi: 10.3201/eid2410.180649
- Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013-August 2016. Am J Transplant. 2017;17:296–299. doi: 10.1111/ajt.14121
- Ruiz-Gaitán A, Moret AM, Tasias-Pitarch M, et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781
- Johnson CJ, Davis JM, Huttenlocher A, et al. Emerging fungal pathogen Candida auris evades neutrophil attack. mBio. 2018;9i:e01403–18.
- Chowdhary A, Sharma C, Duggal S, et al. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis. 2013;19:1670–1673. doi: 10.3201/eid1910.130393
- Araúz AB, Caceres DH, Santiago E, et al. Isolation of Candida auris from 9 patients in Central America: Importance of accurate diagnosis and susceptibility testing. Mycoses. 2018;61:44–47. doi: 10.1111/myc.12709
- Al Maani A, Paul H, Al-Rashdi A, et al. Ongoing challenges with healthcare-associated Candida auris outbreaks in Oman. J Fungi (Basel). 2019;23;5(4). pii: E101.
- Larkin E, Hager C, Chandra J, et al. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother. 2017;24;61(5). pii: e02396-16.