ABSTRACT
Accruing evidence suggests an increased risk of myocarditis in adolescents from messenger RNA COVID-19 vaccines. However, other potential adverse events remain under-researched. We conducted a retrospective cohort study of adolescents aged 12–18 with a territory-wide electronic healthcare database of the Hong Kong population linked with population-based vaccination records and supplemented with age- and sex-specific population numbers. Two age- and sex-matched retrospective cohorts were formed to observe 28 days following the first and second doses of BNT162b2 and estimate the age- and sex-adjusted incidence rate ratios between the vaccinated and unvaccinated. Thirty AESIs adapted from the World Health Organization’s Global Advisory Committee on Vaccine Safety were examined. Eventually, the first-dose cohort comprised 274,881 adolescents (50.25% received the first dose) and the second-dose cohort 237,964 (50.29% received the second dose). Ninety-four (34.2 per 100,000 persons) adolescents in the first-dose cohort and 130 (54.6 per 100,000 persons) in the second-dose cohort experienced ≥1 AESIs. There were no statistically significant differences in the risk of any AESI associated with BNT162b2 except myocarditis [first-dose cohort: incidence rate ratio (IRR) = 9.15, 95% confidence interval (CI) 1.14–73.16; second-dose cohort: IRR = 29.61, 95% CI 4.04–217.07] and sleeping disturbances/disorders after the second dose (IRR = 2.06, 95% CI 1.01–4.24). Sensitivity analysis showed that, with myocarditis excluded as AESIs, no significantly elevated risk of AESIs as a composite outcome associated with vaccination was observed (P = 0.195). To conclude, the overall absolute risk of AESIs was low with no evidence of an increased risk of AESIs except myocarditis and sleeping disturbances/disorders.
The coronavirus disease 2019 (COVID-19) pandemic incurs a huge burden to individuals of all age ranges in maintaining good health [Citation1]. Albeit rarer in younger individuals compared with adults, a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection could possibly lead to serious complications such as multiple organ failure and mortality [Citation2]. Accordingly, the emergency use of a variety of vaccines has been extended to adolescents worldwide [Citation3]. Of note, BNT162b2, one of the most widely used messenger RNA (mRNA) COVID-19 vaccines, is currently used to vaccinate adolescents aged as young as 12 years in numerous countries [Citation4]. In fact, a further extension to younger age groups has been approved to include children as young as five [Citation5].
Nevertheless, the increased risk of myocarditis has been generated with regard to the use of mRNA vaccines and BNT162b2 in particular [Citation6–8]. Case reports and studies comparing the incidence of vaccinated individuals with the background incidence all suggest an elevated risk [Citation9]. A large cohort study of nearly one million individuals [Citation8] has estimated a threefold-increased risk of myocarditis among vaccinated individuals compared with the unvaccinated despite a very low absolute risk, which is consistent with findings from other studies as well [Citation10,Citation11]. There was also a descriptive study on the increased number of myocarditis reports in adolescents using the nationwide passive surveillance system monitoring a range of adverse events [Citation12]. A more recent Danish cohort study, however, did not identify such a risk associated with BNT162b2 but with mRNA-1273 [Citation13]. This widely observed elevated risk has received widespread public attention and aroused concerns over the safety of mRNA vaccines in adolescents overall. Certain adverse events with rare incidence may be difficult to capture in randomized controlled trials on a typically limited number of participants. Nevertheless, post-marketing pharmacovigilance data on the use of BNT162b2 in adolescents remain scant.
Since 14 June 2021, the age limit for BNT162b2 vaccination in Hong Kong has been lowered to 12 years or older [Citation14]. As of 30 September 2021, more than 200,000 adolescents aged under 18 had received the first dose of BNT162b2 [Citation14]. The SARS-CoV-2 infection rate in Hong Kong has remained low, with the daily number of confirmed COVID-19 cases maintained below 200 cases and the total cumulative number of cases kept under 12,500 out of a population of over seven million [Citation15]. Since 16 August 2021 (up to 30 September 2021), there were zero local cases recorded in the daily reports [Citation15]. This low infection rate is facilitative of the pharmacovigilance of BNT162b2 because adverse effects following vaccination would be unlikely induced by a COVID-19 infection. This population-based cohort study aims to describe and compare a range of adverse events of special interest in adolescents between the vaccinated and unvaccinated using a territory-wide electronic health record database with linkage to local vaccination records.
