ABSTRACT
Intestinal epithelial cell interactions with enteric pathogens have been incompletely elucidated owing to the lack of model systems that recapitulate the cellular diversity, architecture and functionality of the intestine. To analyze rotavirus (RV) infection and the subsequent innate immune response, we established cultures of differentiated porcine intestinal epithelial cells in three different variations: basolateral-out enteroids, apical-out enteroids and two-dimensional (2D) filter-grown intestinal epithelial cells. Application of specific antibodies for fluorescent staining indicated that enteroids and enteroid-derived cell cultures contain multiple intestinal epithelial cell types. Infection studies indicated that both apical-out enteroids and 2D intestinal epithelial cells are susceptible to porcine RV infection. However, 2D intestinal epithelial cells are more useful for a detailed characterization and comparison of apical and basolateral infection than apical-out enteroids. Virus-induced apoptosis was observed in apical-out enteroids at 24 h post infection but not at earlier time points after infection. RV infected not only enterocytes but also goblet cells and Paneth cells in apical-out enteroids and 2D intestinal epithelial cells. Interestingly, despite the lack of significant differences in the efficiency of infection after apical and basolateral infection of 2D intestinal epithelial cells, stronger innate immune and inflammatory responses were observed after basolateral infection as compared to infection via the apical route. Therefore, apical-out enteroids and 2D intestinal epithelial cells provide useful primary cell culture models that can be extended to analyze invasion and replication strategies of agents implicated in enteric diseases or to study immune and inflammatory responses of the host induced by enteric pathogens.
Introduction
Rotaviruses (RVs) are a major cause of viral diarrhoea in children or young animals. They are classified into the genus Rotavirus within the family Reoviridae [Citation1]. RVs are 70–75 nm in diameter, triple-layered, and non-enveloped. The genome consists of eleven segments of double-stranded (ds) RNA encoding six structural proteins (VP1–VP4, VP6, and VP7) and six non-structural proteins (NSP1–NSP6) [Citation2]. According to the serological reactivity and genetic variability of VP6, at least ten groups (A – J) have been differentiated [Citation3,Citation4]. RVA, RVB, RVC, RVE, and RVH have been detected in pigs [Citation5–9]. RVA, the most common cause of infections among the five groups, are further classified into different genotypes based on the outer capsid layer protein VP7 (G for glycoprotein) and VP4 (P for protease-cleaved protein), which form the basis of the dual nomenclature system. The porcine rotavirus A strain 4555 used in this study was isolated from a piglet with diarrhoea and was passaged less than ten times to limit the risk of viral adaption to MA104 cells.
RVs are transmitted predominantly through the fecal-oral route; the airborne droplets route has been hypothesized but not proven [Citation10]. Many studies of RVs have been conducted in MA104, IPEC-J2, and Caco-2, since these immortalized cell lines are permissive for viral replication [Citation11–14]. However, these transformed or cancer-derived cell cultures do not differentiate into specialized cell types and often show poor polarity [Citation15]. In vivo, intestinal epithelial cells are highly polarized with a specialized apical side facing the lumen of the gut with its microbiota and a basolateral side facing the subepithelial tissue [Citation16]. Normally, pathogens transmitted through the fecal-oral route attack the apical side, however, the basolateral side can also be attacked when the epithelial barrier is breached [Citation16]. RV infection of cell lines cannot recapitulate numerous aspects of RV infection such as polarized viral entry, cell tropism, mechanisms of epithelial barrier function as well as polarized innate immune response. Therefore, it is necessary to establish culture systems for differentiated intestinal epithelial cells.
An organoid is a three-dimensional construct composed of multiple cell types by means of self-organization and is capable of simulating the architecture and functionality of native organs [Citation17–19]. Enteroids (organoids derived from the small intestine), or “Miniguts” [Citation20], have been applied to model interactions between enteric pathogens and intestinal epithelial cells since enteroids comprise stem cells, proliferating cells and differentiated epithelial cells, including enterocytes, enteroendocrine cells, goblet cells and Paneth cells [Citation21] as well as the M cells of Peyer's patches [Citation20]; therefore they can model viral infections in more detail than immortalized cell lines. Organoids are embedded in Matrigel or basement membrane extract (BME) which are comprised of extracellular matrix (ECM) proteins that help to maintain epithelial polarity with basolateral epithelial surfaces facing outwards and apical surfaces facing the lumen of the spherical organoid [Citation21]. In order to deliver pathogens to organoid cultures, there are four methods available which include (a) microinjection, (b) mechanical disruption of organoids, (c) apical-out organoids, and (d) 2D monolayer cultures of differentiated intestinal epithelial cells grown on filter supports [Citation22].
Enteroids have been used to study gastrointestinal bacteria such as Salmonella [Citation23], Listeria monocytogenes [Citation15], Escherichia coli [Citation24], Clostridioides difficile [Citation25], parasites such as Heligmosomoides polygyrus [Citation26], and Cryptosporidium [Citation27] as well as viral pathogens. Studies involving viral pathogens such as rotaviruses [Citation28,Citation29], reovirus [Citation30], norovirus [Citation31,Citation32], adenovirus [Citation33], influenza virus [Citation34], and coronavirus [Citation35–38] have revealed that infections of organoids recapitulate many characteristic features of host–pathogen interactions. In this study, we developed porcine basolateral-out enteroids, apical-out enteroids, and 2D differentiated intestinal epithelial cells. Among these three cultures, apical-out enteroids and 2D differentiated intestinal epithelial cells are more susceptible to porcine RV and allowed to determine the cell tropism and polarized antiviral response after porcine RV infection and other mechanisms of RV infection such as barrier function and enterocyte regeneration.
Material and methods
Porcine basolateral-out enteroid culture
Porcine enteroids derived from crypts were generated as described [Citation21,Citation36]. Porcine jejunum was obtained from four clinically healthy pigs, sacrificed for scientific interest. Euthanasia of animals for scientific purposes was approved by the local authorities (reference number TiHo-T-2021-8). Small pieces of porcine jejunum were gently scraped to remove mucus and villi from the intestine wall. Then they were rinsed with cold PBS for at least three times. Afterwards, the jejunum samples were incubated with 5 mM EDTA chelating buffer for 30 min on ice. Crypts were isolated by scraping and collected into a falcon tube with cold PBS. Finally, the cells were suspended with enteroids growth medium (Advanced Dulbecco’s modified Eagle medium/F12 (DMEM/F12), 10 mM HEPES, 100U/mL Penicillin, 1× B27, 1 mM N-Acetyl-cysteine, and the following growth factors: 10 nM Gastrin I, 50 ng/mL Epidermal Growth Factor (EGF), 10 mM Nicotinamide, 500 nM A83-01, 10 µM SB202190, and 50% L-WRN (ATCC® CRL3276TM)-conditioned media (contains Wnt3A, R-spondin, and noggin)) and Matrigel at a ratio of 1:1. To culture enteroids, enteroid growth medium was changed every two days. For the first 3–4 days of cultivation, 10 µM Y27632 was also included in the medium.
