ABSTRACT
The first locally acquired case in the Chinese mainland was reported on May 31, 2023, lagging behind other countries. In this study, we aimed to examine the early clinical and epidemiological characteristics of the earliest cases of Mpox in Beijing, China. Additionally, we investigated the sequence and transmission patterns of the Mpox virus (MPXV). We analyzed 37 reported cases of Mpox in Beijing from May 31, 2023 to June 21, 2023. The age range of the subjects was 24–51 years. Thirty-six cases (97.3%) were identified in men who have sex with men (MSM), and 19 cases (51.4%) tested positive for the human immunodeficiency virus. Thirty-three cases were symptomatic, while four were asymptomatic. Skin lesions were observed in 32 cases (97.0%), fever in 26 (78.8%), and swollen lymph nodes in 17 (51.5%). Rash typically appeared in the genital or perianal area 1–3 days before fever onset, with a minimum incubation period of 2 days. For individuals with skin rashes, the skin lesion samples showed 100% positivity and low Ct values. There were high oropharyngeal swab (75.8%) and blood (84.6%) positivity rates. All MPXV strains belonged to the B.1.3 branch of the West African lineage. These strains carried 76–86 nucleotide substitutions compared with the reference human MPXV genome, and genetic diversity was observed. Our findings provide the first insights into the landscape of early transmission of Mpox in Beijing and help inform policy formulation in the Chinese mainland.
Introduction
Mpox, formerly known as monkeypox, is an endemic disease in Central and West Africa [Citation1]. From 1970 to 2017, sporadic cases of Mpox were reported in Africa, typically originating from contact with wildlife, particularly rodents. Since 2017, initial signs of human-to-human Mpox transmission have emerged in West Africa, and sporadic cases were transmitted to countries outside of Africa from 2018 to 2021 [Citation2,Citation3]. In May 2022, the UK and several other European countries reported a Mpox outbreak that was not epidemiologically linked to cases or animals in West Africa, neither was it imported from West Africa [Citation4]. The outbreak quickly spread to the Americas and other regions [Citation5], prompting the World Health Organization to declare a Public Health Emergency of International Concern in July 2022, which was announced an end to on May 11, 2023 [Citation6]. As of June 2023, the current global Mpox outbreak resulted in over 80,000 reported cases across 112 countries and regions[Citation6,Citation7].
From 2020 to 2022, the Chinese mainland consistently implemented border quarantine and inbound traveller isolation policies for coronavirus disease 2019 (COVID-19). In September 2022, a case of imported Mpox was detected in Chongqing City, China [Citation8]. The individual was identified during the COVID-19 quarantine period, and no subsequent transmission occurred. Therefore, the COVID-19 isolation policies effectively mitigated the spread of Mpox in China. It was not until May 31, 2023, that Beijing reported the first locally acquired case of Mpox in the Chinese mainland [Citation9], considerably lagging behind other countries.
Although epidemiological and clinical characteristics of Mpox cases in other countries had been reported previously [Citation4–7], the patterns of Mpox transmission in Asia and Europe/America had shown significant differences, with many Asian countries not experiencing the outbreak seen in European and American countries. In addition, the general picture in China as well as the phylogenetic characteristics remain unclear in light of cultural differences and delayed onset of epidemic in China. The primary objective of this study is to thoroughly examine the early clinical and epidemiological characteristics of Mpox, as well as to investigate the sequence and transmission patterns of the Mpox virus (MPXV) within China. These findings are expected to contribute substantially to the understanding of Mpox in China, and contributes to the early detection and diagnosis of Mpox cases and assists in formulating Mpox epidemic prevention and control measures.
Materials and methods
Surveillance and case report
Following the Technical Guidelines for Mpox Prevention and Control (2022 edition) developed by the National Health Commission of China, a comprehensive surveillance is implemented across healthcare facilities at all levels to monitor Mpox cases. When clinical doctors encounter individuals with Mpox-like symptoms, they report such cases through the online reporting system for notifiable infectious diseases. Patient samples were collected and sent to the laboratory of the Beijing Center for Disease Prevention and Control.
Participants
In this study, we analysed all individuals who tested positive for the MPXV through nucleic acid testing from May 31, 2023, to June 21, 2023. Confirmed diagnoses were based on the presence of skin or mucosal lesions, along with at least one human specimen positive for the MPXV. Asymptomatic cases were defined as individuals who did not exhibit any skin/mucosal lesions or other symptoms associated with Mpox, but that tested positive for the MPXV by oropharyngeal or rectal swabs. These cases were primarily detected through active testing among close contacts and tracing populations.
