 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The specific antibodies induced by SARS-CoV-2 infection may provide protection against a subsequent infection. However, the efficacy and duration of protection provided by naturally acquired immunity against subsequent SARS-CoV-2 infection remain controversial. We systematically searched for the literature describing COVID-19 reinfection published before 07 February 2022. The outcomes were the pooled incidence rate ratio (IRR) for estimating the risk of subsequent infection. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies. Statistical analyses were conducted using the R programming language 4.0.2. We identified 19 eligible studies including more than 3.5 million individuals without the history of COVID-19 vaccination. The efficacy of naturally acquired antibodies against reinfection was estimated at 84% (pooled IRR = 0.16, 95% CI: 0.14-0.18), with higher efficacy against symptomatic COVID-19 cases (pooled IRR = 0.09, 95% CI = 0.07-0.12) than asymptomatic infection (pooled IRR = 0.28, 95% CI = 0.14-0.54). In the subgroup analyses, the pooled IRRs of COVID-19 infection in health care workers (HCWs) and the general population were 0.22 (95% CI = 0.16-0.31) and 0.14 (95% CI = 0.12-0.17), respectively, with a significant difference (P = 0.02), and those in older (over 60 years) and younger (under 60 years) populations were 0.26 (95% CI = 0.15–0.48) and 0.16 (95% CI = 0.14-0.19), respectively. The risk of subsequent infection in the seropositive population appeared to increase slowly over time. In conclusion, naturally acquired antibodies against SARS-CoV-2 can significantly reduce the risk of subsequent infection, with a protection efficacy of 84%.
Registration number: CRD42021286222
Introduction
The COVID-19 pandemic has caused devastating impacts on global health and the economy [Citation1]. As of 15 February 2022, 412.3 million confirmed cases of COVID-19 and approximately 5.8 million deaths have been reported worldwide [Citation2]. SARS-CoV-2 infection induces the production of detectable levels of specific antibodies in most patients within 3–6 weeks after infection, and only 2.0-8.5% of patients remained seronegative after 60 days post-infection [Citation3]. Several studies reported that specific antibodies in patients with COVID-19 are continuously detected for at least 6–13 months after COVID-19 infection [Citation4-6]. Previous studies indicated that naturally acquired antibodies after SARS-CoV-2 infection might protect against virus reinfection [Citation7-9]. However, as these studies often included relatively small sample sizes with different follow-up times, populations and serology measurement methods, the actual extent to which primary infection protects against reinfection and how long protection lasts are still being debated.
As of 16 February 2022, 61.9% of the global population has received at least one dose of a COVID-19 vaccine. However, vaccine distribution is extremely uneven, with only 10.6% of people in low-income countries receiving at least one dose [Citation10]. As the number of COVID-19 cases accumulates and vaccine campaigns continue, the extent and durability of the protective effect of natural immunity elicited by infection is critical to the pandemic prediction process, estimates of herd immunity and development of vaccination strategies [Citation11-13]. Therefore, we conducted a systematic review and meta-analysis to evaluate the protective efficacy of naturally acquired humoral immunity against subsequent SARS-CoV-2 infection in unvaccinated individuals.
Methods
Search strategy
We systematically searched for the relevant literature published before 07 February 2022 in six databases, including three peer-reviewed databases (PubMed, Embase, and Web of Science) and three preprint platforms (medRxiv, bioRxiv, and Europe PMC). Key search terms included the following: SARS-CoV-2, immunity, protection, reinfection and nucleic acid testing. The full search strategy was described in Supplementary Table 1. We also conducted a secondary reference search on all eligible studies and nine relevant review articles [Citation11,Citation14-21]. The protocol of this systematic review and meta-analysis has been registered at PROSPERO (Registration number: CRD42021286222) [Citation22].
Selection criteria
Two investigators (“QC” and “KXZ”) independently assessed all retrieved publications. Discrepancies were resolved by discussion with a third investigator (“XHL”). Eligible studies must meet all of the following criteria: (1) longitudinal study in population without a history of COVID-19 vaccination, (2) SARS-CoV-2 serology testing at baseline to discriminate the previously infected and uninfected populations, (3) confirmation of COVID-19 cases by nucleic acid testing during follow-up, (4) the study must have compared the risk of SARS-CoV-2 reinfection/infection between baseline seropositive and seronegative groups, and (5) a sample size of >10 participants in each group. We excluded studies that met any of the following criteria: (1) the study used odds ratio as an effect size indicator and did not report original data, and (2) the baseline seronegative group included subjects with a known COVID-19 history.