Porcine apical-out enteroid culture
After embedding in Matrigel for 7–10 days, porcine enteroids were collected and incubated in 5 mM EDTA in PBS for 1 h at 4°C on a rotating platform. After this step, the samples were centrifuged at 400 g for 5 min and the supernatant was removed. The pellet was resuspended in enteroid growth medium on ultra-low attachment 24-well tissue culture plates (Corning); 10 µM Y27632 were also included in the media for the cultivation. The EDTA was used to disrupt the polymerization of the extracellular matrix (ECM) protein laminin [Citation15]. ECM is a main component of Matrigel and it has been reported that ECM proteins can regulate the polarity of epithelial cells [Citation39].
2D differentiated intestinal epithelial cell culture derived from enteroids
After culture in Matrigel for a week, porcine enteroids were collected into cold PBS and incubated on ice for at least 1 h. After centrifugation at 400 g for 5 min, the supernatant containing the Matrigel was removed. The pellet was trypsinized by TrypLETM Express Enzyme (GibcoTM) for 10 min in a 37°C water bath. Enteroids were dissociated to single cells and resuspended in monolayer medium (DMEM/F12, 20% FBS, 50% L-WRN – conditioned media, 50 ng/mL EGF). 3–4× 105 cells were seeded per transwell filter and cultured with monolayer medium. After four days of incubation, the medium was replaced by differentiation medium (DMEM/F12, 5% L-WRN-conditioned media, 20% FBS, 50 ng/mL EGF, 5 μM DAPT.) and the cells were incubated for another four days.
Measurement of transepithelial resistance
Transepithelial electrical resistance (TEER) of 2D cell culture monolayers was measured using the Millicell® ERS-2 (Electrical Resistance System) device. The electrodes were disinfected with 70% ethanol and rinsed with PBS. After one washing procedure with warm PBS, 200 and 500 μL PBS were added to the apical and basolateral compartments, respectively. For each well, TEER was measured three times. To monitor the TEER of 2D intestinal epithelial cells, the respective values were determined every two days before and after differentiation.
Haematoxylin and eosin (H&E) and immunohistochemical (IHC) staining
2D monolayers were fixed in 10% neutral-buffered formalin, as described previously. After embedding in paraffin, the samples were sectioned at 2 μm thickness and prepared for haematoxylin and eosin (H&E) and IHC staining using a standard protocol [Citation40,Citation41]. To localize the distribution of enterocyte in 2D differentiated intestinal epithelia cells, antibody specific for Villin was used.
Immunofluorescence microscopy
Cells were washed with PBS and fixed in 3% paraformaldehyde for 30 min at room temperature, then washed three times with PBS. Cells were permeabilized with 0.2% TritonX-100 for 30 min at 37°C and blocked in PBS with 5% normal goat serum (or normal donkey serum from Abcam). Primary antibodies were applied overnight at 4°C. After three washes with PBS-Tween 20, cells were incubated with secondary antibodies for 1 h at 37°C. Antibodies used in this study are listed in . For staining of cell markers, different sets of enteroids were used because several antibodies against the different cell markers derived from mouse and several other antibodies derived from rabbit (); therefore, it was not possible to use the same set of cells (enteroids) for immunofluorescence staining of three or four markers. After three washes with PBS-Tween 20, cells were stained with DAPI for 10 min at room temperature. Cells were mounted in ProLongTM Gold Antifade Mountant (InvitrogenTM), and images were collected using a 20× or 100× oil immersion objective of a Nikon Eclipse Ti-S microscope (Nikon).
Figure 1. Characterization of the cellular composition of undifferentiated basolateral-out porcine enteroids and apical-out enteroids. (A) Bright field photographs of porcine enteroids after different times of development (4–13 days). (B) Bright field photographs of Matrigel-embedded basolateral-out enteroids (left) and suspended apical-out enteroids (right); Scale bars: 100 µm. The symbols ★, ● and ▾ indicate enteroids which were considered as apical-out enteroids, enteroids with mixed polarity and basolateral-out enteroids, respectively. (C) At day 0 and 3, basolateral-out enteroids, apical-out enteroids and enteroids with mixed polarity in suspension culture were quantified. (D) Basolateral-out enteroids and apical-out enteroids were analyzed by immunofluorescence microscopy for apical tight junction protein (ZO-1, green) and for the presence of proliferative cells (KI-67, red), enterocytes (Villin, red), enteroendocrine cells (CHGA, green), goblet cells (MUC2, red), and Paneth cells (LYS, red). Nuclei were stained with DAPI (blue). Scale bars: 50 µm.
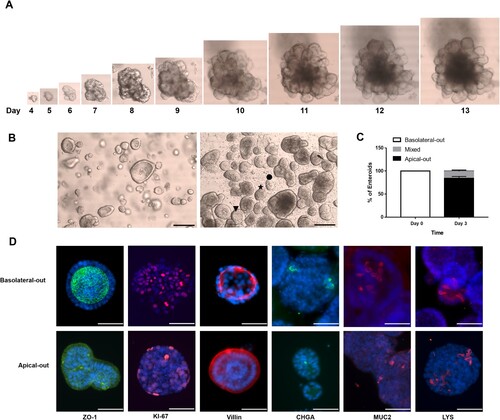
Table 1. Antibodies used for immunofluorescence analysis.
RV infection of porcine enteroids and 2D differentiated intestinal epithelial cells
Porcine RVA strain 4555 was kindly provided by Dr. Peter Otto (Friedrich-Loeffler-Institute, Federal Research Institute for Animal Health, Jena, Germany). MA104 cells were used for propagation and titration of RV. Basolateral-out enteroids were collected into cold PBS and incubated on ice for 1 h. After centrifugation at 400 g for 5 min, the supernatant containing the Matrigel was removed, and pellets were washed two times with cold PBS. The basolateral-out enteroids in the pellet fraction were resuspended in DMEM/F12 medium containing the virus (with 10 µg/ml trypsin) and incubated at 37°C for 3 h. To remove unbound virus, basolateral-out enteroids were centrifuged and pellets were washed with DMEM/F12 medium three times. Finally, the pellets were resuspended in enteroid growth medium and Matrigel at a ratio of 1:1, and the enteroids were seeded on 24-well tissue culture plates. For infection of apical-out enteroids, suspended apical-out enteroids were cultured on ultra-low attachment 24-well tissue culture plates for three days prior to infection. After centrifugation at 400 g for 3 min, the supernatant was removed, and the pellets were washed three times with PBS. The apical-out enteroids in the pellet fraction were resuspended in DMEM/F12 medium containing the virus (with 10 µg/ml trypsin) at the indicated MOI, and incubated at 37°C for 3 h. Apical-out enteroids were centrifuged to remove unbound virus as described above and washed with DMEM/F12 medium three times. The final pellet fraction was resuspended in maintenance medium and seeded on ultra-low attachment 24-well tissue culture plates.
For 2D monolayer cultures, cells were differentiated for four days prior to infection and filters were washed three times with warm PBS; the virus inoculum (with 10 µg/ml trypsin) was applied from either the apical or the basolateral compartment at 37°C for 3 h. Filters were washed at least three times to remove unbound virus and maintenance medium was used for the whole infection period. All infection experiments were repeated three times.