Tracing of close contacts and the source of infection
Close contacts were identified and the source of infection was traced. Follow-up was conducted for 21 days after their exposure, and specimens were collected and tested at the time of identification, as well as 7, 14, and 21 days after exposure. Close contacts refer to individuals who have had close contact with the patient within 4 days prior to the onset of symptoms. This includes sexual partners, family members, and cohabitants who may have had direct or indirect skin or mucous membrane contact with the patient, as well as individuals who have shared a confined space with the patient for an extended period of time, and healthcare workers who were not wearing appropriate personal protective equipment. The source of infection refers to individuals who have had direct or indirect skin contact with the patient within 21 days prior to the onset of symptoms. This includes sexual partners, family members, and cohabitants.
Data collection
A comprehensive epidemiological investigation was performed for all individuals who tested positive for MPXV nucleic acid. When a case of illness was identified, the Beijing Centre for Disease Control and Prevention organized public health professionals to conduct thorough epidemiological investigations through face-to-face or telephone interviews. The investigations were used to obtain a broad range of information, such as demographic information, clinical symptoms prior to diagnosis, medical attention, exposure history during the 21 days before illness onset, and close contacts during the period from 4 days before illness onset to the time of diagnosis.
Specimen collection and laboratory testing
Blood samples, vesicular or pustular fluid, skin lesion swabs, and oropharyngeal swabs were collected in confirmed cases of Mpox. In asymptomatic carriers, rectal or perianal swabs and oropharyngeal swabs were collected. Viral DNA was extracted using a commercial kit (Qiagen, Dusseldorf, Germany) and detected using the MPXV polymerase chain reaction (PCR) kit (Biogerm Co. Ltd., Shanghai, China; Kinghawk Co. Ltd., Beijing, China) targeting F3L using the ABI 7500 system (Applied Biosystems, Carlsbad, CA, US) following the manufacturer’s protocol.
Next-generation sequencing and phylogenetic analysis
The amplicon sequencing method was used to generate the MPXV genomes. Viral DNA was amplified by multiplex PCR. PCR products were purified using AMpure XP Beads (Beckman, Brea, CA, US) and quantified using Qubit 3.0 (Thermo Fisher Scientific, Waltham, MA, US). The sequencing libraries were prepared with the Nextera DNA Library Prep Kit and sequenced using the Illumina Nextseq 2000 (Illumina, San Diego, CA, US). Consensus sequences were extracted from each mapping and assembly for each sample using CLC Genomics Workbench, version 22.0 (Qiagen). Nucleotide substitutions were identified when compared to the reference human MPXV genome (Accession number: NC_063383.1). The 31 sequences obtained in this study were compared to the representative sequences of different MPXV lineages from Japan, Thailand, the United Kingdom, Germany, the United States, Brazil, and Mexico collected from the GISAID database. A phylogenetic tree was constructed by the maximum likelihood method using IQ-TREE software [Citation10]. The best tree was constructed after performing 1,000 ultrafast bootstrap replicates. A subcluster was defined as a subset of genomic sequences clustered together on the phylogenetic tree with no more than two single-nucleotide polymorphisms.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study population. Median and interquartile range (IQR) were reported for continuous variables, while frequency and percentage were reported for categorical variables. Patient information was recorded using Microsoft Word and then summarized in Microsoft Excel to create a database. All descriptive statistics were performed using SPSS software, version 18 (IBM Corp., Armonk, NY, US).
Results
Demographic information
On May 31, 2023, the first confirmed locally acquired case of Mpox was reported in Beijing, China. As of June 21, 2023, a total of 37 Mpox cases have been reported, with daily reports ranging from 1 to 5 cases per day and no surge in new cases (). These cases are distributed among the 11 districts of Beijing. The highest number of cases is in Chaoyang District, accounting for 54.1% (20 of 37) of all cases ().
Figure 1. Onset and report time of Mpox cases in Beijing between May 31, 2023 and June 21, 2023.
Note: 37 Mpox cases are included in A and 33 symptomatic cases of Mpox are included in B.
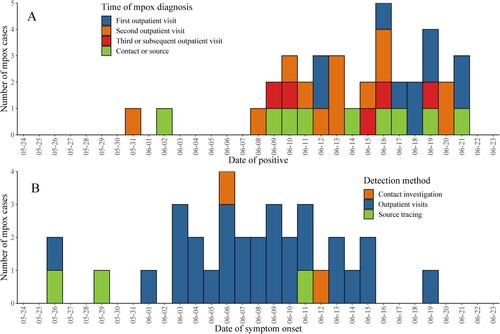
Table 1. Demographics, medical history, and exposure travel information among confirmed Mpox cases of Beijing, China as of June 21, 2023.