Data extraction and quality assessment
Two investigators (“KXZ” and “XCH”) extracted the following data using a standardized electronic data collection form: study title, publication or preprint date, journal or preprint platform, authors, study location, demographic characteristics of the study population, follow up time, the method of the serology measurement, sample sizes and reinfection/infection cases in baseline seropositive or seronegative groups, the definition of reinfection, whether researchers attempted to adjust for any potential covariates, IRR and 95% confidence interval (95% CI). If the IRR was not able to be obtained from the study, a 2×2 contingency table was constructed to calculate it. When no cases of reinfection/infection were detected in the baseline seronegative or seropositive groups during follow-up, “0.05” was used to evaluate an estimate of the relative risk. The quality of the included studies was independently evaluated using the Newcastle–Ottawa Scale (NOS) by two investigators (“KXZ” and “XCH”). The NOS contains three categories (8 subcategories), and a maximum of 9 stars can be allotted to each study. A score of 0–3 stars was considered a low-quality study, a score of 4–6 stars was considered a moderate-quality study, and a score of 7–9 stars was considered a high-quality study. Extracted data and quality assessment scores were checked by a third investigator (“XHL”), and disagreements were resolved through discussion.
Statistical analysis
The primary outcome was the risk of SARS-CoV-2 reinfection/infection between the baseline seropositive and seronegative groups. The second outcome was the risk of symptomatic and asymptomatic COVID-19 between the two groups. The subjects with a positive PCR test for SARS-CoV-2 after the baseline seropositive result were defined as reinfection cases. Heterogeneity was assessed using the Q and I² statistics, with P < 0.05 and I²>50% indicating significant heterogeneity. A suitable model was used to generate pooled IRR and 95% CIs. Subgroup analyses of the primary outcome were performed in the following groups: peer-review status (peer-review or preprint), the type of target antibodies (S protein or N protein), population (HCWs or general population), age (< 60 years old or ≥ 60 years old), whether studies reported adjusted results (adjusted or unadjusted), the method for monitoring infection cases (passive monitoring or active monitoring), and the definition of reinfection (strict definition or loose definition). The classification criteria for each subgroup are described in the Supplementary Table 2. Bubble plots were used to explore the changing trends of the protection provided by naturally acquired antibodies. Funnel plots (log risk ratio against standard errors) and Begg’s test were used to examine the potential for publication bias. The one-study-at-a-time method (OAT) was used to assess the reliability of the results in the sensitivity analysis.
Statistical analyses were conducted using meta libraries in the R programming language 4.0.2 [Citation23]. A two-sided P value < 0.05 was considered statistically significant.
Results
A total of 15021 relevant records were identified, of which 6093 duplicate records were removed. According to the titles and abstracts, 8776 irrelevant records were excluded, and the full texts of 152 articles were assessed. Additionally, 245 relevant records were identified from the second citation search. Finally, 19 studies were eligible and included in this meta-analysis ().
Figure 1. Flow diagram of the included studies. One study identified from the second citation search was included in the meta-analysis. [Citation24].
![Figure 1. Flow diagram of the included studies. One study identified from the second citation search was included in the meta-analysis. [Citation24].](/cms/asset/e9319764-0717-489d-8db2-c0f4ebe95252/temi_a_2046446_f0001_oc.jpg)
The 19 eligible studies included more than 3.5 million COVID-19 unvaccinated individuals without the history of COVID-19 vaccination. The sample sizes of the included studies ranged from 209 to 3257478 (median: 3249, IQR: 653-10582). Eight studies were conducted in the UK, four in the USA, three in Switzerland, one in Italy, one in Qatar, one in Nicaragua, and one in Sweden. The mean/median ages of the enrolled participants in fifteen studies (15/19) were less than 60 years old, those in another two studies (2/19) were more than 60 years old, and the remaining two studies (2/19) separately analyzed both the two age groups. The study populations mainly included HCWs, the general population, residents of a care home, marine recruits and hemodialysis patients. Most studies initiated between January 2020 and June 2020, and only one study started in October 2020 [Citation24]. The length of the follow-up time ranged from 4.0–13.0 months [Citation24,Citation25]. Two main methods, enzyme-linked immunosorbent assay (ELISA) and chemiluminesent micropaticle immunoassay (CMIA), were used to measure titers of blinding antibodies against SARS-CoV-2, and no studies grouped participants according to the baseline status of neutralizing antibodies. As most studies started during the early stage of the COVID-19 pandemic, the persistency of SARS-CoV-2 RNA has not been clearly understood, so varied window periods between the positive PCR test and the baseline seropositive or previous positive RNA result were adopted in defining reinfection in different studies. The main characteristics of all included studies are summarized in .
