ABSTRACT
Dexamethasone implementation for COVID-19 management represented a milestone but data regarding its impact and safety have not been consistently reproduced. We aimed to evaluate in-hospital mortality before and after the implementation of corticosteroid treatment (CS-T) for severe and critical COVID-19. We conducted a cohort study that included patients admitted with severe and critical COVID-19. The primary outcome was death during hospitalization. Secondary outcomes included the length of stay (LOS), need for invasive mechanical ventilation (IMV), time to IMV initiation, IMV duration, and development of hospital-acquired infections (HAIs). Bivariate, multivariate, and propensity-score matching analysis were performed. Among 1540 patients, 688 (45%) received CS-T. Death was less frequent in the CS-T group (18 vs 31%, p < .01). Among patients on IMV, death was also less frequent in the CS-T group (25 vs 55%, p < .01). The median time to IMV was longer in the CS-T group (5 vs 3 days, p < .01). HAIs occurred more frequently in the CS-T group (20 vs 10%, p < .01). LOS, IMV, and IMV duration were similar between groups. Multivariate analysis revealed an independent association between CS-T and lower mortality (aOR 0.26, 95% CI 0.19–0.36, p < .001). Propensity-score matching analysis revealed that CS-T was independently associated with lower mortality (aOR 0.33, 95% CI 0.22–0.50, p < .01). Treatment with corticosteroids was associated with reduced in-hospital mortality among patients with severe and critical COVID-19, including those on IMV.
Introduction
Since the early months of the SARS-CoV-2 pandemic, the fact that severe forms of COVID-19 are associated with systemic inflammation [Citation1,Citation2] prompted great efforts to evaluate the effect of numerous anti-inflammatory and immunomodulating therapies [Citation3]. The broad anti-inflammatory effect of steroids in COVID-19 has been evaluated in numerous trials [Citation4–11]. Fortunately, the RECOVERY trial showed decreased mortality with dexamethasone in hypoxaemic COVID-19 patients [Citation4]. Dexamethasone has also been associated with increased ventilator-free days among critically ill patients [Citation7]. A meta-analysis, including the RECOVERY [Citation4] and six other clinical trials, concluded that the administration of systemic corticosteroids was associated with lower 28-day mortality [Citation12]. Updated guidelines recommend the use of dexamethasone in hypoxaemic patients with COVID-19 [Citation13–15]. The implementation of dexamethasone as the standard of care represents a milestone in the rapidly evolving therapeutic strategies for COVID-19. Corticosteroid treatment (CS-T) is the only proven intervention to reduce COVID-19-associated mortality, but such results have not been consistent across trials [Citation4–11]. Likewise, data regarding secondary infections have not been consistently evaluated and are still lacking. As the number of cases continues to increase, COVID-19 remains a major issue in global health. As of 31 October 2021, more than 246 million cases of confirmed COVID-19 and nearly 5 million deaths have been reported by the World Health Organization [Citation16]. We conducted a cohort study to evaluate in-hospital mortality before and after the implementation of CS-T for severe and critical COVID-19.