RNA extraction and RT-qPCR
RNA of Matrigel-embedded or suspended enteroids (containing only 1–3 × 105 cells/dome) was extracted by using the RNeasy Plus Micro Kit (QIAGEN) which is particularly suited for very small sample amounts. For RNA extraction from 2D monolayers (containing at least 5 × 105 intestinal epithelial cells) the RNeasy Mini Kit (QIAGEN) was used. The transcription levels were analyzed on Bio-Rad CX96 with QuantiTect SYBR Green RT-PCR Kit (Qiagen). GAPDH (GAPDH, Glycerinaldehyd-3-phosphat-Dehydrogenase) was used as a reference gene in all SYBR Green RT-qPCR analyzes. To assess differentiation of 2D monolayer cultures, the transcription level at 2 days post differentiation was set to 1, and for the other time points relative transcription levels were calculated.
For infection experiments, data from apical infection were compared with data from basolateral infection. The RV RNA genome copy numbers were determined by Taqman RT-qPCR using the QuantiTect Probe RT-PCR Kit (QIAGEN). For analysis of the course of RV infection on porcine enteroids and 2D differentiated intestinal epithelial cells, the RV RNA genome copy numbers determined at 3.5 h.p.i. (comprising 3 h of virus inoculation and 0.5 h of virus incubation) are considered as input values. Primers and probes used in this study are listed in [Citation36,Citation38,Citation42,Citation43]. Further details of the individual RT-PCR protocols can be obtained upon request.
Table 2. Primers and probes used for RT-qPCR.
Statistical analyzes
All infection experiments were conducted at least three times. For comparison of two groups (apical infection and basolateral infection), unpaired Student’s t-test (and nonparametric tests) was applied in GraphPad Prism. Results are shown as means with standard deviations. A p-value < 0.05 was considered statistically significant: *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Establishment of porcine basolateral-out enteroids and apical-out enteroids
To analyze the infection by porcine RV in the target tissue of their host, we established porcine enteroids from crypts [Citation36]. Crypts were isolated from the jejunum of a porcine intestine, embedded in Matrigel and cultured with enteroid growth medium. As shown in (A), at day 4 after seeding, enteroids started to grow larger and adopted a complex structure with a central lumen and buds protruding from the surface, and significantly increased in size until day 13. Enteroids were propagated to a large cell number (around 8 × 106–1 × 107 cells/ 24 well plate) and cryopreserved to be used for later experiments.
Enteric pathogens preferentially infect epithelial cells via the apical surface, which is facing the lumen enclosed by the cells of an enteroid. Therefore, it is challenging to apply the virus to the respective membrane domain. To facilitate viral infections, apical-out enteroids were established as previously described [Citation15,Citation36] and cultured in enteroid growth medium. After 3 days of suspension culture in growth media using low-attachment plates, a change of the morphology of enteroids was observed resulting in the formation of apical-out enteroids lacking a central lumen, and edges of columnar epithelial cells were visible ((B)). As shown in (B) (right), enteroids with ★, ▾, and ● were considered as apical-out enteroids, basolateral-out enteroids, and enteroids with mixed polarity, respectively. After 3 days in suspension, around 84% of enteroids showed apical-out orientation, while around 15% of enteroids exhibited a mixed polarity ((C)). Immunofluorescence staining revealed that zonula occludens-1 (ZO-1) protein, which is an apical protein, is expressed at the inner surface of basolateral-out enteroids ((D) upper panel) and at the outer surface of apical-out enteroids ((D) lower panel), respectively. Villin is expressed at the inner surface of basolateral-out enteroids and at the outer surface of apical-out enteroids ((D)). Immunofluorescence staining of Marker of Proliferation Ki-67, Chromogranin A (CHGA), Mucin 2 (MUC2) and Lysozyme (LYS) revealed that proliferative cells, enteroendocrine cells, goblet cells and Paneth cells are present in both basolateral-out and apical-out enteroids, respectively ((D)).
Apical-out enteroids are susceptible to infection by porcine RV
In order to investigate the susceptibility of porcine enteroids to infection by RV, basolateral out enteroids and apical-out enteroids were inoculated with RV (MOI = 0.1). At different time points after infection, cells were analyzed by immunofluorescence microscopy as well as by RT-qPCR. RV antigen (red) was detected in either basolateral-out enteroids or apical-out enteroids at 24 and 48 h post infection (h.p.i.); ZO-1 antigen (green) was used to confirm the polarity of enteroids ((A), upper panel: basolateral-out enteroids, lower panel: apical-out enteroids). RT-qPCR analysis at different time points p.i. revealed that the number of RV genomes produced from apical-out entroids increased during the infection period and peaked at 48 h.p.i, while no increase of viral RNA genomes was detected after infection of basolateral-out enteroids ((B)). Taken together, RV antigen was detected after infection of both basolateral-out and apical-out enteroids, while efficient viral RNA replication was detected only in apical-out enteroids.
Figure 2. Apical-out enteroids are susceptible to infection by porcine rotavirus (RV). Basolateral-out and apical-out enteroids were infected with porcine RV. (A) At 24 h post infection (h.p.i.) and 48 h.p.i., infected enteroids and mock enteroids were subjected to immunostaining of rotavirus antigen (red); tight junctions were stained by ZO-1 specific antibody (green), and the nuclei were stained with DAPI (blue). RV positive cells have been pointed by white arrows. (B) The course of infection was analyzed by RT-qPCR to determine the number of viral RNA copies (encoding RV non-structural protein 3, NSP3) produced per well at the indicated time points post infection in basolateral-out and apical-out enteroids. The experiments were conducted three independent times. (C) Assessment of enterocyte infection. RV antigen-containing cells (left, red), Villin-containing cells (middle, green), and merged images (right) are shown. (D) Assessment of goblet cells infection. RV antigen-containing cells (left, red), MUC2-containing cells (middle, green), and merged images (right) are shown. (E) Assessment of Paneth cells infection. RV antigen-containing cells (left, red), Lysozyme-containing cells (middle, green), and merged images (right) are shown. (F) Apoptosis induced by rotavirus was visualized by immunostaining of RV antigen (left, red) and cleaved caspase-3 (middle, green); merged images are shown on the right. (C–F) Mock infected (upper panel) and RV infected (lower panel) organoids are shown. Merged images: nuclei were stained by DAPI (blue); scale bars: 50 µm.