Table 1. Description of included studies and reported outcomes.
The two independent investigators evaluated study quality according to the NOS. The consistency of the evaluated NOS items was 95.4% (147/152), which suggested that interrater reliability was high (Supplementary Table 3). The scores for study quality ranged from 5 to 8. Nine studies were determined to be with high quality, ten studies with moderate quality, and no study was judged as with low quality (Supplementary Table 4).
The protection of naturally acquired antibodies against SARS-CoV-2 reinfection
presents the pooled results for the protection of naturally acquired antibodies against future SARS-CoV-2 infection. In fixed effect meta-analysis models (I2 = 15%, P = 0.27), we observed significant protection against SARS-CoV-2 reinfection in the seropositive population compared with seronegative individuals (pooled IRR = 0.16, 95% CI = 0.14-0.18). In the sensitivity analysis, the pooled IRRs of remaining studies ranges from 0.14–0.16 after removing any one of the studies, which suggested the good reliability of the pooled IRR (Supplementary Figure 1). Except for one extreme value, the funnel plot indicated that most of the included studies were symmetrically distributed in the triangular area (Supplementary Figure 2). Begg’s test similarly suggested no publication bias in the included studies (P = 0.22).
Figure 2. Forest plot of the pooled incidence rate ratio for SARS-CoV-2 infection comparing baseline seropositive and seronegative individuals.
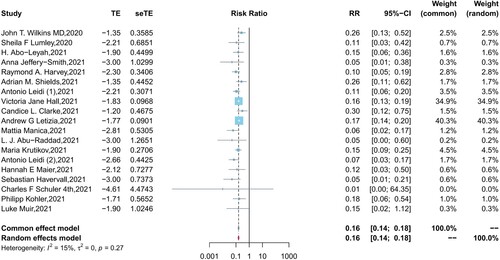
In the subgroup analyses, the pooled IRRs in HCWs and the general population were 0.22 (95% CI = 0.16–0.31, I2 = 19%, P = 0.28) and 0.14 (95% CI = 0.12–0.17, I2 = 41%, P = 0.13), respectively, with a significant difference (P = 0.02) observed between the two populations (Supplementary Figure 3). The incidence risk ratio of reinfection was lower in participants aged less than 60 years than in participants aged greater than 60 years (0.16, 95% CI = 0.14-0.19 vs 0.26, 95% CI = 0.15–0.48), however, differences (P = 0.12) between the two age groups were not significant (Supplementary Figure 4). In addition, no significant differences (all P > 0.05) were observed in the subgroup analysis of the peer review status of articles, the types of target antibodies, the definition of reinfection cases, and whether studies reported adjusted results (Supplementary Figures 5-9).
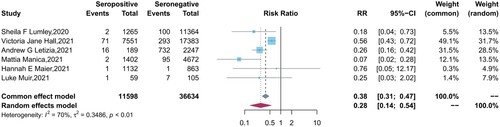
Six studies reported the protection of the antibodies induced by a previous infection against future symptomatic and asymptomatic reinfections between baseline seropositive and seronegative groups. Similar to the results of vaccine effectiveness, natural infections provided a lower level of protection against asymptomatic infection (pooled IRR = 0.28, 95% CI = 0.14-0.54) than symptomatic COVID-19 cases (pooled IRR = 0.09, 95% CI = 0.07-0.12) ().
Figure 3. Forest plot of the protection provided by naturally acquired antibodies against future symptomatic COVID-19 between baseline seropositive and seronegative individuals.
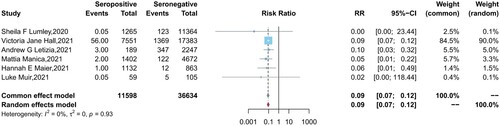
Figure 4. Forest plot of the protection provided by naturally acquired antibodies against future asymptomatic COVID-19 between baseline seropositive and seronegative individuals.
Ten studies that reported the mean/median follow-up times were included in the bubble plot to explore the changing trends of the protection provided by naturally acquired antibodies after a prior COVID-19 infection, the protection appeared to decrease slowly over time (Supplementary Figure 10).
