To the editor: Erysipelothrix genus, a kind of gram-positive facultative anaerobes, is known to cause Erysipelas in pigs and Erysipeloid in humans [Citation1]. Currently, Erysipelothrix genus is composed of five species, including Erysipelothrix rhusiopathiae, Erysipelothrix tonsillarum, Erysipelothrix inopinata, Erysipelothrix larvae, and Erysipelothrix piscisicarius[Citation2]. E. piscisicarius is a novel pathogen in fish aquaculture, causing orofacial ulceration and necrosis, necrotizing dermatitis, myositis, and cellulitis in fish [Citation3]. So far, E. piscisicarius infection has not been documented in humans. Herein, we reported a case of E. piscisicarius infection in a woman detected by metagenomics next-generation sequencing (mNGS) and confirmed by whole genome sequencing (WGS), and it is the first case of E. piscisicarius infection in humans.
A 72-year-old female patient was admitted to our hospital with a complaint of high fever and chills of unknown origin (Day 1). In the emergency department, she reached a body temperature of 40.2°C, combined with headache, vertigo, anhelation, lags in response, drowsiness, and urine retention. Physical examination showed pulse of 125 bpm, 39 breaths/min, and blood pressure of 89/45 mmHg. After blood routine testing (Table S1), the patient was transferred to EICU for further treatment. Piperacillin-tazobactam (4.5 g q8h) was empirically administrated for the patient, combined with symptomatic treatment like reducing sputum and nutrition support. The initial symptoms of the patient had been relieved, including dizziness, headache, and anhelation (Day 2). However, she remained a fever with a cold, with a maximum body temperature of 38.0°C. With active treatment, the patient's body temperature gradually returned to normal (Day 3).
Evaluations to determine the microbiological cause of fever included blood culture and mNGS. The detailed methods were shown in the Technical Appendices. The mNGS achieved 20,849,484 reads; after quality control processing, we obtained 19,247,085 clean reads for the downstream analysis. Nine reads of E. piscisicarius were detected in the peripheral blood by mNGS (Day 4). In contrast, the blood culture result showed an infection of E. rhusiopathiae, verified by the Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry subsequently (Figure S1). Due to this inconsistency, the cultured microorganisms were sent for WGS. The acquired sequence matched the E. piscisicarius whole genome at a coverage rate of 91.2% (A), confirming the mNGS result. Just then, a red and swollen mass (1 × 0.5 cm) at the fingertip of the patient's left thumb was noted (B). The patient complained that the finger had been punctured when washing shrimps, which was later identified as Penaeus vannamei, three days before the onset of the disease. She denied the contact history with other seafood, poultry, or livestock. Thus, the patient was diagnosed with erysipeloid based on the clinical manifestation and laboratory findings. Moreover, the antimicrobial susceptibility test suggested that the isolated bacteria are susceptible to imipenem, ciprofloxacin, and piperacillin-tazobactam (Figure S2). Concerning the treatment against E. rhusiopathiae infection, piperacillin-tazobactam remained administered for anti-infection. Finally, the patient was discharged from the hospital after her blood culture testing and mNGS were both negative (Day 11). The timeline of the clinical course of the patient was shown in C.
Figure 1. The diagnosis of E. piscisicarius infection and the genome analysis. (A) The whole genome sequencing yielded a total genome coverage of 91.2%. (B) Red and swollen mass on the patient's left thumb fingertip with a size of about 1 × 0.5 cm. (C) The timeline of the clinical course of the patient. (D) ANI heatmap showed that DNK211006EB014 shared an ANI greater than 0.95 with E. piscisicarius_15TAL0474. (E) E. piscisicarius is identified by phylogenetic analysis. (F) Circular map of the genome.
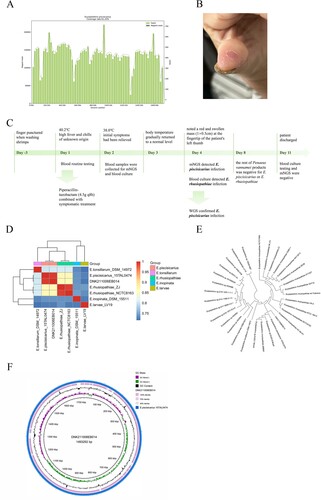
To further understand the pathogenicity and virulence of the microorganism (DNK211006EB014) isolated from the patient, genome analysis was conducted. The genome sequence has been deposited in NCBI Sequence Read Archive (SRR19882239, SRR19882240, and SRR19882241). As shown in D, DNK211006EB014 shared an average nucleotide identity (ANI) value of 99.41% with E. piscisicarius 15TAL0474. The phylogenetic tree further identified the acquired sequence was E. piscisicarius (E). The assembled genome (F) was 1,493,292 bp in length, with a GC content of 37.52%. It contains 1418 genes. 147 virulence-associated genes (VAGs) were detected by comparing in the virulence factors database (VFDB). The prediction revealed that these VAGs encoding the proteins participated in several functional classes, including immune modulation (43), nutritional/metabolic factor (29), adherence (16), exotoxin (11), regulation (11), stress survival (10), effector delivery system (9), biofilm (6), exoenzyme (5), as shown in Table S2. These products are involved in the biosynthesis of capsular polysaccharides (cps4A, cps4H, cap8E, cap8F, cap8G), and surface protein (pspA), closely associated with virulence. All these VAGs were also presented in E. piscisicarius 15TAL0474.