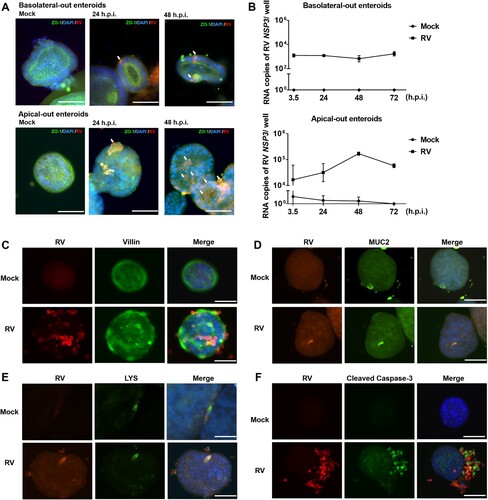
Previous in vivo studies have shown that mature enterocytes of the intestinal epithelium are a major target of RV [Citation44,Citation45]. As shown in (C), some enterocytes were infected by RV, while RV antigen was also detected in cells not stained by the Villin-specific antibody, suggesting that other cell types are susceptible to RV infection. Immunofluorescence co-staining of RV antigen in cells expressing MUC2 or LYS indicated that both goblet cells and Paneth cells were infected by RV as well ((D and E), respectively).
To analyze the detrimental effect of RV infection (MOI = 0.1) on apical-out enteroids, immunofluorescence staining was performed to detect apoptotic signals during the early and late stages of RV infection. Co-staining with RV and cleaved caspase-3 indicates that RV induced apoptosis at 24 h.p.i. ((F)) but not at 7 h.p.i. (data not shown).
2D monolayers of enteroid-derived cells recapitulate the differentiated intestinal epithelium
In vivo, polarized intestinal epithelial cells display a unique apical membrane facing the lumen of the gut and a basolateral membrane facing the lamina propria [Citation30]. Enteric pathogens usually challenge intestinal epithelial cells through apical surface. However, loss of epithelial barrier function can result in the access of invasive pathogens to the basolateral side of intestinal epithelial cells [Citation30]. To apply infection to apical and basolateral membranes, 3D enteroids (basolateral-out and apical-out enteroids) were used as a first attempt. However, 3D enteroids do not represent suitable models to study polarized infection and polarized immune response, due to the following limitations and reasons: (i) while basolateral-out enteroids are susceptible to RV, efficient viral RNA synthesis was not detectable ((A and B)); (ii) infection of basolateral-out enteroids via the apical side would require mechanical disruption of the enteroids prior to incubation with virus; however, such manipulations would not allow a clear separation of infections from either the basolateral side or, alternatively, from the apical side; (iii) while the majority of enteroids in suspension represent apical-out enteroids, there are still about 15% of enteroids lacking a clear apical-out polarity and instead exhibit a mixed polarity ((C)). To make both the apical and basolateral surfaces of intestinal epithelial cells accessible to experimental manipulation, we established 2D monolayers of differentiated intestinal epithelial cells. The expression of different cell markers was determined to monitor the process of differentiation. Enteroids were dissociated to single cells and the obtained cell suspensions were seeded on pre-coated transwell filters. After four days, intestinal epithelial cells were completely confluent and the TEER reached values of about 500 Ω×cm2. After differentiation, the TEER increased until day 4 post differentiation ((A)). At later time points, the TEER started to decrease. TEER is the measurement of electrical resistance across a cellular monolayer, which is a multifactorial phenomenon and not strictly related to a specific marker protein. The barrier function of the monolayer was also evident from the network of tight junctions visualized by staining of ZO-1 ((B)).
Figure 3. Characterization of the cellular composition of 2D monolayers of differentiated porcine intestinal epithelial cells. (A) The barrier function of the polarized epithelial cells was assessed by measuring the transepithelial electric resistance (TEER) at the indicated time points post differentiation. The TEER measurement was conducted three independent times. (B) Tight junctions were stained with an antibody directed against ZO-1. Scale bar: 10 µm. Inserted small image: nuclei were stained by DAPI (blue). (C) H&E staining (upper panel) and immunohistochemistry analysis with an antibody against Villin (lower panel) of a vertical section of 2D polarized porcine intestinal epithelial cells after four days of culture in monolayer medium followed by four days in differentiation medium. Scale bars: 20 µm. (D) The transcriptional levels of cell markers in differentiated intestinal epithelial cells were determined via RT-qPCR at the indicated time points. The transcription level at 2 days post differentiation was set to 1 and relative transcription levels are shown. LGR5 represents stem cells, SGLT1 represents enterocytes, CHGA represents enteroendocrine cells and MUC2 represents goblet cells. All data were calculated using the 2−ΔΔCT method. The experiments were conducted two independent times. (E) Assessment of 2D monolayers for the presence of cells that are characteristic for the intestinal epithelium (from left to right): enterocytes (staining of Villin, red), enteroendocrine cells (staining of CHGA, green), goblet cells (staining of MUC2, red) and Paneth cells (staining of LYS, green). Nuclei were stained by DAPI (blue). Scale bars: 100 µm.
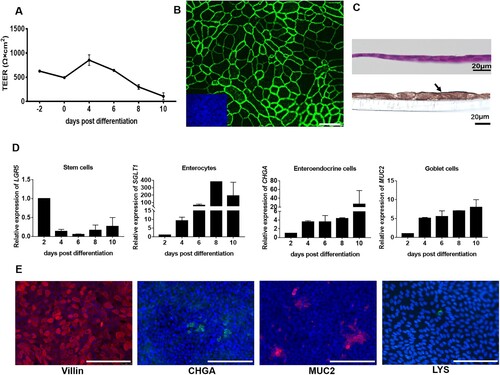
Several cell types that are important for the function of the intestinal epithelium are generated from stem cells undergoing a differentiation process. To include such cells in the intestinal epithelium, the cell monolayers were incubated with differentiation medium. To monitor the appearance of differentiated cells, we applied RT-qPCR to detect the expression of cell markers at the transcription level. 2D intestinal epithelial cells were collected at different days post differentiation and RT-qPCR was applied. The results show that the transcriptional expression of stem cell marker LGR5 decreased after differentiation. In contrast, the transcriptional expression of cell markers for enterocytes (SGLT1), enteroendocrine cells (CHGA), and goblet cells (MUC2) significantly increased after differentiation ((D)). Based on the results of the TEER measurement and the transcription level of cell markers, the conditions for experiments with differentiated intestinal epithelial cells were set as follows: intestinal epithelial cells were cultured in monolayer growth medium for four days and then cultured in differentiation medium for another four days. (C) shows a vertical section of 2D differentiated intestinal epithelial cells; H&E staining ((C) upper panel) shows the thin monolayer structure. The presence of mature enterocytes, the main cell type of the intestinal epithelium, is demonstrated by immunofluorescence staining of Villin ((C), lower panel). The characteristic cell types of an intestinal epithelium are also present in the 2D differentiated intestinal epithelial cells: enterocytes, enteroendocrine cells, goblet cells, and Paneth cells visualized by staining of Villin, CHGA, MUC2, and LYS, respectively ((E)). Taken together, we have established 2D differentiated intestinal epithelial cells and subsequent analyses showed that day 4 post differentiation is a suitable time point to initiate infection studies.