Discussion
This systematic review and meta-analysis, including 19 studies and > 3.5 million individuals without the history of COVID-19 vaccination, provided a synthesis of the evidence that naturally acquired antibodies against SARS-CoV-2 significantly reduce the risk of subsequent infection. The efficacy of natural infection with detectable antibodies against reinfection was estimated at 84%, with higher efficacy against symptomatic COVID-19 cases than asymptomatic infection. Natural humoral immunity seems to provide similar or greater protection than COVID-19 vaccines in the ensuing thirteen months after a prior infection [Citation25,Citation26].
However, it is noted that there was high heterogeneity (I² = 70%, P<0.01) among the asymptomatic reinfection studies. After further subgroup analyses, we found the too stringent definition of asymptomatic cases in one study with large sample size (enrolled 25661 HCWs), which excluded PCR positive subjects who were pauci-symptomatic from asymptomatic cases, lead to the low protection efficacy of natural immunity in this study [Citation27]. However, although excluding this study decreases the heterogeneity (I² = 0%, P = 0.42), it does not change the conclusion a lot (pooled IRRs = 0.21 vs 0.28), which indicates the reliability of the result.
In the subgroup analyses, we found that the protection of antibodies in HCWs was lower than that of the general population (pooled IRRs of 0.22 vs 0.14). Many studies have found that HCW populations have a higher seropositive rate and infection risk than the general population [Citation28-30]. High-risk medical procedures with repeated and close contact with patients significantly increased the frequency and intensity of exposure for HCWs, which may directly affect the protective effect of antibodies. Furthermore, HCW populations usually have a high frequency of COVID-19 nucleic acid testing, which may lead HCWs with asymptomatic COVID-19 to be more likely to be identified [Citation31]. This increase in testing is another possible cause of the low level of protection of antibodies against SARS-CoV-2 that was observed in the HCW population.
We also observed relatively lower protection against reinfection in the seropositive population aged greater than 60 years compared with younger individuals. A large amount of strong evidences suggested that the reductions in the antibody response induced by vaccination are significantly associated with increasing age [Citation32,Citation33]. However, the dynamic change in antibodies acquired from natural infection differs from that induced by vaccinations. Antibodies were detected at higher levels in the population over 60 years of age in the early recovery period than in the younger population but then decayed at a faster rate [Citation34,Citation35]. This difference may be correlated with the negative alterations in the immune system with increasing age, which is known as immunosenescence [Citation36]. The differences in the dynamics of antibody responses may be a cause of the lower level of protection in the population aged greater than 60 years. These results suggest that even with a history of SARS-CoV-2 infeciton, elderly individuals and HCWs need to be vaccinated in a timely manner to obtain stronger protection against future infection.
Notably, most studies included in this systematic review were conducted between January 2020 and March 2021, indicating that the majority of the subjects included in those studies had not experienced the outbreaks of Delta, Omicron and other new variants during the pandemic. According to data from the Global Initiative on Sharing All Influenza Data (GISAID), as of 6 February 2022, Omicron (approximately 92%) and Delta (approximately 7%) were the dominant strains in the COVID-19 pandemic [Citation37]. Substantial mutations of the spike and nucleocapsid proteins lead to higher viral loads and increased transmission and adversely affect the protection provided by antibodies acquired from prior infection [Citation38,Citation39]. A retrospective study in South Africa indicated that a higher risk of reinfection was observed for Omicron than for Beta and Delta, suggesting that Omicron has a stronger immune escape capacity [Citation40,Citation41]. The herd immunity constructed from COVID-19 vaccination and natural infection may become a remote goal. Further research is needed to determine the level of protection provided naturally acquired antibodies against mutant strains, as well as the persistence of protection.
This study was also subject to other limitations. Firstly, the requested window periods between the positive RNA test and the baseline seropositive or previous positive RNA result in the definitions of reinfection were inconsistent in the included studies. A looser definition of reinfection in some studies may lead to some patients with prolonged viral shedding being misunderstood as reinfection cases and underestimating the protection of antibodies against SARS-CoV-2 [Citation16,Citation42]. However, in the subgroup analysis of studies with strict or loose definition, the protection efficacy were similar in the two subgroups which implies the negligible impact of this factor. Secondly, adaptive immunity activated by SARS-CoV-2 infection, including B cells, CD4+ T cells, and CD8+ T cells, provides long-term protection against reinfection [Citation5,Citation43]. Some hidden COVID-19 infectors who were seronegative but positive for cellular immunity were included in the baseline seronegative group, which may have a negative effect on the estimates of protection efficacy.
