ABSTRACT
Exposure to poultry in live poultry markets is strongly associated with human infection with avian influenza virus. To effectively prevent the transmission of viruses from live poultry to humans, people have been forced to change their living habits from purchasing live poultry for consumption to purchasing freshly slaughtered poultry after the permanent closure of live poultry markets in China. In this study, we reported a case of human infection by the H5N6 virus in Hangzhou after exposure to a freshly slaughtered chicken, defying the traditional hypothesis that human infection requires a history of exposure to live poultry and indicating a novel route of infection. Rapid genomic characterization of H5N6 influenza A variants from the patient and the associated environment suggested that these viral variants were of avian origin, belonged to clade 2.3.4.4b H5 and were adapting to the human host after infection. Comparative analysis of the local H5N6 genomes showed that viral contamination in the associated environment and the poultry market was complex. Considering this case of H5N6 infection, conducting surveillance for any possible new avian influenza virus reassortment spillover to humans or other animal species is critical, and awareness of the risk of exposure to possible viral variants from infected slaughtered poultry or the associated environment must be seriously improved.
Highlights
We reported the first case of human infection with avian-origin influenza A (H5N6) virus in Zhejiang Province, southeastern China.
Rapid genomic characterization of H5N6 influenza A variants from a patient and the associated environment suggested that these viral variants were of avian origin and were adapting to the human host after infection.
Comparative analysis of the H5N6 genomes showed that viral contamination in the associated environment and poultry market was complex.
Considering this case of H5N6 infection, the risk of exposure to possible viral variants from infected slaughtered poultry or the associated environment must be seriously considered.
To the editor
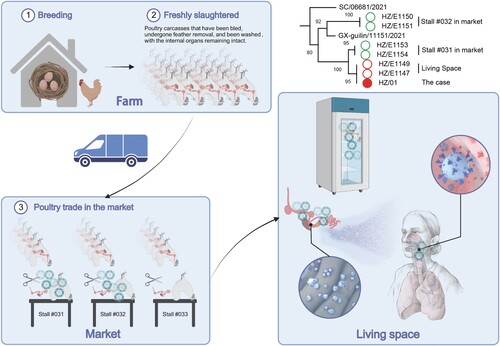
Avian influenza viruses (AIVs) pose significant risks to public health due to their high mortality rates. Novel AIV reassortants, namely, H5N1, H7N9, H10N8 and H5N6, are currently circulating in poultry flocks and occasionally infect humans [Citation1–3]. Since the first epidemic of H7N9 influenza A virus in March 2013, substantial concern has been raised over the possible occurrence of novel AIV reassortants with enhanced poultry-human or limited human-human transmissibility. Exposure to poultry in live poultry markets (LPMs) is strongly associated with human AIV infection [Citation4]. Therefore, all of the LPMs in Hangzhou have been permanently closed since February 2014 to effectively prevent the transmission of AIVs from live poultry to humans. As a result, people have been forced to change their living habits from purchasing live poultry for consumption to purchasing freshly slaughtered poultry, which is defined as a poultry carcase that has been bled, undergone feather removal, and been washed, with the internal organs remaining intact. Avian H5N6 influenza A virus infection, which was first confirmed in Sichuan Province in April 2014 [Citation5], has caused a total of 57 laboratory-confirmed cases as of December 17, 2021, including 27 deaths (case fatality rate (CFR) of 47.37%) [Citation6]. Herein, we report a case of human infection by the H5N6 virus in Hangzhou after exposure to a freshly slaughtered chicken, defying the traditional hypothesis that human infection requires a history of exposure to live poultry and indicating a novel route of infection.