Materials and methods
Study design
A retrospective cohort design was adopted to examine the incidence rate ratio (IRR) of vaccination-related adverse events of special interest (AESI) within a 28-day observation period between the vaccinated and the unvaccinated.
Data source
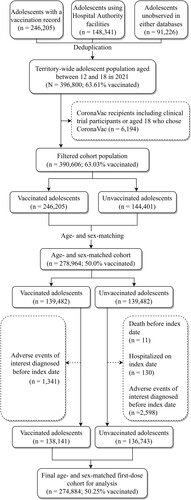
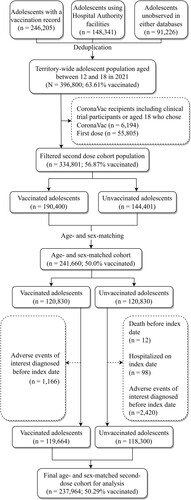
Territory-wide vaccination records of adolescents aged 12–18 were provided by the Department of Health (DH) of the Hong Kong Government. They were then linked with electronic health records provided by the Hospital Authority (HA), the sole provider of public inpatient services and specialist ambulatory clinics as well as a major provider of public general outpatient services, by matching the encrypted Hong Kong Identity Card number between the two databases. Our database contained essential information for the pharmacovigilance of COVID-19 vaccines such as clinical diagnoses, medication use, healthcare utilization, etc. AESIs were identified using this comprehensive database which has been used to conduct population-based COVID-19 vaccines pharmacovigilance studies on various outcomes [Citation16–24, http://dx.doi.org/10.1111/joim.13453]. We further included adolescents without any vaccination record or healthcare utilization record in Hong Kong (unobserved in the above two data sources) from the age- and sex-specific population numbers provided by the Census and Statistics Department (C&SD) as of mid-2021 [Citation25].
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West (UW 21–149 and UW 21-138) and the Department of Health Ethics Committee (LM 21/2021).
Cohort selection and formation
Two retrospective cohorts were formed, with the observation periods starting from the first dose and from the second dose of BNT162b2, respectively. Both of these cohorts adopted the unvaccinated individuals as the comparison group to estimate the IRR. We retrieved the vaccination records of individuals aged 12–18 during the study period from DH and merged them with data of patients who received HA services from 1 January 2018 to 30 September 2021. CoronaVac recipients, consisting of those aged 18 years who chose to receive CoronaVac or clinical trial participants, were excluded from the merged cohort. For the second-dose cohort, those who had received only one dose were excluded. According to the age- and sex-specific population sizes provided by the C&SD, we further included individuals unobserved in our databases who were unvaccinated and did not use any public healthcare services by the HA since 2018. These individuals were operationalized as additional rows in the datasets coded as unvaccinated without any AESI.
Age (in years) and sex were then used to randomly match the unvaccinated with the vaccinated at the ratio of one to one by randomly selecting an adolescent of the same age and sex from the vaccinated adolescent(s) for each unvaccinated adolescent without replacement, with the vaccinated considerably outnumbering the unvaccinated. The vaccination dates (first or second dose for the respective cohort) were then mapped to the matched unvaccinated individuals as the index date, based on which the individual follow-up periods were determined. Accordingly, we removed those who died before the index date, were hospitalized on the index date (in the unvaccinated group) or had any AESI records before the index date. This approach has been used in previous large-scale pharmacovigilance studies as well [Citation17,Citation20,Citation21].