2D monolayers of differentiated intestinal epithelial cells are susceptible to infection by porcine RV
RV infection of 2D monolayers of differentiated porcine intestinal epithelial cells was analyzed by applying the virus at an MOI of 0.5 to either the apical or the basolateral side of the cells. Intestinal epithelial cells were fixed at 24 h.p.i. and analyzed by immunofluorescence microscopy. RV was able to infect the 2D differentiated intestinal epithelial cells from the apical as well as from the basolateral side ((A)). To obtain quantitative data about the viral replication, the amount of viral RNA was determined by RT-qPCR. Results illustrate that the amount of RV genomes increased with the incubation time until it reached a peak at 24 h.p.i; the course of the infection was the same irrespective of the side of virus application ((B)). In contrast, after infection of apical-out enteroids, the number of RV genomes peaked at 48 h.p.i ((B)). It can be speculated that in addition to the different MOIs used for the infections of 3D enteroids (MOI = 0.1; (B)) and 2D cultures (MOI = 0.5; (B)), floating of the apical-out enteroids in medium may result in a delay of virus spread due to limited contact, while cells of 2D cultures are directly connected with each other and support efficient spread.
Figure 4. Porcine rotavirus (RV) infection of 2D monolayers of differentiated intestinal epithelial cells. 2D intestinal epithelial cells were infected with porcine RV from either the apical or the basolateral surface. (A) At 24 h.p.i., mock monolayers and infected monolayers were subjected to immunostaining of rotavirus antigen (red) and staining of nuclei by DAPI (blue). Scale bars: 100 µm. (B) The courses of apical and basolateral RV infections were analyzed by RT-qPCR to determine the amount of viral RNA accumulated at the indicated time points. The experiments were conducted three independent times. (C) Infection of enterocytes was assessed by visualizing rotavirus antigen-containing cells (left, red) and Villin-containing cells (middle, green); a merged image is shown on the right. (D) Infection of goblet cells was assessed by visualizing rotavirus antigen-containing cells (left, red) and MUC2-containing cells (middle, green); a merged image is shown on the right. (E) Infection of Paneth cells was assessed by visualizing rotavirus antigen-containing cells (left, red) and Lysozyme-containing cells (middle, green); a merged image is shown on the right. (C–E) Nuclei were stained by DAPI (blue). Scale bars: 100 µm.
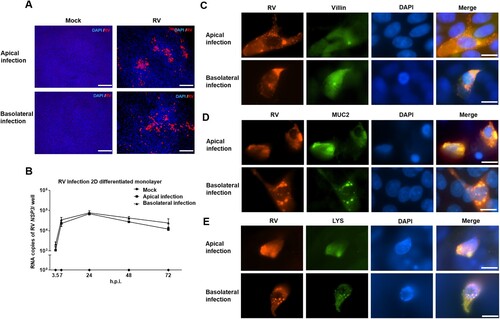
In order to get information about the cell tropism of RV, 2D intestinal epithelial cells were inoculated with RV via the apical or via the basolateral plasma membrane domain. Since RV infection results in cytopathic effects, low MOI (MOI of 0.1) was used in the experiments performed for detection of cell tropism to avoid lysis of cells. By using apical-out enteroids, we found that in addition to enterocytes, goblet cells and Paneth cells are also susceptible to infection by RV ((C–E)). Co-staining of RV antigen and cell markers in 2D monolayers of differentiated intestinal epithelial cells confirmed that Villin-positive enterocytes, MUC2-positive goblet cells and LYS-positive Paneth cells were infected by RV ((C–E)).
Innate immune response after infection of porcine intestinal epithelial cells by RV
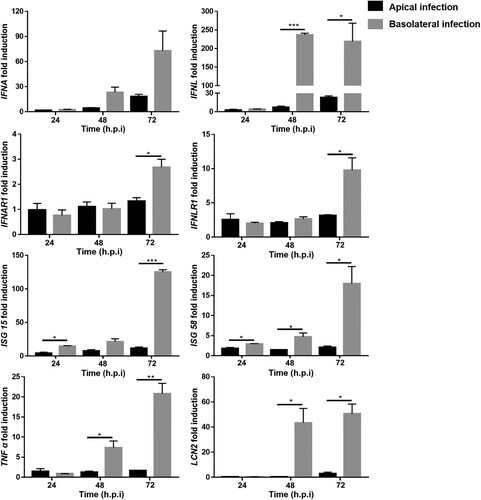
The susceptibility of 2D monolayers to RV infection via both the apical and basolateral side provides an opportunity to investigate antiviral defense and host intrinsic immune responses in differentiated intestinal epithelial cells. The roles of type I and type III IFNs (IFN, interferon) in RV infections have been a controversial issue for many years without a widely accepted conclusion. For human RV, a significant increase of type III IFNs, but not of type I IFNs was reported after infection of human enteroids [Citation46]. However, up-regulation of both type I and type III IFNs upon RV infection was observed in primary murine intestinal cells [Citation47]. In order to evaluate which types of IFNs are induced by porcine RV in porcine enteroids, porcine RV was applied at an MOI of 1 at either the apical or the basolateral surface of intestinal epithelial cells. Samples were collected at 24, 48, and 72 h.p.i. and RT-qPCR was applied to detect the transcriptional expression of IFNs, IFN receptors, interferon-stimulated genes (ISGs) and inflammatory factors. As shown in , type I and type III IFNs (IFNA, IFNL), interferon receptors (IFNAR1, IFNLR1) and interferon-stimulated genes (ISG15 and ISG58) were increased upon RV infection. Interestingly, RT-qPCR results showed a significant higher upregulation of interferon-related genes following basolateral infection, demonstrating a stronger interferon-mediated immune response after basolateral infection. Moreover, the transcription levels of diarrhoea-related inflammatory marker lipocalin-2 (LCN2) and pro-inflammatory cytokine tumour necrosis factor alpha (TNF α) were determined following apical and basolateral infection with RV. Similar to the IFN-mediated innate immune response, the induction of inflammatory response related genes was significantly stronger after basolateral infection of differentiated porcine intestinal cells by RV. Taken together, the results of our study show that type I IFN, type III IFN-ISG signalling and inflammatory pathways are induced in RV-infected 2D porcine differentiated intestinal epithelial cells and that basolateral infection induces a significantly stronger interferon-mediated immune response and inflammatory response in comparison with apical infection.
Figure 5. Porcine intestinal epithelial cells respond to rotavirus (RV) infection. 2D monolayers of differentiated porcine intestinal epithelial cells were infected with porcine RV at MOI = 1 from either the apical surface or the basolateral surface. Samples were collected at 24, 48, and 72 h.p.i.. The relative transcriptional expression of IFNA, IFNL, IFNAR1, IFNLR1, ISG 15, ISG 58, TNF α, and LCN2 was evaluated by RT-qPCR. The experiments were conducted three independent times. All data were calculated using the 2−ΔΔCT method. Results are shown as the mean +/− s.d. and are expressed as fold induction of expression related to transcription levels of mock infected cells which were set to 1. For comparison of response after apical and basolateral infections, P values were determined using unpaired Student’s t-test (and nonparametric tests) in GraphPad Prism. Comparative indicator labels are only shown when p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001. In the IFNA panel, comparative indicator labels are not shown because P values at 48 and 72 h.p.i. are above 0.05 (at 48 h, p = 0.0516; at 72 h, p = 0.0817).