Of the 37 cases of Mpox, 28 (75.7%) were diagnosed via outpatient visit, 6 (16.2%) were traced through contact investigation, and 3 (8.1%) were identified through source tracing efforts. All cases tested positive for Mpox viral nucleic acid by reverse transcription PCR (RT–PCR).
All reported cases of Mpox were in individuals aged between 24 and 51 years, with a median age of 30.0 years (IQR 26.5–34.5 years), and 16.2% (6 of 37) affected individuals were aged >40 years. Among the individuals aged >40 years, the three oldest patients (48, 48, and 51 years old, respectively) were vaccinated against smallpox before 1981 (). The vaccination history was verified by a thorough examination of the upper-arm scar and a comprehensive interview conducted during the epidemiological inquiry.
Of the 37 cases of Mpox, 19 (51.4%) were human immunodeficiency virus (HIV)-positive, with one case being newly diagnosed with HIV during the medical visit. Among the 12 individuals who provided information on their HIV anti-retroviral treatment, 11 received regular medication (). Out of these 10 individuals with recent test results, 9 showed undetectable HIV levels, while 1 had a viral load of >20 copies/mL. As for the CD4+ cell count, three individuals had counts of 300–500/mm3 and six individuals had counts of >500/mm3, and 1 individual did not provide it.
Clinical findings
Thirty-three individuals with Mpox were symptomatic, while four were asymptomatic. Rash, fever, and swollen (painful) lymph nodes were the most typical early symptoms in the 33 symptomatic cases. Skin lesions were observed in 32 cases (97.0%), fever in 26 cases (78.8%), and swollen (painful) lymph nodes in 17 cases (51.5%) ().
Table 2. Clinical characteristics of Mpox cases.
Among the patients with skin rashes, the rashes had the characteristic appearance of Mpox rashes and were typically localized to the genital or perigenital and perianal regions. The number of lesions before confirming Mpox was typically low, with only one exception in which a patient displayed dense rashes over the mons pubis and lower abdomen. The remaining 31 patients had a single-digit number of lesions scattered mainly around the genitals or perianal region, with occasional cases displaying sparse rash on the head or extremities. The lesions were mostly in the pustular or crusted stage, with different stages of rash observed on different parts of the patient’s body. For example, while genital rashes in some patients had already formed crusts, facial rashes were still in the papular stage.
Fever caused by the MPXV can be either low-grade or high-grade. Among the 23 cases with fever that underwent temperature measurement, 16 (69.6%) had a temperature of ≥38.0°C and 7 (30.4%) had a temperature of ≥39.0°C (). Interestingly, fever did not always precede skin rash, as observed in traditional descriptions of Mpox symptoms. Instead, in some cases (13 of 33), local skin rash appeared first on or around the genitals and anus, followed by fever onset within 1–3 days (median 2 days) ().
Swollen (painful) lymph nodes (17 cases), particularly in the inguinal region (16 cases), were also common. Additionally, there were two cases with swollen lymph nodes in the submandibular region, accompanied by facial rashes.
Among the 19 cases with a known exposure time, the incubation period ranged from 2 to 20 days, with a median of 9 days (IQR 7–13 days). Two cases of Mpox with an incubation period of 2 days presented with atypical initial symptoms, including genital swelling in one case and a rash on the perianal mucosa in the other. Four asymptomatic cases were identified from close contacts of index cases and tested positive for MPXV on oropharyngeal and rectal swabs. Two individuals tested positive by oropharyngeal swab and negative by rectal swab, while the other two individuals showed the opposite pattern (negative by oropharyngeal swab and positive by rectal swab). With the exception of one patient with a Ct value of 24 in the rectal swab specimen, the remaining three individuals had Ct values of >35 in either their oropharyngeal or rectal swab. Two asymptomatic cases were vaccinated against smallpox prior to 1981. Three asymptomatic cases were HIV-positive, and one HIV-negative case was taking HIV pre-exposure prophylaxis.
Diagnostic timeliness and accuracy
Twenty-eight cases of Mpox were identified at outpatient clinics, with a median time from symptom onset to initial consultation of 4 days (IQR 2.5–6.5 days; range 0–15 days). The median time from symptom onset to diagnosis was 6 days (IQR 5–8.5 days; range 2–15 days).
The rate of misdiagnosis on the first outpatient visit was high as 64.3%. Of the 28 cases, only 10 cases (35.7%) were diagnosed on their first visit, while the remaining 18 had two or more visits before diagnosis. Among them, 13 cases (46.4%) were diagnosed on the second visit, 4 (14.3%) on the third visit, and 1 (3.6%) on the fourth visit.