Outcome: adverse events of special interest
The selection of adverse events was based on the World Health Organization’s Global Advisory Committee on Vaccine Safety (GACVS), [Citation26] from which a list of 30 AESIs were adopted to identify those who were diagnosed with any of the listed potential adverse reactions using the International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Primary Care (ICPC), except mortality and COVID-19, to define the primary composite outcome of this study (please see eTable 1 for the codes for the operationalization of AESI), i.e. any AESI. The observation period started from the index date and ended with 28 days after receiving the vaccine, mortality, receiving the second dose (only applicable to the first-dose cohort), or 30 September 2021 (end of data availability), whichever came earliest. Analysis on eight sub-categories of the AESI, namely, autoimmune diseases, cardiovascular system diseases, circulatory system diseases, hepato-renal system diseases, nerves and central nervous system diseases, skin and mucous membrane, bone and joints system diseases, respiratory system diseases, and diseases of other systems, as well as each specific AESI were conducted as the secondary outcomes.
Exposure: vaccinated versus unvaccinated
Receiving BNT162b2 (one dose or two doses for the two respective cohorts) was compared with being unvaccinated as the exposure of this study given that BNT162b2 was the only approved vaccine for distribution for residents aged 12 or above in Hong Kong (as of the last day of available data) [Citation27].
Statistical analysis
Poisson regression models were performed to generate age- and sex-adjusted IRR to examine the association between vaccination and AESI. The same analyses were replicated on all sub-categories of AESI and each specific AESI as secondary outcomes. As a sensitivity analysis, we repeated the analysis on the primary outcome (an AESI) in the second-dose cohort with myocarditis removed from the list of AESIs in the operationalization to infer about the importance of myocarditis in the potential elevation of AESI risks.
All analyses were conducted using the R (version 4.1.1) statistical environment.
Results
As of 30 September 2021, a total of 252,399 (63.61%) of the 396,800-adolescent population were vaccinated at least one dose, with 190,400 (56.87%) having received the second dose.
For the first-dose cohort, after age- and sex-matching for mapping the index date from the unvaccinated to the vaccinated group, 278,964 individuals remained. Eventually, the cohort comprised 274,884 patients, with 50.25% of the adolescents vaccinated at least one dose after subsequent removal of ineligible individuals. Following the same selection procedures, the final second-dose cohort consisted of 237,964 individuals with 50.29% vaccinated with two doses. displays the flowchart of the cohort selection for the first-dose cohort and shows that for the second-dose cohort.
Cohort characteristics: age and sex
The cohort characteristics for both cohorts are summarized in . For the first-dose cohort, the male proportion of the vaccinated and unvaccinated groups was 50.36%% and 50.27%, respectively. For the second-dose cohort, it was 51.12% and 51.02%. The mean age was highly similar between the vaccinated and unvaccinated in both cohorts.
Table 1. Sex and age distribution between the vaccinated and unvaccinated groups.
Adverse events of special interest
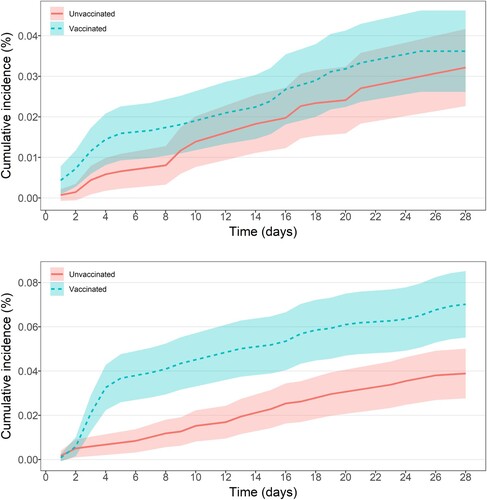
Over the observation period for the first-dose cohort, 94 adolescents experienced at least one AESI, constituting an overall incidence of 0.3 per 1000 persons [95% confidence interval (CI) 0.3–0.4]. For the second-dose cohort, the incidence was 0.5 (95% CI 0.5–0.6). shows that the estimated cumulative incidence (of any AESI) over the observation period, with the vaccinated group largely overlapping with the unvaccinated in the first-dose cohort. Nevertheless, an elevated cumulative incidence of AESI was observed among the vaccinated group compared with the unvaccinated group for the second-dose cohort.