The patient (female, aged 51 years) was a purified water delivery person in Shangcheng district and presented with respiratory symptoms, including a high fever (39.1 °C), fatigue, chills and a cough, on December 15, 2021. The symptoms were not relieved with rest at home. By December 18, other symptoms had developed (heavy cough, shortness of breath, and yellow and sticky sputum), and she was admitted to a local hospital. According to radiographic lung changes, she was diagnosed with community-acquired severe acute interstitial pneumonia. The patient had no contact with live poultry prior to illness onset. However, considering that she had a history of exposure to slaughtered chicken sections half a month previously, an influenza A RNA test was performed. By means of type- and subtype-specific PCR assays via viral RNA tests of bronchoalveolar lavage fluid collected prior to oseltamivir treatment, H5N6 influenza A infection was laboratory confirmed on December 22: A (Ct = 23.23), H5 (Ct = 23.58), and N6 (Ct = 22.23). The patient was transferred to the isolation ward of the intensive care unit (ICU) 7 days after disease onset. Oseltamivir was administered during her stay in the ICU. Due to unexpected results after treatment for severe acute respiratory distress syndrome (ARDS) and mixed infection, treatment was terminated, and she was discharged from the hospital on January 12, 2022 (Figure S1). Informed consent was obtained from the spouse of the patient.
To trace the source, 49 environmental samples were collected from the epidemiologically associated living space of the patient and a nearby poultry market on December 23, of which 12 samples were positive for the H5N6 virus (Table S1). Among the 29 samples from the poultry market, five from sectioning sites of two poultry stalls were H5N6-positive. Twenty samples were collected from the home of the patient, among which 35% of the swabs (7/20) were positive for the H5N6 virus. Positive swabs were concentrated in the area linked to the slaughtered chicken. The highest viral load was found in the sample collected from the peritoneal lavage fluid of the slaughtered chicken: A (Ct = 23.17), H5 (Ct = 23.60), and N6 (Ct = 22.33).
The epidemiological investigation preliminarily identified a total of 24 close contacts of the patient, including seven family members (her husband, son and his family, and daughter and her family), eight colleagues and nine poultry market workers. To date, none of the above have developed symptoms such as fever or cough, and no H5N6 was identified by throat swab sampling, indicating that the H5N6 subtype has only limited poultry-human transmission ability, although the mortality rate of the disease is very high. The close contacts were placed under medical observation for ten days.
To study the genetic basis of human infection with the H5N6 virus, seven complete viral genomes were obtained using PathAmp FluA reagents (Life Technologies, USA) and nanopore sequencing on the Gridion platform (one from the patient and six from the environment linked to the case). Mutations were confirmed by Sanger sequencing performed by Sangon (Shanghai, China) using specific previously described primers [Citation7]. The genomes of these H5N6 variants have been submitted to the Global Initiative on Sharing Avian Influenza Data (GISAID) under accession numbers EPI1946727-34 and EPI1951343-90. To determine the evolutionary relationship, these sequences were compared with those of other H5N6 variants downloaded from the GISAID database. The maximum likelihood phylogenetic tree generated by RAxML v8.2 [Citation8] and MEGA X [Citation9] revealed that all of the local H5N6 variants belonged to clade 2.3.4.4b H5 (Figure S2). Hemagglutinin (HA), neuraminidase (NA) and all internal genes of these viruses clustered with the previously reported H5N6 isolate A/GX-guilin/11151/2021 (GISAID accession number EPI1887786-93) collected from a person in Guangxi Province [Citation10] (Figure S2 and S3), with high nucleotide identity ranging from 98.55%–99.80%. Through comparative analysis, three different variants were found among the seven genomes, suggesting that viral contamination in the associated environment was complex. According to the phylogenetic tree, HZ/E1150 and HZ/E1151 collected from stall #032 of the poultry market were more similar to GX/11151 than to HZ/01 (H5N6 in this study). HZ/01, HZ/E1147, HZ/E1149, HZ/E1153 and HZ/E1154 were clustered in the same subclade, while HZ/E1147 and HZ/E1149, collected from the patient’s living space, were closely related to that collected from the patient, suggesting that the freshly slaughtered poultry may have been the direct source of infection, not the contaminated stalls of the poultry market.