After the announcement of the first Mpox case and the increase in the awareness of Mpox among men who have sex with men (MSM), there was a trend toward a gradual shortening of the time taken to seek medical attention (). Furthermore, suspected cases tended to choose the two specialized hospitals for infectious diseases in Beijing (21 of 28) for outpatient visits.
Mode of transmission
Among the 37 individuals with Mpox, 36 identified themselves as MSM. Among them, 2 individuals identified as bisexual, while the remaining 34 identified as homosexual (). Among the 36 individuals who self-identified as MSM, 32 acknowledged having engaged in sexual relationships with other men before contracting Mpox. The primary avenues through which these sexual partners met included dating apps, online support groups catering to individuals with similar experiences, and chance encounters at bars. There was one individual who identified himself as heterosexual and mentioned that before contracting the MPXV, he had engaged in sexual intercourse with a woman.
A total of 33 close contacts were identified for the 37 Mpox cases. These contacts included 8 regular male sexual partners, 10 casual male sexual partners, 6 family members, 6 roommates, and 3 healthcare workers. Among these close contacts, six (18.2%), including one regular and five casual sexual partners, tested positive for Mpox, either upon detection or on day 7 after their last exposure. Among these six secondary cases, four were asymptomatic, while the remaining two exhibited symptoms, such as genital and trunk rash in one case, and oral mucosal rash in another. Importantly, no transmission to family members, roommates, or healthcare workers was observed.
A total of 39 general contacts were identified, comprising 12 healthcare workers, 16 coworkers, 6 social contacts, 2 cohabitants, and 3 individuals involved in the handling of case waste. None of these general contacts developed any symptoms related to Mpox.
MPXV detection
For individuals with skin rashes, the samples taken from the lesion site, including vesicular fluid, lesion swabs, and scabs, showed 100% positivity. Additionally, the Ct values for these samples were consistently low (). The MPXV was detected in 84.6% of the blood samples, indicating the presence of viremia, while oropharyngeal swabs were positive in 75.8% of instances, indicating that the patient’s nasal and oral secretions contained the MPXV. Among the four asymptomatic cases without skin lesions, two were positive only on the oropharyngeal swab, while another case was positive only on the rectal swab (perianal swab).
Table 3. Positive rates for different specimen types in Mpox cases.
Phylogenetic analysis
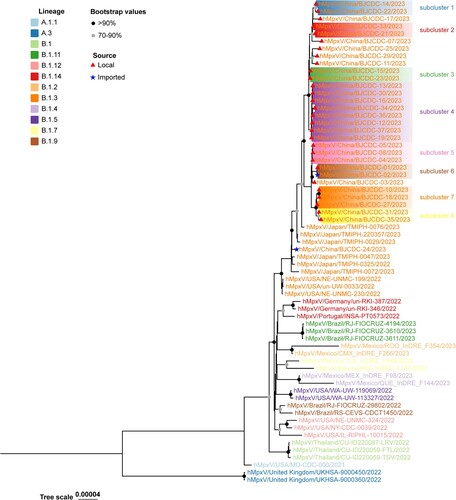
Thirty-one MPXV genomes were obtained from the infected individuals by next-generation sequencing. All of the viruses belonged to the B.1.3 branch of the West African lineage. There were 76–86 nucleotide substitutions carried by these strains compared with the reference human MPXV genome (Accession number: NC_063383.1). These 31 genomes were phylogenetically related to the strains recently isolated from in Japan (). Among the 31, 29 genomes were obtained from local cases, while two were obtained from imported cases from Thailand. The phylogenetic analysis showed that 29 local strains were clustered into the same clade, but they could be further divided into eight subclusters (supported by two single-nucleotide polymorphisms). The imported strain (hMpxV/China/BJCDC-02/2023) was obtained from a patient who returned to Beijing from Thailand, which caused the first local case (hMpxV/China/BJCDC-01/2023) in the Chinese mainland. Another imported strain (hMpxV/China/BJCDC-24/2023) was also obtained from a patient who returned to Beijing from Thailand, but it was not phylogenetically related to the foregoing strain (hMpxV/China/BJCDC-02/2023) or other local strains ().
Figure 2. Phylogenetic tree based on the genome sequences of the Mpox virus.
Note: Phylogenetic analysis of 29 local strains and 2 imported strains in Beijing, 2023. Different-colored branch names represent the different lineages. The black circles indicate bootstrap values of >90%, and the grey circles indicate bootstrap values of >70% and <90%. The red triangles indicate local strains and the blue stars indicate imported strains.