In conclusion, antibodies acquired from prior infection can provide strong protection against reinfection with an efficacy of 84%. Meanwhile, the protection provided by naturally acquired antibodies in HCWs and elderly individuals aged greater than 60 years was relatively low compared with the general population and younger population. During this evolving pandemic, the protection of naturally acquired antibodies against mutant strains, as well as the persistence of protection, requires further study.
Contributions
All authors reviewed the manuscript critically and approved it for submission. They contributed significantly to this work.
Supplemental Material
Download MS Word (465.5 KB)Acknowledgement
We are grateful for the support of the Bill & Melinda Gates Foundation [INV-005834], Fujian Provincial Science and Technology Project [2020L3001], and the CAMS Innovation Fund for Medical Sciences [No.2019RU022]. We also thank all authors for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data extracted from included studies can be obtained from https://github.com/chenqi160/Data.
Additional information
Funding
References
- Xiang L, Tang M, Yin Z, et al. The COVID-19 pandemic and economic growth: theory and simulation. Front Public Health. 2021 Sep;9(1355.
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard [cited Feb 15 2022]. Available from: https://covid19.who.int/.
- Staines HM, Kirwan DE, Clark DJ, et al. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis. 2021 Jan;27(1.
- Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 Dec;370(6521):1227-1230.
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063.
- Gallais F, Gantner P, Bruel T, et al. Anti-SARS-CoV-2 Antibodies Persist for up to 13 Months and Reduce Risk of Reinfection. medRxiv. 2021.
- Bao L, Deng W, Gao H, et al. Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2. bioRxiv. 2020.
- Om E, Byrne P, Walsh KA, et al. Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review. Rev Med Virol. 2021 Mar;31(2):e2162.
- Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020 Nov;26(11):1691–1693.
- Our World In Data. Coronavirus (COVID-19) Vaccinations [cited Feb 16 2022]. Available from: https://ourworldindata.org/covid-vaccinations.
- Babiker A, Marvil CE, Waggoner JJ, et al. The importance and challenges of identifying SARS-CoV-2 reinfections. J Clin Microbiol. 2021 Mar 19;59(4):e02769–20.
- Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. The Lancet. 2020 May;395(10236):1527–1529.
- Overbaugh J. Understanding protection from SARS-CoV-2 by studying reinfection. Nat Med 2020 Nov;26(11):1680–1681.
- Khoshkam Z, Aftabi Y, Stenvinkel P, et al. Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection. J Adv Res. 2021;31:49–60.
- Krishna E, Pathak VK, Prasad R, et al. COVID-19 reinfection: Linked Possibilities and future outlook. J Family Med Prim Care. 2020 Nov;9(11):5484–5449.
- Murchu EO, Byrne P, Carty PG, et al. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. 2021 May: e2260.
- Chivese T, Matizanadzo JT, Musa OAH, et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery – a comprehensive systematic review and meta-analysis. medRxiv. 2021.
- Ruiz-Galiana J, Ramos PDL, García-Botella A, et al. Persistence and viability of SARS-CoV-2 in primary infection and reinfections. Rev Esp Quimioter. 2021.
- Farrukh L, Mumtaz A, Sana MK. How strong is the evidence that it is possible to get SARS-CoV-2 twice? A systematic review. Rev Med Virol. 2021.
- Sciscent BY, Eisele CD, Ho L, et al. COVID-19 reinfection: the role of natural immunity, vaccines, and variants. J Community Hosp Intern Med Perspect. 2021.
- Perry J, Osman S, Wright J, et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. medRxiv. 2022.
- PROSPERO [cited Nov 10 2021]. Available from: https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
- Guido S, Theodore L, FBartos, jbkang. meta: General Package for Meta-Analysis [cited Nov 29 2021]. Available from: https://github.com/guido-s/meta/.
- Krutikov M, Palmer T, Tut G, et al. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long-term care facilities (VIVALDI): a prospective cohort study. Lancet Healthy Longev. 2021;2(6):e362–e370.
- Maier HE, Kuan G, Saborio S, et al. Clinical spectrum of SARS-CoV-2 infection and protection from symptomatic re-infection. Clin Infect Dis. 2021 Aug:ciab717.
- Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid COVID-19 immunity. medRxiv. 2021.
- Manica M, Pancheri S, Poletti P, et al. Risk of symptomatic infection during a second coronavirus disease 2019 wave in severe acute respiratory syndrome coronavirus 2–seropositive individuals. Clin Infect Dis. 2021:ciab556.
- Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089–1094.