Figure 3. (a) Cumulative incidence with 95% confidence interval (shaded area) of any AESI of first-dose vaccinated and unvaccinated groups within 28-day observation period. (b) Cumulative incidence with 95% confidence interval (shaded area) of any AESI of second-dose vaccinated and unvaccinated groups within 28-day observation period.
For the first-dose cohort, the most common AESI was sleeping disturbance/disorder (n = 18 for the vaccinated group; n = 16 for the unvaccinated group), followed by anaphylaxis (n = 8 for vaccinated group; n = 7 unvaccinated group), myocarditis (n = 8 for vaccinated group; n = 1 for unvaccinated group), and arrhythmia (n = 7 for vaccinated group; n = 2 for unvaccinated group). Likewise, for the second-dose cohort, the most common AESI was sleeping disturbance/disorder (n = 23 for the vaccinated group; n = 11 for the unvaccinated group), followed by myocarditis (n = 30 for vaccinated group; n = 1 for unvaccinated group), and arrhythmia (n = 12 for vaccinated group; n = 6 for unvaccinated group).
Poisson regression model
As shown in , Poisson regression analyses suggested that, for the first-dose cohort, there were no marked differences in the risk of any AESI upon receiving the vaccine during the observation period of 28 days except for myocarditis (IRR = 9.15, 95% CI 1.14–73.16, P = 0.037). Similarly, the sub-category analyses indicated that the vaccinated group had a five-fold higher incidence rate of 5.34 (95% CI 1.53–18.56) in the cardiovascular system AESIs compared with the unvaccinated group (p = 0.009). All other sub-category analyses suggested no significant differences in the association of vaccination status with any other types of AESIs (P > 0.05). The incidence of myocarditis was estimated at 2.91 (95% CI 1.26–5.73) per 100,000 vaccinated persons compared with 0.36 (95% CI 0.01–2.03) among the unvaccinated.
Table 2. Incidence rate ratios with 95% confidence intervals of adverse events of special interest (AESI) from age- and sex-adjusted Poisson regressions censoring on receiving second dose and 28-day of first dose inoculation.
For the second-dose cohort as shown in , a similar pattern was observed and the IRR for myocarditis was estimated at 29.61 (95% CI 4.04–217.07) and that for cardiovascular system AESIs at 5.92 (95% CI 2.66–13.17). In addition, the IRR for sleeping disturbance/disorder was estimated at 2.06 (95% CI 1.01–4.24). The incidence of myocarditis was estimated at 12.61 (95% CI 8.51–18.00) per 100,000 vaccinated persons compared with 0.42 (95% CI 0.01–2.34) among the unvaccinated.
Table 3. Incidence rate ratios with 95% confidence intervals of adverse events of special interest (AESI) from age- and sex-adjusted Poisson regressions comparing two-dose vaccination with non-vaccination censoring on 28-day of second dose inoculation.
Sensitivity analysis
With myocarditis excluded from the composite AESI outcome for the second-dose cohort, no significant association between vaccination and AESI was observed (IRR = 1.29, 95% CI 0.87–1.90, P = 0.195).
Discussion
This study is one of the first cohort studies describing and comparing AESIs after receiving BNT162b2 among adolescents. Overall, the BNT162b2 is found to have an acceptable safety profile for adolescents in Hong Kong, as evidenced by a very low incidence of AESIs following vaccination without a marked elevation of AESI risk compared with the unvaccinated, except there is an increased risk of myocarditis, especially following the second dose. A marginally significant heightened risk of sleeping disturbance/disorder was also observed following the second dose.
Compared with previous research in Western populations, the observed absolute risk of myocarditis following BNT162b2 use, especially the second dose, was apparently higher i.e. above 10 per 100,000 vaccinated persons. For instance, in a recent Israeli study, among those aged 16–29, the cumulative incidence over 42 days was estimated at 5.49 per 100,000 vaccinated individuals. In a British study, only one additional event per one million persons was estimated to be induced by the use of BNT162b2 [Citation10]. This difference may have arisen from a potential underestimation of the risk in the Western populations, a younger cohort adopted in this current study, or unknown genetic differences between the Chinese and Western populations. These speculations warrant further research to substantiate or refute.