We also evaluated whether the local H5N6 variants had gained any important amino acid substitutions promoting mammalian adaptation (Table S2). Regarding the HA segment, the viral isolates from the patient, the slaughtered chicken and the stalls of the poultry market all possessed multiple basic amino acid motifs, such as REKRRKR↓G or KEKRRKR↓G, at the cleavage site, indicating high pathogenicity in poultry. Some key amino acids within HA and NA were the same as those in GX/11151, including Q226 and G228 in HA, which are believed to affect the binding affinity of the α-2,6-linked sialic acid receptor; the deletion of 11 amino acids at the stalk of NA (positions 59-69), associated with virulence in mice; E119, I222, H274, R292 and N294 in NA, involved in susceptibility to oseltamivir and zanamivir; and S31 in the M2 protein, indicating sensitivity to adamantine. Substitutions at host-specific related sites, such as E627 K, D701N and Q591 K in the PB2 fragment, were not observed in any of the viral isolates, indicating that the isolates from the bronchoalveolar lavage fluid of the patient, the slaughtered chicken and the associated environment retained features of avian origin. Notably, the H5N6 sample collected from the patient seven days after illness onset contained two substitutions in the PA (R635 K) and NP (E434 K) proteins compared with those from the slaughtered poultry, suggesting that the avian-origin H5N6 variant was adapting to the host after infection.
Human infection with avian H5N6 influenza A virus is preventable, controllable and curable. However, due to the lack of population immunity in humans and the ongoing evolution of the virus, there is a continuous risk that clade 2.3.4.4b H5 viruses could cause an influenza pandemic if these viruses develop the ability to efficiently transmit among humans. The slaughtered poultry trade has replaced the live poultry trade in China. Poultry markets have converted original live poultry trading areas to centralized trading areas for frozen slaughtered poultry. This case of H5N6 infection indicates that the one-health approach needs to be strengthened to trace the source of infection in time, prevent cross-species transmission of viruses, reduce risks, and protect people’s health. (1) Conducting surveillance for any possible new AIV reassortment spillover to humans or other animal species is pivotal. (2) The management of poultry breeding sites and live poultry distribution chains should be strengthened, including comprehensive rectification, cleaning, and disinfection of all the live poultry distribution chains in districts and counties. (3) The awareness of the risk of exposure to possibly contaminated poultry carcases or associated environments must be seriously improved (). In poultry infected by AIVs before slaughter, the virus may reside in virus-contaminated internal organs, even if the carcase is thoroughly cleaned. If the carcase is not handled properly, infection may occur during the sectioning process. Therefore, it is recommended to purchase frozen poultry through official channels. Workers in poultry markets who dissect and clean slaughtered poultry should don personal protective equipment, such as masks and rubber gloves. After dissection, the wastewater and internal organs of fresh poultry must be disinfected. (4) To reduce the risk of human infection with avian influenza viruses, some effective measures should be taken, such as strengthening health education through knowledge popularization of avian influenza prevention and control measures among society members and improving disease prevention awareness among poultry trade workers.
Supplemental Material
Download Zip (770.5 KB)Acknowledgments
This work was supported by the Hangzhou Key Medicine Discipline Fund for Hygienic Microbiology Laboratory (2020-2024), the Zhejiang Provincial Programme for the Cultivation of High-level Innovative Health Talents (2018-2022) and the Zhejiang Provincial Public Welfare Technology Application Research Project of China (LGF19H190003).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Liu D, Shi W, Shi Y, et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet. 2013;381(9881):1926–1932.
- Chen H, Yuan H, Gao R, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383(9918):714–721.
- Yang ZF, Mok CK, Peiris JS, et al. Human infection with a novel avian influenza A(H5N6) virus. N Engl J Med. 2015;373(5):487–489.
- Liu B, Havers F, Chen E, et al. Risk factors for influenza A(H7N9) disease--China, 2013. Clin Infect Dis. 2014;59(6):787–794.
- World Health Organization. WHO China statement on H5N6. 7 May 2014. http://www.wpro.who.int/china/mediacentre/releases/2014/20140507/en/ [2021–12–29].
- WHO. Avian influenza weekly update number 823. https://www.who.int/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20211217.pdf?sfvrsn=5f006f99_72#38;sfvrsn=5f006f99_42. [2021–12–29].
- Hoffmann E, Stech J, Guan Y, et al. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146(12):2275–2289.
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313.
- Kumar S, Stecher G, Li M, et al. MEGA x: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549.
- Bi F, Jiang L, Huang L, et al. Genetic characterization of Two human cases infected with the avian influenza A (H5N6) viruses - Guangxi zhuang autonomous region, China, 2021. China CDC Wkly. 2021;3(44):923–928.