- Houlihan CF, Vora N, Byrne T, et al. SARS-CoV-2 virus and antibodies in front-line Health Care Workers in an acute hospital in London: preliminary results from a longitudinal study. medRxiv. 2020.
- Eyre DW, Lumley SF, O'Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020 Aug;9:e60675.
- Hellewell J, Russell TW, Investigators S, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021 Apr;19(1):106.
- Collier DA, Ferreira I, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021 Aug;596(7872):417–422.
- Chen Q, Zhao H, Yao X, et al. Comparing immunogenicity of the escherichia coli-produced bivalent human papillomavirus vaccine in females of different ages. Vaccine. 2020 Sep;38(39):6096–6102.
- Laing ED, Epsi NJ, Richard SA, et al. SARS-CoV-2 antibodies remain detectable 12 months after infection and antibody magnitude is associated with age and COVID-19 severity. medRxiv. 2021.
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with Age and disease severity. Cell. 2020 Nov;183(4):996–1012.e19.
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007 Apr;120(4):435-46.
- Global Initiative on Sharing All Influenza Data (GISAID). Genomic epidemiology of novel coronavirus - Global subsampling [cited Feb 15 2022]. Available from: https://nextstrain.org/ncov/gisaid/global.
- Li Q, Wu J, Nie J, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020 Sep;182(5):1284-1294.e9 e9.
- Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021 Aug;385(7):585-594.
- Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021.
- Quarleri J, Galvan V, Delpino MV. Omicron variant of the SARS-CoV-2: a quest to define the consequences of its high mutational load. Geroscience. 2021 Dec: 1–4.
- U.S. Centers for Disease Control and Prevention. Protocol for Investigating Suspected SARS-CoV-2 Reinfection [cited Oct. 27 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/php/reinfection.html.
- Stephens DS, McElrath MJ. COVID-19 and the path to immunity. JAMA. 2020.
- Wilkins JT, Hirschhorn LR, Gray EL, et al. Serologic status and SARS-CoV-2 infection over 6 months of follow Up in healthcare workers in Chicago: A cohort study. Infect Control Hosp Epidemiol. 2021 Aug: 1–9.
- Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in Health care workers. N Engl J Med. 2021 Feb;384(6):533–540.
- Abo-Leyah H, Gallant S, Cassidy D, et al. The protective effect of SARS-CoV-2 antibodies in scottish healthcare workers. ERJ Open Res. 2021;7(2):00080–02021.
- Jeffery-Smith A, Iyanger N, Williams SV, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill. 2021 Feb;26(5.
- Harvey RA, Rassen JA, Kabelac CA, et al. Association of SARS-CoV-2 seropositive antibody test With risk of future infection. JAMA Intern Med. 2021 May;181(5):672–679.
- Shields AM, Faustini SE, Kristunas CA, et al. Longitudinal protection following natural SARS-CoV-2 infection and early vaccine responses: insights from a cohort of community based dental health care professionals. medRxiv. 2021.
- Leidi A, Koegler F, Dumont R, et al. Risk of reinfection after seroconversion to SARS-CoV-2: A population-based propensity-score matched cohort study. 2021 May:2021.03.19.21253889.
- Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS-CoV-2 immune responses in haemodialysis patients and protection against reinfection. medRxiv. 2021.
- Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in england: a large, multicentre, prospective cohort study (SIREN). The Lancet. 2021;397(10283):1459–1469.
- Letizia AG, Ge Y, Vangeti S, et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: a prospective cohort study. Lancet Respir Med. 2021 Jul;9(7):712–720.
- Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021 May;35:100861.
- Leidi A, Berner A, Dumont R, et al. Occupational risk of SARS-CoV-2 infection and reinfection during the second pandemic surge: a cohort study. Occup Environ Med. 2021 Dec:oemed-2021-107924.
- Havervall S, Ng H, Jernbom Falk A, et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J Intern Med. 2022 Jan;291(1):72–80.
- Schuler CFT, Gherasim C, O'Shea K, et al. Mild SARS-CoV-2 illness Is Not associated with reinfections and provides persistent spike, nucleocapsid, and virus-neutralizing antibodies. Microbiol Spectr. 2021 Oct;9(2):e0008721.
- Kohler P, Güsewell S, Seneghini M, et al. Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent Covid-19 – a prospective multicentre cohort study. medRxiv. 2021.
- Muir L, Jaffer A, Rees-Spear C, et al. Neutralizing antibody responses after SARS-CoV-2 infection in End-stage kidney disease and protection against reinfection. Kidney Int Rep. 2021;Jul6(7):1799–1809.