Discussion
Intestinal organoids can be derived either from pluripotent stem cells or from intestinal epithelial crypts. The surrounding layer of epithelial cells of the organoid derived from pluripotent stem cells are encased by mesenchymal cells which are not included in organoids derived from crypts [Citation48]. Although crypt-derived enteroids lack a mesenchymal component, they are able to undergo the differentiation process to a mature status which is not yet achievable with organoids derived from pluripotent stem cells. Thus, crypt-derived enteroids are crucial for advancing our understanding of the interactions between intestine and infectious agents [Citation22]. Both pluripotent cells derived enteroids and, more frequently, crypt-derived enteroids have been used to analyze the infection by different RVs [Citation28,Citation29]. Histopathological images of intestines of mice infected with murine RV have shown that RV predominantly infects mature enterocytes of the middle and top of intestinal villi [Citation45]. In addition to enterocytes, human RV also infects enteroendocrine cells in human enteroids [Citation29]. In our study, porcine enteroids are derived from crypts and we established three different culture models: basolateral-out enteroids, apical-out enteroids, and 2D monolayers of differentiated epithelial cells. Major types of the differentiated epithelial cells, enterocytes, enteroendocrine cells, goblet cells, and Paneth cells, are included in all three culture models, while other cells (e.g. tuft cells, and M cells) were not detected yet. We have shown that RV infected both basolateral-out and apical-out enteroids, but efficient viral replication was observed only in apical-out enteroids. RV strain 4555 used in this study can cause cytopathic effects. Therefore, in order to avoid lysis of any cells, we used low MOI in the experiments for detection of cell tropism. By using apical-out enteroids and 2D differentiated intestinal epithelial cells, we found that porcine RV strain 4555 not only infects enterocytes but also goblet cells and Paneth cells ((C–E), (C–E)). In future studies, it will be interesting to investigate whether other RV strains are able to infect goblet cells and Paneth cells to explore if the cell tropism of different RV strains varies. RV infection induced apoptosis as demonstrated with apical-out enteroids. Molecular insights into the mechanisms of enterocyte damage and apoptosis are limited due to the lack of model systems that recapitulate the small intestine. Organoids provide interesting and useful opportunities to study not only the cell tropism but also the mechanisms of cell damage induced by enteric viruses as well as the subsequent regeneration of the epithelial cell layer [Citation37].
Most enteric pathogens attack the epithelial cell layer via the apical cell surface. In addition, pathogens can invade into intestinal epithelial cells from the basolateral side when the barrier function of epithelium is lost. In enteroids, the apical plasma membrane is facing the lumen and the basolateral cell surface is exposed to the surrounding environment. Therefore, it is difficult to apply infectious agents to the apical surface. To overcome this problem, human apical-out enteroids as well as enteroid-derived 2D monolayer cultures of differentiated intestinal epithelial cells were established and have been described previously [Citation15,Citation24]. Apical-out enteroids have been reported to be susceptible to Mycobacterium avium ssp. Paratuberculosis [Citation49], Listeria monocytogenes [Citation15] and enteric viruses (e.g. transmissible gastroenteritis virus (TGEV H165) [Citation36]). In the present study we have demonstrated that porcine apical-out enteroids are also susceptible to infection by porcine RV, however, they cannot be used to analyze infections via the basolateral route. This route of infection is relevant for pathogens approaching from the blood stream and for agents that have destroyed the epithelial barrier and thus get access to the basolateral surface of the epithelium. With regard to infections of basolateral-out enteroids, enteroids can be mechanically disrupted into fragments prior to incubation with pathogens. However, due to this mechanical manipulation, it cannot be clearly determined whether viral infections of individual enteroids were initiated on apical or basolateral surfaces. Although apical-out enteroids provided a chance to apply infection from apical side, evaluation of such organoid suspensions revealed the presence of enteroids with a mixed polarity (about 15%; (B and C)) and even a very low number of basolateral-out enteroids in addition to the majority of apical-out enteroids; similar observations have been reported in previous studies [Citation15,Citation36]. Thus, neither basolateral-out nor apical-out enteroids represent suitable models to study polarized infections and polarized host cell responses. In contrast, the 2D culture system of well-differentiated intestinal epithelial cells grown on filter supports showed a clear separation of the apical and basolateral surfaces and therefore provides a more useful model for studies of polarized infections than 3D enteroids. Surprisingly, we found that RV replication is severely limited on basolateral-out enteroids, but is efficient after basolateral infection of 2D monolayers of intestinal epithelial cells. Possible explanations for this difference may be: (i) 2D cultures of intestinal cells present a larger number of susceptible cells compared to basolateral-out enteroids. (ii) Basolateral-out enteroids are embedded in Matrigel, which may result in limited virus spread.
RV infections of polarized cells have been analyzed by several groups and conflicting conclusions have been reported. An early study from Svensson showed that a sialic acid (SA) – dependent Rhesus Rotavirus (RRV) strain enters Caco-2 cells via both the apical and the basolateral surface with the same efficiency [Citation50,Citation51]. Another group reported that SA-independent RVs enter via both the apical and the basolateral surface of polarized cells, whereas SA-dependent RVs infect most efficiently via the apical surface [Citation52]. Recently, another two studies suggested that human RV Wa and porcine RV efficiently enter via the basolateral surface of IPEC-J2 and primary enterocytes [Citation11,Citation51]. So far, all cultures used to study polarized RV infection are either immortalized cells or non-differentiated primary cells which are limited in their value to reflect the properties of the intestinal epithelium. In this study, enteroid-derived 2D monolayer cultures of well-differentiated epithelial cells were used that contain the cell types characteristic for an intestinal epithelium. We have found that 2D monolayers are susceptible to both apical and basolateral infection by RV strain 4555 which is SA-dependent ((A and B)). Accordingly, this cell culture system provides a promising tool for more detailed studies about polarized intestinal infection in the future.
Only a few studies have addressed the polarized immune response after infection or stimulation of intestinal epithelial cells [Citation30]. In this study, we demonstrate that basolateral infection of porcine intestinal epithelial cells by RV results in a higher expression of interferons and inflammatory related genes as compared to an apical infection (). A similar observation was reported for mammalian reovirus, which elicited a stronger innate immune response after basolateral infection of human intestinal epithelial cells (hIECs) in comparison to apical infection. In the case of reovirus infection of hIECs, the observed difference correlated with lower levels of replication and production of progeny virus after basolateral infection [Citation30]. Interestingly, in our studies we did not observe such a significant difference in the efficiency of viral replication after apical and basolateral infection. Therefore, the factors responsible for the different immune responses after apical/basolateral infection of porcine intestinal epithelial cells with RV may be different from those involved in the reovirus-induced immune response of hIECs. The established 2D porcine intestinal epithelial cells described in the present study provide a promising tool to study infections of the intestine by various pathogens and to elucidate the mechanisms behind the enhanced activation of the innate immune response originating from basolateral infection. In future studies, it will be interesting to investigate if interferons and diarrhoea-related inflammatory factors are released in a side-specific manner. Asymmetric distribution of cellular pattern recognition receptors, for example Toll-like receptors (TLRs), on epithelial cells has been proven as a significant factor leading to different induction of interferons after infection or stimulation from different sides. In addition, it has been reported that intestinal epithelial cells express higher levels of IFNLR1 and lower levels of IFNAR1 and IFNAR2, which may explain a rather weak response to type I IFN [Citation53]. It will be interesting to study if there is any asymmetric distribution of IFN receptors on apical and basolateral surfaces of intestinal epithelial cells and to detect downstream signal transduction after application of exogenous type I or type III IFNs to the apical or basolateral side.