Materials and methods
Patients and settings
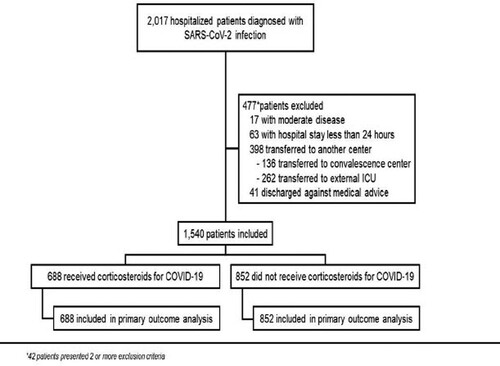
We conducted a prospective cohort study in a COVID-19 reference centre in Mexico City. Our centre was converted into a COVID-19 dedicated facility on 16 March 2020. Data of all consecutive patients admitted with a positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) between 18 March and 9 November were prospectively registered using the electronic medical record. Patients with severe and critical COVID-19 were included. According to previous definitions, a case was considered severe when SpO2 was <93%, PaO2/FiO2 ratio < 300, respiratory rate ≥ 30 breaths per minute, or ≥50% lung involvement was seen in chest CT; a case was considered critical when either shock, invasive mechanical ventilation (IMV), or multi-organ failure were present [Citation17]. According to institutional protocols, all patients underwent SARS-CoV-2 RT-PCR testing on nasopharyngeal swab samples. Nucleic acid extraction was performed using NucliSens easyMAG system (bioMérieux, Boxtel, The Netherlands) and RT-PCR was processed on Applied Biosystems 7500 thermocycler (Foster City, CA, USA) according to specifications described elsewhere [Citation18]. Each patient was followed-up from admission to death or discharge. The primary outcome was in-hospital death. Secondary outcomes included length of stay (LOS), IMV during follow-up, time to IMV initiation, days on IMV, and development of culture-proven hospital-acquired infection (HAI). A HAI was considered after review by an infectious diseases (ID) specialist to ensure it met accepted criteria [Citation19–22]. Patients who were transferred to other facilities before discharge or death, had an LOS < 24 h, or were diagnosed with moderate disease, were excluded. Because of the observational nature of the study, written informed consent was waived. The study was approved by the Institutional Board Review (Ref. number 3333).
Corticosteroid administration
After 17 June 2020, following the preliminary results of the RECOVERY trial [Citation23], our centre implemented the use of dexamethasone 6 mg QD for up to 10 days for hypoxaemic patients with COVID-19 as the standard of care. Before that date, steroid use for COVID-19 was not standardized. For this study, CS-T was considered when intravenous dexamethasone ≥ 6 mg QD, prednisone ≥ 40 mg QD, or methylprednisolone ≥ 32 mg QD were used for COVID-19 treatment according to a case-by-case decision by the treating physicians. All patients received the standard of care according to available evidence at the time of admission.
Statistical analysis
A non-probabilistic, consecutive sampling of all admitted patients that fulfilled the inclusion criteria was implemented. As the study planning started in March 2020, before widespread data were available, no prespecified sample size was calculated. As the study progressed, a sample size calculation was made. Considering a known mortality of 30% in the non-treated (previously published local data [Citation24]) and of 23% in the treated group [Citation4], a probability of type I error (α) of 0.05, and a statistical power (1−β) of 80%, we calculated a sample size of at least 1246 patients. Data were described using mean, standard deviation (SD), median, and interquartile range (IQR) according to variables’ distribution. Comparisons between patients who received CS-T and those who did not (NCS-T) were made using χ2, Fisher’s exact test, independent samples t-test, and two-sample rank-sum tests. Bivariate analysis to calculate relative risk (RR) and 95% confidence interval (95% CI) of in-hospital death were performed. A multivariate analysis using a multiple logistic regression model including variables with a p-value < .2 in bivariate analysis and of biological importance was performed. Additionally, a propensity score (PS) using a matching method was calculated. To estimate the PS, CS-T was regressed in a logistic regression model. Confounding variables that could affect the outcome and treatment selection were included in the model. For the matching process, we used the logit of the PS using ≤0.1 width calipers of the estimated PS SD. A matching ratio of 1:1 and a non-replacement method were used. To assure balance within the matched sample, a comparison between means, variances, and standardized absolute differences was made. A standardized absolute difference < 0.1 was considered for adequate balance. A post-regression analysis within the matched sample was performed. Finally, the average treatment effect was estimated. Two-sided p-values < .05 were considered statistically significant. Missing data were not replaced and were reported in the results. STATA version 15.1 (Texas, USA) was used.
Results
A total of 1540 patients with severe or critical COVID-19 were included, of which 688 (45%) received CS-T (). CS-T was more frequent in patients admitted after 16 June [657/743 (88%) vs. 31/797 (2%)]. In the CS-T group, 665 (96.7%) received dexamethasone, 20 (2.9%) methylprednisolone, and 3 (0.4%) prednisone.