Discussion
We report a human case with E. piscisicarius infection through mNGS identification and WGS confirmation. To the best of our knowledge, this is the first case of an E. piscisicarius infection that causes severe symptoms in humans.
The patient was punctured in the finger when dealing with Penaeus vannamei three days before the onset of the disease, which might be the most probable means of E. piscisicarius infection. However, all the specimens of the rest of Penaeus vannamei products that remained in the patient's house were negative for E. piscisicarius or E. rhusiopathiae. It might be interpreted that only a part of the shrimp was infected with the E. piscisicarius and the pathogen infected the patient due to the wound caused by these shrimps, which had already been cooked and consumed. For illnesses caused by E. rhusiopathiae infection, appropriate antibiotics, adequate debridement, and surgical drainage are the recommended therapies [Citation4]. In this case, we performed the antimicrobial susceptibility test of the isolated bacteria referring to the Clinical and Laboratory Standards Institute (CLSI) criteria for Erysipelothrix rhusiopathiae, which indicated that the bacteria are susceptible to piperacillin-tazobactam. Piperacillin is one of the most active agents against E. rhusiopathiae infection [Citation5]. Our clinical experience proved that the therapeutic regimen against E. piscisicarius is similar to that against E. rhusiopathiae.
Culture and culture-based methods have been used to identify clinical pathogens for a long time, however it remains barriers when identifying novel pathogens. In our case, differences arose between the identification results from culture and mass spectrometry and mNGS, considering that the criteria for identification of E. piscisicarius through culture are not well established. Compared with the low positive rate and low accuracy of microbiology laboratory cultures [Citation6,Citation7], mNGS contributes to quickly and precisely detect E. piscisicarius, suggesting its advantage over the traditional culture in identifying novel pathogens.
It has been reported that capsular polysaccharides [Citation8] and cell surface proteins [Citation9] play essential roles in the virulence of E. rhusiopathiae. In our case, 147 VAGs were detected, some of which putatively participated in the biosynthesis of capsular polysaccharide and cell surface protein in E. piscisicarius, contributing to its virulence. Considering the scarce information on E. piscisicarius in the database, it is suggested to further investigate the pathogenic virulence and antimicrobial resistance.
In conclusion, E. piscisicarius can cause human infections and diseases and aquatic animal infections. Once cutaneous lesions are formed after indecent exposure to marine products, it must be paid attention to and treated adequately, and be vigilant to emerging pathogens like E. piscisicarius. Compared with conventional methods, mNGS exhibits better detection performance, which is beneficial to clinical practice.
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Declaration of interest statement
The authors report there are no competing interests to declare.
Supplemental Material
Download Zip (2.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pomaranski EK, Soto E. The formation, persistence, and resistance to disinfectant of theerysipelothrix piscisicariusbiofilm. J Aquat Anim Health. 2020;32(1):44–49. doi:10.1002/aah.10097 PubMed PMID: 31991024.
- Chang EK, Camus AC, Pomaranski E, et al. Pathogenesis of Erysipelothrix piscisicariusinfection in tiger barbs (ntigrus tetrazona). J Fish Dis. 2021;44(11):1681–1688. doi:10.1111/jfd.13485. PubMed PMID: 34251051.
- Pomaranski EK, Griffin MJ, Camus AC, et al. Description of Erysipelothrix piscisicarius sp. nov., an emergent fish pathogen, and assessment of virulence using a tiger barb (Puntigrus tetrazona) infection model. Int J Syst Evol Microbiol. 2020;70(2):857–867. doi:10.1099/ijsem.0.003838. PubMed PMID: 31682217.
- Hofseth K, Dalen H, Kibsgaard L, et al. Infectious tenosynovitis with bloodstream infection caused by erysipelothrix rhusiopathiae, a case report on an occupational pathogen. BMC Infect Dis. 2017;17(1):12, doi:10.1186/s12879-016-2102-1. PubMed PMID: 28056818; PubMed Central PMCID: PMCPMC5217415.
- Venditti M, Gelfusa V, Tarasi A, et al. Antimicrobial susceptibilities of Erysipelothrix rhusiopathiae. Antimicrob Agents Chemother. 1990;34(10):2038–2040. doi:10.1128/AAC.34.10.2038. PubMed PMID: 2291674; PubMed Central PMCID: PMCPMC171989.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign. Crit Care Med. 2013;41(2):580–637. doi:10.1097/CCM.0b013e31827e83af. PubMed PMID: 23353941.
- Fenollar F, Raoult D. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int J Antimicrob Agents. 2007 Nov;30(Suppl 1):S7–15. doi:10.1016/j.ijantimicag.2007.06.024 PubMed PMID: 17707613.
- Shimoji Y, Yokomizo Y, Sekizaki T, et al. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Microb Inf. 1994;62(7):2806–2810. doi:10.1128/iai.62.7.2806-2810.1994.
- Shimoji Y. Pathogenicity of erysipelothrix rhusiopathiae: virulence factors and protective immunity. Microbes Infect. 2000;2(8):965–972. doi:10.1016/S1286-4579(00)00397-X.