There are numerous reports discussing the ability of type I and type III IFNs to control RV infection and replication in humans and mice, but only few studies are addressing other species such as pigs [Citation46,Citation47,Citation54,Citation55]. Type I IFNs (IFN α, IFN β) can be produced and sensed by all cell types as their heterodimeric receptor IFNAR1/IFNAR2 is widely expressed. Type III IFNs (IFN λ) can also be produced by all cell types, but their sensing is restricted to epithelial cells and a subset of immune cells [Citation54,Citation56,Citation57]. Studies in human enteroids revealed that RV infection mainly induced type III IFNs [Citation46]. However, this endogenous response does not correlate with a restriction of viral replication. This is in contrast to the effect of exogenous type I and type III IFNs treatment which restricts viral replication, with type I IFNs being more potent than type III IFNs [Citation46]. In our study, we found that both type I and type III IFNs were induced in porcine intestinal epithelial cells by apical and basolateral RV infection. While apical infection resulted in similar levels of expression of IFN α and IFN λ1 at transcript level, basolateral infection induced a significantly higher transcriptional expression of IFN λ1 (). Up-regulation of both type I and type III IFNs upon RV infection was also observed in primary murine intestinal cells, however, further experiments have shown that only mice lacking IFNLR1 exhibited severe tissue damage and produced higher amounts of RV than wild type mice and mice lacking IFNAR1 [Citation47]. In contrast, another study has shown that two types of IFNs cooperate to control intestinal replication and extra-intestinal spread of simian RV in neonatal mice, while adult mice only rely on type III IFNs to control infection [Citation55]. The results of our study show that IFNAR1 and IFNLR1 were induced in porcine intestinal epithelial cells by apical and basolateral RV infections, and that basolateral infection induced a significantly higher transcriptional expression of IFNLR1 at 72 h.p.i.. Taken together, the roles of type I and type III IFNs in restricting RV infection and replication are still contentious, and many studies focus on human and mice but rarely address pigs due to the lack of intestinal cell models. One explanation of these different conclusions is that type I and type III IFNs may play different roles in different animals infected by different RV strains. The established 2D and 3D porcine intestinal organoid culture systems described in the present study provide promising tools to study infections of the intestine by various pathogens and to elucidate the roles of two type IFNs to control porcine RV infections.
Acknowledgements
We want to thank Peter Otto for providing the porcine rotavirus A strain 4555 E12, Katrin Künnemann for excellent technical assistance, as well as Nelia Libowski, Malte Pitters and Henrik Thomsen for kind support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Estes MK, Palmer EL, Obijeski JF. Rotaviruses - a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi:10.1007/978-3-642-69159-1_3
- Amimo JO, Raev SA, Chepngeno J, et al. Rotavirus interactions with host intestinal epithelial cells. Front Immunol. 2021 Dec 22;12:793841. doi:10.3389/fimmu.2021.793841
- Banyai K, Kemenesi G, Budinski I, et al. Candidate new rotavirus species in Schreiber's bats, Serbia. Infect Genet Evol. 2017 Mar;48:19–26. doi:10.1016/j.meegid.2016.12.002
- Matthijnssens J, Otto PH, Ciarlet M, et al. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch Virol. 2012 Jun;157(6):1177–1182. doi:10.1007/s00705-012-1273-3
- Janke BH, Nelson JK, Benfield DA, et al. Relative prevalence of typical and atypical strains among rotaviruses from diarrheic pigs in conventional swine herds. J Vet Diagn Invest. 1990;2(4):308–311. doi:10.1177/104063879000200410
- Marthaler D, Rossow K, Culhane M, et al. Widespread rotavirus H in commercially raised pigs, United States. Emerg Infect Dis. 2014 Jul;20(7):1195–1198. doi:10.3201/eid2007.140034
- Smitalova R, Rodak L, Smid B, et al. Detection of nongroup A rotaviruses in faecal samples of pigs in the Czech Republic. Vet Med (Praha). 2009 Jan;54(1):12–18. doi:10.17221/3081-VETMED
- Wakuda M, Ide T, Sasaki J, et al. Porcine rotavirus closely related to novel group of human rotaviruses. Emerg Infect Dis. 2011 Aug;17(8):1491–1493. doi:10.3201/eid1708.101466
- Vlasova AN, Amimo JO, Saif LJ. Porcine rotaviruses: epidemiology, immune responses and control strategies. Viruses. 2017 Mar 18;9(3):48. doi:10.3390/v9030048
- Ward RL, Bernstein DI, Young EC, et al. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis. 1986 Nov;154(5):871–880. doi:10.1093/infdis/154.5.871
- Cui T, Theuns S, Xie J, et al. Porcine rotavirus mainly infects primary porcine enterocytes at the basolateral surface. Vet Res. 2019 Dec 19;50(1):110. doi:10.1186/s13567-019-0728-x
- Obert G, Peiffer I, Servin AL. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J Virol. 2000 May;74(10):4645–4651. doi:10.1128/JVI.74.10.4645-4651.2000
- Liu F, Li G, Wen K, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23(2):135-49. doi:10.1089/vim.2009.0088
- Estes MK, Graham DY, Gerba CP, et al. Simian rotavirus SAl replication in cell cultures. J Virol. 1979;31(3):810–815. doi:10.1128/jvi.31.3.810-815.1979
- Co JY, Margalef-Catala M, Li X, et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 2019 Feb 26;26(9):2509–2520e4. doi:10.1016/j.celrep.2019.01.108
- Kayisoglu O, Schlegel N, Bartfeld S. Gastrointestinal epithelial innate immunity-regionalization and organoids as new model. J Mol Med (Berl). 2021 Apr;99(4):517–530. doi:10.1007/s00109-021-02043-9
- Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell. 2016 Sep 26;38(6):590–600. doi:10.1016/j.devcel.2016.08.014
- Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014 Jul 18;345(6194):1247125. doi:10.1126/science.1247125
- Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013 Jun 7;340(6137):1190–1194. doi:10.1126/science.1234852
- de Lau W, Kujala P, Schneeberger K, et al. Peyer's patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol Cell Biol. 2012 Sep;32(18):3639–3647. doi:10.1128/MCB.00434-12
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262–265. doi:10.1038/nature07935
- Blutt SE, Estes MK. Organoid models for infectious disease. Annu Rev Med. 2022 Jan 27;73:167–182. doi:10.1146/annurev-med-042320-023055