The median age was 55 years (IQR 45–65) and 941/1540 (61%) were male. Obesity, type 2 diabetes mellitus (T2DM), and hypertension were present in 681 (44%), 440 (29%), and 528 (34%), respectively. Immunosuppression was present in 87 (6%), being pharmacologic, HIV infection, and malignancy the most common cause in 52/87 (60%), 15/87 (17%), and 15/87 (17%), respectively. The median oxygen saturation, assessed by pulse oximetry (SpO2), on admission was 84% (IQR 72–88). The median glucose concentration was 129 mg/dL (IQR 109–183). Except for increased lactate dehydrogenase (LDH) levels in the CS-T group, baseline laboratory results were not different between groups. Patients in the CS-T group had a history of T2DM [225/688 (33%) vs 215/851 (25%), p < .01], and a higher ( > 2 points) Charlson comorbidity index score [256/688 (37%) vs 225/851 (26%), p < .01], more frequently, and a longer symptom onset on admission [7 days (IQR 6–10) vs 7 days (IQR 5–10), p < .05]. Other interventions were different between groups on admission. The use of IMV within 24 h of admission was more frequent in the CS-T [119 (17%) vs 108 (13%), p < .05], as well as enrolment in COVID-19 clinical trials [196 (28%) vs 124 (15%), p < .01]. Treatment with empiric antibiotics, chloroquine/hydroxychloroquine and tocilizumab, either alone or in combination was more frequent in the NCS-T group [704 (83%) vs 210 (31%), 209 (25%) vs 5 (1%), and 86 (10%) vs 11 (2%), respectively, p < .01 for all comparisons] ().
Table 1. Baseline characteristics of the entire cohort.
Outcomes
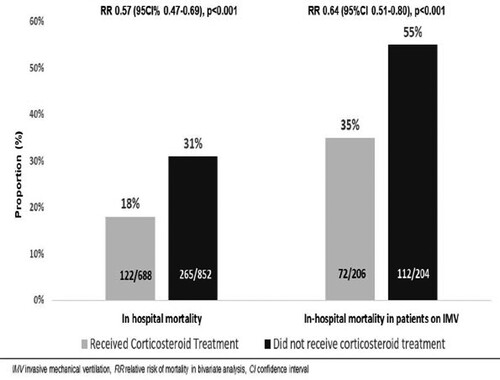
Outcomes are described in . In-hospital death occurred in 122/688 (18%) patients in the CS-T group and 265/852 (31%) in the NCS-T group (p < .01). Among 410 patients who received IMV, 72/206 (25%) patients in the CS-T group and 112/204 (55%) in the NCS-T group died (p < .01) (). Among 1153 survivors, LOS was similar between groups [8 days (IQR 5–16) in the CS-T group vs 7 days (IQR 5-13) in the NCS-T group, p = .05]. Although the frequency of IMV during follow-up was similar between groups [87/560 (15%) in the CS-T group vs 96/744 (13%) in the NCS-T group, p = .22], the median time from admission to IMV was longer in the CS-T group [5 days (IQR 3–7) vs 3 days (IQR 2–4), p < .01] (Figure S1, supplementary material). The median IMV duration was similar between groups [13 days (IQR 8–20) in the CS-T group vs 14 days (IQR 11–20) in the NCS-T group, p = .41].
Table 2. Outcomes in the entire cohort.
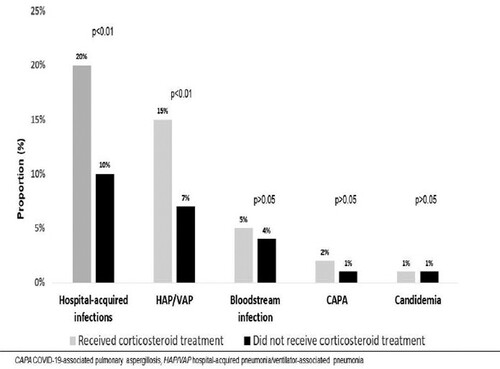
Of note, HAIs occurred more frequently in the CS-T group [139/688 (20%) vs 82/852 (10%), p < .01], even after adjusting for IMV status. The most common type of HAI, hospital-acquired/ventilator-associated pneumonia (HAP/VAP), was more frequent in the CS-T group [103/688 (15%) vs 55/852 (7%), p < .01]. No differences were seen in bloodstream infections, COVID-19-associated pulmonary aspergillosis (CAPA), or candidaemia (5%, 2%, and 1% in the CS-T group vs 4%, 1%, and 1% in the NCT-S group, respectively) (). Microbiological data of 236 episodes of pneumonia and 73 episodes of bloodstream infections are described in . An episode of blood glucose concentration of 180 mg/dL or greater at day 3 after admission occurred in 65 of 344 patients with available data (19%) in the CS-T group. No major consequences of hyperglycaemia were registered.