A recent study conducted in Hong Kong examining the risk of myocarditis and pericarditis following BNT162b2 vaccination showed that there was an overall increased risk of myocarditis among adolescents (overall incidence rate 18.52 (95% CI 11.67–29.09) per 100,000 persons vaccinated). Amongst the 33 cases described, all had mild diseases and recovered spontaneously. The risk was particularly prominent among males after the second dose [incidence rate 37.32 (95% CI 26.98–51.25) per 100,000]. All cases were mild, and did not require inotropic, ventilatory and circulatory support [Citation18]. This was consistent with the observation in this study, as well as in other jurisdictions, including the United States [Citation28–31] and Israel [Citation7,Citation11]. A recent murine model study has demonstrated the association between mRNA vaccine and myopericarditis, probably because of higher systemic levels of mRNA lipid nanoparticles due to inadvertent intravenous injection or rapid return from the lymphatic circulation [Citation32]. This finding, together with our current observations, provides evidence to support the recommendation for adolescents between 12 and 17 years in Hong Kong (since 16 September 2021) to receive one dose of BNT162b2 only [Citation27]. These adolescents who suffered from vaccine-related myocarditis shall warrant long-term follow-up for any potential cardiovascular sequelae.
Despite the increased risk of myocarditis and the marginally increased risk of sleeping disturbance/disorder the other AESIs recorded among the vaccinated group were similar to the unvaccinated group. Such observation was consistent with local studies conducted in the adult population [Citation16,Citation17]. Narcolepsy, one of the conditions covered by our definition of sleeping disturbance/disorder, has not been reported in other studies on COVID-19 vaccine-related AESI so far, but reported previously to be possibly associated with influenza vaccination [Citation33]. In our study, no patients coded as having sleeping disturbance/disorder had this particular condition (ICD-9 347.xx). Contrary to observation in the West, our study did not reveal an increased risk of thromboembolism following BNT162b2 vaccination [Citation34], which is likely due to a younger population and possibly the inherently lower risk of thromboembolism among Asians to Caucasians [Citation35]. However, this absence of risk elevation might be due to insufficient statistical power as well. Continuous safety monitoring is still needed.
This study has several limitations. First, adolescents who experienced AESIs may have sought medical attention in the private sector. Nevertheless, data collected in this study is considered representative as the HA constitutes approximately 80% of the inpatient services in Hong Kong [Citation36]. Second, in common with all observational studies, residual confounders cannot be completely excluded in this retrospective database analysis. Third, similar to other large-scale pharmacovigilance studies using electronic medical record databases, we only relied on the diagnostic codes and other records for the operationalization of the diseases despite the demonstrated accuracy of those codes [Citation37]. Fourth, the number of adolescents not using HA services and without a vaccination record was calculated using the C&SD population estimates by age and sex. The validity of the study results is therefore subject to the accuracy of those numbers. Last, as the population of Hong Kong is predominantly Chinese, replication of the analyses in other world populations is warranted to test for the generalizability of the results.
Conclusion
Adolescents receiving BNT162b2 vaccine had an increased risk of myocarditis and sleeping disturbance/disorder, but not other AESIs. The overall risk of COVID-19 infection in different jurisdictions should be weighed against these specific risks in adolescents.
Supplemental Material
Download MS Word (12.5 KB)Supplemental Material
Download MS Word (16.6 KB)Acknowledgements
This study was regulatory-initiated pharmacovigilance and was funded by a research grant from the Food and Health Bureau of The Government of the Hong Kong Special Administrative Region (Reference COVID19F01). The funding organization provided record-linkage data via the Department of Health and Hospital Authority but has no role in the design and conduct of the study, management, analysis, and interpretation of the data. The funding organization reviewed and approved the manuscript but had no role in the preparing of the manuscript nor the decision to submit the manuscript for publication. FTTL and ICKW are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by the by AIR@InnoHK administered by Innovation and Technology Commission. We would like to thank the Hong Kong Department of Health and the Hospital Authority for providing vaccination and clinical data.