- Verma S, Senger S, Cherayil BJ, et al. Spheres of influence: insights into salmonella pathogenesis from intestinal organoids. Microorganisms. 2020 Apr 1;8(4):504. doi:10.3390/microorganisms8040504
- VanDussen KL, Marinshaw JM, Shaikh N, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015 Jun;64(6):911–920. doi:10.1136/gutjnl-2013-306651
- Wang X, Yamamoto Y, Wilson LH, et al. Cloning and variation of ground state intestinal stem cells. Nature. 2015 Jun 11;522(7555):173–178. doi:10.1038/nature14484
- Drurey C, Lindholm HT, Coakley G, et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J Exp Med. 2022 Jan 3;219(1):e20211140. doi:10.1084/jem.20211140
- Heo I, Dutta D, Schaefer DA, et al. Modelling cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol. 2018 Jul;3(7):814–823. doi:10.1038/s41564-018-0177-8
- Finkbeiner SR, Zeng XL, Utama B, et al. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 2012;3(4):e00159–12. doi:10.1128/mBio.00159-12
- Saxena K, Blutt SE, Ettayebi K, et al. Human intestinal enteroids: a New model To study human rotavirus infection, host restriction, and pathophysiology. J Virol. 2016 Jan 1;90(1):43–56. doi:10.1128/JVI.01930-15
- Stanifer ML, Mukenhirn M, Muenchau S, et al. Asymmetric distribution of TLR3 leads to a polarized immune response in human intestinal epithelial cells. Nat Microbiol. 2020 Jan;5(1):181–191. doi:10.1038/s41564-019-0594-3
- Lin SC, Qu L, Ettayebi K, et al. Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc Natl Acad Sci USA. 2020 Sep 22;117(38):23782–23793. doi:10.1073/pnas.2010834117
- Ettayebi K, Crawford S E, Murakami K, et al. Replication of human noroviruses in stem cell–derived human enteroids. Science. 2016;353(6306):1387–1393. doi:10.1126/science.aaf5211
- Holly MK, Smith JG. Adenovirus infection of human enteroids reveals interferon sensitivity and preferential infection of goblet cells. J Virol. 2018 May 1;92(9):e00250-18. doi:10.1128/JVI.00250-18
- Huang L, Hou Q, Ye L, et al. Crosstalk between H9N2 avian influenza virus and crypt-derived intestinal organoids. Vet Res. 2017 Nov 2;48(1):71. doi:10.1186/s13567-017-0478-6
- Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020 Jul;26(7):1077–1083. doi:10.1038/s41591-020-0912-6
- Li Y, Yang N, Chen J, et al. Next-Generation porcine intestinal organoids: an apical-out organoid model for swine enteric virus infection and immune response investigations. J Virol. 2020 Oct 14;94(21):e01006-20. doi:10.1128/JVI.01006-20
- Yang N, Zhang Y, Fu Y, et al. Transmissible gastroenteritis virus infection promotes the self-renewal of porcine intestinal stem cells via Wntβ-catenin pathway. J Virol. 2022;96(18):e0096222. doi:10.1128/jvi.00962-22
- Li L, Fu F, Guo S, et al. Porcine intestinal enteroids: a new model for studying enteric coronavirus porcine epidemic diarrhea virus infection and the host innate response. J Virol. 2019 Feb 19;93(5):e01682-18. doi:10.1128/JVI.01682-18
- Wang AZ, Ojakian GK WJN. Steps in the morphogenesis of a polarized epithelium II. disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):153–165. doi:10.1242/jcs.95.1.153
- Pavasutthipaisit S, Stoff M, Ebbecke T, et al. CARD9 deficiency increases hippocampal injury following acute neurotropic picornavirus infection but does not affect pathogen elimination. Int J Mol Sci. 2021 Jun 29;22(13):6982. doi:10.3390/ijms22136982
- Su A, Yan M, Pavasutthipaisit S, et al. Infection studies with airway organoids from carollia perspicillata indicate that the respiratory epithelium Is Not a barrier for interspecies transmiss. Microbiol Spectr. 2023 Mar 14;11(2):e0309822. doi:10.1128/spectrum.03098-22.
- Pang XL, Lee B, Boroumand N, et al. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J Med Virol. 2004 Mar;72(3):496–501. doi:10.1002/jmv.20009
- Gonzalez LM, Williamson I, Piedrahita JA, et al. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One. 2013;8(6):e66465. doi:10.1371/journal.pone.0066465
- Lundgren O, Svensson L. Pathogenesis of rotavirus diarrhea. Microbes Infect. 2001 Nov;3(13):1145–1156. doi:10.1016/S1286-4579(01)01475-7
- Crawford SE, Ramani S, Tate JE, et al. Rotavirus infection. Nat Rev Dis Primers. 2017 Nov 9;3:17083. doi:10.1038/nrdp.2017.83
- Saxena K, Simon LM, Zeng XL, et al. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci USA. 2017 Jan 24;114(4):E570–E579. doi:10.1073/pnas.1615422114
- Pott J, Mahlakoiv T, Mordstein M, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011 May 10;108(19):7944–7949. doi:10.1073/pnas.1100552108
- Blutt SE, Crawford SE, Ramani S, et al. Engineered human gastrointestinal cultures to study the microbiome and infectious diseases. Cell Mol Gastroenterol Hepatol. 2018 Mar;5(3):241–251. doi:10.1016/j.jcmgh.2017.12.001
- Blake R, Jensen K, Mabbott N, et al. The development of 3D bovine intestinal organoid derived models to investigate Mycobacterium avium ssp Paratuberculosis pathogenesis. Front Vet Sci. 2022;9:921160. doi:10.3389/fvets.2022.921160
- Svensson L, Finlay BB, Bass D, et al. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991 Aug;65(8):4190–4197. doi:10.1128/jvi.65.8.4190-4197.1991
- Porta DC, Lopez S, Arias CF, et al. Polarized rotavirus entry and release from differentiated small intestinal cells. Virology. 2016 Dec;499:65–71. doi:10.1016/j.virol.2016.09.010
- Ciarlet M, Crawford SE, Estes MK. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J Virol. 2001 Dec;75(23):11834–11850. doi:10.1128/JVI.75.23.11834-11850.2001
- Mahlakoiv T, Hernandez P, Gronke K, et al. Leukocyte-derived IFN-alpha/beta and epithelial IFN-lambda constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog. 2015 Apr;11(4):e1004782. doi:10.1371/journal.ppat.1004782
- Doldan P, Dai J, Metz-Zumaran C, et al. Type III and not type I interferons efficiently prevent the spread of rotavirus in human intestinal epithelial cells. J Virol. 2022 Sep 14;96(17):e0070622. doi:10.1128/jvi.00706-22
- Lin JD, Feng N, Sen A, et al. Distinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infections. PLoS Pathog. 2016 Apr;12(4):e1005600. doi:10.1371/journal.ppat.1005600
- Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019 Apr 16;50(4):907–923. doi:10.1016/j.immuni.2019.03.025
- Stanifer ML, Guo C, Doldan P, et al. Importance of type I and III interferons at respiratory and intestinal barrier surfaces. Front Immunol. 2020;11:608645. doi:10.3389/fimmu.2020.608645