Table 3. Bacterial microorganisms isolated in infectious episodes in the entire cohort.
In-hospital mortality
Bivariate analysis showed increased hospital mortality associated with male gender, increasing age, previous comorbidities (T2DM, hypertension, cardiovascular disease, and ischaemic heart disease), higher Charlson comorbidity index score, lower SpO2 on admission, baseline lymphocyte count < 800 cells/µL, C-reactive protein (CRP) > 10 mg/dL, ferritin > 500 ng/mL, DHL > 245 U/L, D-dimer > 1000 ng/mL, SpO2/FiO2 ratio < 300, ICU admission, use of IMV, and development of HAI. CS-T and enrolment in any clinical trial were associated with lower mortality (RR 0.57, 95 CI% 0.47–0.69, and RR 0.29, 95% CI 0.20–0.41, p < .01, respectively). Complete results of bivariate analysis are reported in the supplementary material (Table S1). In multivariate logistic regression analysis, CS-T was independently associated with reduced mortality [adjusted odds ratio 0.26 (95% CI 0.19–0.36), p < .01]. Enrolment in any clinical trial was also associated with reduced mortality. Male gender, age, lymphocyte count < 800 cells/µL, CRP > 10 mg/dL, D-dimer > 1000 ng/mL, SpO2 < 90%, and IMV at any point were independently associated with increased mortality. Development of an HAI was not independently associated with mortality ().
Table 4. Multivariate regression analysis for mortality in the entire cohort.
A PS analysis using data from 1360/1540 (88%) patients was estimated. A total of 968 patients were matched in 484 pairs. The variables included in the model and balance diagnostics are described in and Figure S2. Adequate balance within the matched sample was achieved. A logistic regression analysis for in-hospital mortality using the matched sample showed that CS-T was independently associated with lower mortality [adjusted odds ratio of 0.33 (95% CI 0.22–0.50, p < .01)] (Table S2). A significant treatment effect was observed within the matched sample regarding in-hospital mortality [82/484 (17%) vs 138/484 (29%), difference −12%, p < .01].
Table 5. Balance within the matched sample
Discussion
This prospective cohort study evaluated the impact of CS-T on hospital mortality of patients with severe and critical COVID-19. We observed that CS-T was associated with decreased in-hospital mortality in patients with severe and critical COVID-19 in the entire cohort and a PS-matched comparative sample. Our results are compatible with the RECOVERY Collaborative Group report [Citation4], which concluded that dexamethasone was associated with a lower mortality in patients receiving supplementary oxygen, including patients on IMV. Our results underscore the importance of inflammatory response in COVID-19-associated mortality. Other factors, such as male gender, increasing age, lower SpO2, higher inflammatory markers, and IMV were independently associated with increased in-hospital mortality after multivariate analysis. Such factors have been previously associated with mortality [Citation24–27]. In our study, toclilizumab was not associated with a lower mortality; contradictory results have been reported regarding tocilizumab treatment for COVID-19 [Citation28–31].
Several baseline differences, such as prevalence of T2DM, time from symptom onset to admission, LDH concentration, enrolment in COVID-19-related clinical trials, and IMV initiation within 24 h after admission, were noted between the CS-T and the NCS-T groups. Even though time from symptom onset to admission showed a statistical difference, the difference is not clinically significant.