Disclosure statement
FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and the Hong Kong Research Grants Council, outside the submitted work. XL has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme, unrelated to this work. EWYC reports honorarium from Hospital Authority; and grants from Research Grants Council (RGC, Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region, outside the submitted work. PI has received funding from the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and Hong Kong Jockey Club Charities Trust. ICKW receives research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. All other authors declare no competing interests.
Data availability statement
Data will not be available for third parties as permission has not been obtained from the data custodians.
Additional information
Funding
References
- Miller IF, Becker AD, Grenfell BT, et al. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26:1212–1217.
- Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873.
- Opel DJ, Diekema DS, Ross LF. Should we mandate a COVID-19 vaccine for children? JAMA Pediatr. 2021;175:125–126.
- World Health Organization. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. Geneva; 2021. https://apps.who.int/iris/bitstream/handle/10665/341786/WHO-2019-nCoV-vaccines-SAGE-recommendation-BNT162b2-2021.2-eng.pdf?sequence=1&isAllowed=y.
- Food & Drug Administration. FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. Washington DC; 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age.
- Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021. DOI:10.1001/jamacardio.2021.2821.
- Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021. 23–385.
- Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090.
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484.
- Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021. DOI:10.1038/s41591-021-01630-0.
- Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021. DOI:10.1056/NEJMoa2110737.
- Hause AM, Gee J, Baggs J, et al. COVID-19 vaccine safety in adolescents aged 12-17 years - United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1053–1058.
- Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665.
- Centre for Health Protection. Hong Kong vaccination dashboard; 2021. [cited 2021 Aug 27]. https://www.covidvaccine.gov.hk/en/dashboard/totalFirstDose.
- World Health Organization. WHO coronavirus (COVID-19) dashboard; 2021. [cited 2021 Oct 3]. https://covid19.who.int/.
- Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021. DOI:10.1016/S1473-3099(21)00451-5.
- Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021. DOI:10.1136/annrheumdis-2021-221571.
- Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021. DOI:10.1093/cid/ciab989.
- Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Ann Intern Med. 2022. DOI:10.7326/M21-3700.
- Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13:411.
- Li X, Tong X, Wong ICK, et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut. 2022. DOI:10.1136/gutjnl-2021-326860.
- Sing C-W, Tang CTL, Chui CSL, et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol. 2022. DOI:10.1002/ajh.26478.
- Xiong X, Wong CKH, Au ICH, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022. DOI:10.1089/thy.2021.0684.
- Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Heal - West Pacific. 2022;21:100393.
- Census and Statistics Department. Population estimates; 2021. [cited 2021 Nov 9]. https://www.censtatd.gov.hk/en/scode150.html.
- World Health Organization. Background paper on Covid-19 disease and vaccines: prepared by the Strategic Advisory Group of Experts (SAGE) on immunization working group on COVID-19 vaccines, 22 December 2020. World Health Organization; 2020.
- Hong Kong Government. COVID-19 Vaccination Programme; 2021. [cited 2021 Aug 6]. https://doi.org/10.1001/jamapediatrics.2022.0101.
- Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982.
- Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 20. MMWR Morb Mortal Wkly Rep. 2021;70:1094–1099.
- Perez Y, Levy ER, Joshi AY, et al. Myocarditis Following COVID-19 mRNA vaccine: a case series and incidence rate determination. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2021. DOI:10.1093/cid/ciab926.
- Diaz GA, Parsons GT, Gering SK, et al. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021;326:1210–1212.
- Li C, Chen Y, Zhao Y, et al. Intravenous injection of COVID-19 mRNA vaccine can induce acute myopericarditis in mouse model. Clin Infect Dis. 2021. DOI:10.1093/cid/ciab707.
- Sarkanen TO, Alakuijala APE, Dauvilliers YA, et al. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177–186.
- Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931.
- White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123:S11-7.
- Leung GM, Tin KYK, O’Donnell O. Redistribution or horizontal equity in Hong Kong’s mixed public–private health system: a policy conundrum. Health Econ. 2009;18:37–54.
- Wong AYS, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352. DOI:10.1136/bmj.h6926.