To minimize confounders and bias, a PS matching analysis was performed. After ensuring an adequate balance within the PS-matched sample, CS-T remained independently associated with a lower mortality risk and a significant treatment effect was observed. Of note, treatment with empiric antibiotics and chloroquine/hydroxychloroquine were not included in the PS model because reports have shown that they have no beneficial impact in COVID-19-associated outcomes [Citation32–36]. The differences in empiric antimicrobial use and chloroquine/hydroxychloroquine may reflect the continuous learning process and standard treatment changes according to the rapidly evolving evidence during the pandemic.
In our study, even though CS-T was associated with a longer time from admission to mechanical ventilation, it was not associated with hospital LOS, IMV during follow-up, and IMV duration, as it has been described in previous reports [Citation4–7].
In our study, 20% of the patients that received CS-T developed an HAI, which is similar to previously reported frequencies among COVID-19 patients treated with corticosteroids (21.9–37.7%) [Citation6,Citation7]. An association between CS-T and development of HAIs in COVID-19 patients has been described [Citation37]. In this study, an association between CS-T and development of HAP/VAP, but not bloodstream infection, CAPA, or candidaemia was observed. The development of new infections is a known adverse effect of immunosuppressive therapy [Citation38]. In contrast to recent reports that associated HAIs with a higher mortality in critically ill patients [Citation39,Citation40], the development of HAI was not independently associated with mortality in this cohort. We believe that a prompt ID consult for all patients with COVID-19 along a sound antimicrobial stewardship program may in part explain this finding.
The high prevalence of T2DM, hypertension, and obesity in COVID-19 patients is consistent with reports from our country [Citation41], where such comorbidities are common in the general population [Citation42].
Limitations in our study must be acknowledged. This was an observational cohort study with unbalanced groups so additional PS matching analysis to minimize bias was performed to increase comparability between groups, rendering favourable results in the CS-T group. Before 17 June 2020, corticosteroid use was not systematic regarding the type, dose, and timing, which led to the variability of prescriptions, although this happened in only 5% of the CS-T group. Although hyperglycaemia was frequent, we cannot assess the impact of CS-T because these data were unavailable for analysis in the NCS-T group, since it was not routinely measured. Additionally, data on biochemical markers of bone turnover were not available. Finally, we cannot separate the effect of the learning curve process and the changing standard of care resulting in improved outcomes within the evaluated time frame, in addition to the expected benefit of corticosteroid use. Still, we believe that our study reflects the improved outcomes after the implementation of a standardized CS-T for severe and critical COVID-19. Nevertheless, the search for COVID-19 effective and safe treatments must continue. As new evidence arises, the impact of CS-T for COVID-19 on comorbidities such as diabetes mellitus and bone metabolism disorders must be studied.
In conclusion, treatment with corticosteroids was associated with reduced in-hospital mortality among patients with severe and critical COVID-19, including those on IMV.
Geolocation information
Mexico City, Mexico.
Supplemental Material
Download MS Word (217.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, (J.S.-O.), upon reasonable request.
Additional information
Funding
References
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. https://doi.org/10.1001/jamainternmed.2020.0994.
- Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. https://doi.org/10.1038/s41591-020-1051-9.
- Zhang Y, Chen Y, Meng Z. Immunomodulation for severe COVID-19 pneumonia: the state of the art. Front Immunol. 2020;11:577442. https://doi.org/10.3389/fimmu.2020.577442.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
- Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. https://doi.org/10.1001/jama.2020.17022.
- Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:1298–1306. https://doi.org/10.1001/jama.2020.16761.
- Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. https://doi.org/10.1001/jama.2020.17021.
- Ikeda S, Misumi T, Izumi S, et al. Corticosteroids for hospitalized patients with mild to critically-ill COVID-19: a multicenter, retrospective, propensity score-matched study. Sci Rep. 2021;11:10727. https://doi.org/10.1038/s41598-021-90246-y.
- Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. https://doi.org/10.1016/j.ejphar.2021.173947.
- Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–6428. https://doi.org/10.1172/JCI140617.
- Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2021;72:e373–e381. https://doi.org/10.1093/cid/ciaa1177.
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. https://doi.org/10.1001/jama.2020.17023.
- Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Infect Dis Soc Am. 2021; Version 5.1.1. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Date last updated: September 3, 2021. Date last accessed September 7, 2021.
- World Health Organization. COVID-19 clinical management: living guidance, 25 January 2021. No.WHO/2019-nCoV/clinical/2021.1. World Health Organization,2021.15. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Date last updated: January 25, 2021. Date last accessed September 7, 2021.
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Date last updated: September 3, 2021. Date last accessed September 7, 2021.
- WHO. COVID-19 weekly epidemiological update. World Heal Organ. 2021;(58):1–23. Available at https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---2-november-2021. Date last updated: November 2, 2021. Date last accessed November 5, 2021.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. https://doi.org/10.1001/jama.2020.2648.
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045.
- Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European respiratory society (ERS), European society of intensive Care Medicine (ESICM), European society of clinical microbiology and infectious diseases (ESCMID) and asociación latinoamericana del tórax (ALAT). Eur Respir J. 2017;50:1700582. https://doi.org/10.1183/13993003.00582-2017.
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;49:1–45. https://doi.org/10.1086/599376.
- Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. https://doi.org/10.1016/S1473-3099(20)30847-1.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62:e1–e50. https://doi.org/10.1093/cid/civ933.
- Goverment United Kingdom. World first coronavirus treatment approved for NHS use by government. Press Release June 16, 2020. Available at: https://www.gov.uk/government/news/world-first-coronavirus-treatment-approved-for-nhs-use-by-government. Date last updated: June 18, 2020. Date last accessed August 15, 2021.
- Olivas-Martínez A, Cárdenas-Fragoso JL, Jiménez JV, et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS One. 2021;16:e0245772. https://doi.org/10.1371/journal.pone.0245772.
- Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. https://doi.org/10.1016/j.jaci.2020.04.006.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. https://doi.org/10.1038/s41586-020-2521-4.
- Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15:e0241541. https://doi.org/10.1371/journal.pone.0241541.
- Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384:20–30. https://doi.org/10.1056/NEJMoa2030340.
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. https://doi.org/10.1016/S0140-6736(21)00676-0.
- Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. https://doi.org/10.1056/NEJMoa2028700.
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383:2333–2344. https://doi.org/10.1056/NEJMoa2028836.
- RECOVERY Collaborative Group, Horby P, Mafham M, et al. Effect of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;383:2030–2040. https://doi.org/10.1056/NEJMoa2022926.
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383:2041–2052. https://doi.org/10.1056/NEJMoa2019014.
- Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. https://doi.org/10.1001/jama.2020.8630.
- Hinks TSC, Cureton L, Knight R, et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med. 2021;9: S2213-2600(21)00263-0. https://doi.org/10.1016/S2213-2600(21)00263-0.
- Lozano-Cruz OA, Jiménez JV, Olivas-Martinez A, et al. Adverse effects associated with the use of antimalarials during the COVID-19 pandemic in a tertiary care center in Mexico city. Front Pharmacol. 2021;12:668678. https://doi.org/10.3389/fphar.2021.668678.
- Smith L, Karaba SM, Amoah J, et al. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2021: 1–4. https://doi.org/10.1017/ice.2021.148.
- Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63:655–670. https://doi.org/10.4187/respcare.06314.
- Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–465. https://doi.org/10.1016/j.chest.2021.04.002.
- Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. https://doi.org/10.1016/j.cmi.2020.07.041.
- Peña JE, Rascón-Pacheco RA, Ascencio-Montiel IJ, et al. Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico. Arch Med Res. 2021;52:443–449. https://doi.org/10.1016/j.arcmed.2020.12.002.
- Alcalde-Rabanal JE, Orozco-Núñez E, Espinosa-Henao OE, et al. The complex scenario of obesity, diabetes and hypertension in the area of influence of primary healthcare facilities in Mexico. PLoS One. 2018;13:e0187028, https://doi.org/10.1371/journal.pone.0187028.