Discussion
This study elaborated the clinical features, epidemiological characteristics, and virological features of the earliest cases of Mpox in Beijing, China. Our findings provide the first insights into the landscape of early transmission of Mpox in Beijing and help inform policy formulation in the Chinese mainland.
As of June 21, 2023, a total of 37 confirmed cases of Mpox have been reported in Beijing. These cases are predominantly concentrated among MSM, with transmission occurring through male-to-male sexual contact. These cases also exhibited a higher prevalence of HIV infection, similar to previous reports [Citation4,Citation7]. Therefore, promptly incorporating Mpox into the existing acquired immune deficiency syndrome management network for comprehensive control and management would likely yield substantial benefits and optimize efforts.
The three main clinical features of Mpox are skin rash, fever, and swollen (painful) lymph nodes. The distinctive appearance of blisters, particularly with a central umbilication, makes them highly recognizable. Therefore, with adequate training, healthcare personnel are able to easily recognize such lesions. Additionally, if rapid diagnostic test kits are available for public use, early identification and self-isolation for Mpox will become more accessible.
We also observed that some of the clinical features of affected individuals differed from traditional descriptions. First, in the initial stages, individuals typically exhibited a small number of skin rashes, which were primarily concentrated around the genital and anal regions [Citation11]. The relatively mild symptoms are likely attributed to the fact that the infected individuals are predominantly young adult males. Additionally, the localization of lesions is associated with male-to-male sexual transmission. Second, the sequence of fever and rash could be reversed, with rash appearing before fever in some cases. This is unlike the traditional pattern in which symptoms like fever and headache precede the appearance of rash [Citation12]. The early detection of cases may be facilitated by the symptom characteristic of rash preceding non-specific fever, although the intrinsic pathogenesis remains unclear. Third, the rash localized in the perianal area and other symptoms can rapidly manifest within 2 days of exposure, which is shorter than the previously documented minimum incubation period of 3 days [Citation4]. Lesions on specific mucosal surfaces may be more easily perceived, and health education on Mpox knowledge can also promote early detection of symptoms. These two factors may contribute to a possible reduction in the incubation period. Finally, the existence of asymptomatic infection in the early stages of Mpox suggests that Mpox cases exhibit a broad spectrum of illnesses and asymptomatic carriers may play an important role in transmission, which should be focused on when implementing prevention and control measures. If the measure close contact tracing was absent, asymptomatic infections would be overlooked.
Another point to note is that some individuals with Mpox seek medical attention primarily to screen for sexually transmitted diseases, such as HIV and syphilis [Citation13]. This indicates that the population of MSM or individuals with a history of risky sexual behaviour have a higher awareness of protecting themselves against sexually transmitted diseases [Citation14]. This helps to identify MPXV infection within these groups. However, this also underscores an additional concern, which is that individuals from other populations may exhibit a lesser inclination to seek medical attention compared to MSM, thus resulting in potential underdiagnosis. Therefore, it is important to pay attention to close contacts and casual contacts who do not engage in male-to-male sexual contact as well. Literature reports suggest a risk of transmission among household members and other cohabitants [Citation15]. We are also concerned about the potential spread of Mpox from high-risk groups, such as MSM, to women or other groups.
The main method currently used to detect the MPXV is RT–PCR. In cases of Mpox where skin lesions are present, the collection of vesicular fluid or swabs from the skin lesions has demonstrated a high level of sensitivity and specificity for diagnostic testing. However, in asymptomatic individuals, sample collection often requires more caution, such as collecting samples from multiple sites. Currently, we collect oropharyngeal and rectal swabs from asymptomatic individuals, and these methods can complement each other. Although this study found a high positive rate of MPXV nucleic acid in oropharyngeal swabs, the probability of airborne transmission of Mpox should be very low. In fact, previous cases of transmission through respiratory secretions were mostly associated with direct contact with respiratory secretions rather than transmission through droplets or aerosols in the air [Citation16].
The current globally prevalent MPXV is the clade IIb strain, which is phylogenetically distinct from the prior endemic strains in Central and West African nations [Citation17]. Recently, the clade IIb strain further evolved into multiple branches, indicating the ongoing evolution of this prevalent MPXV [Citation18]. In this study, all of the viruses identified in Beijing belonged to the B.1.3 lineage, which is prevalent in Europe, North America, and Asia. All of the strains carried 76–86 nucleotide substitutions and were highly homologous to the strain recently isolated in Japan, and no local cases were reported in Beijing as of the end of May 2023, suggesting that the virus that is prevalent in Beijing was recently introduced from external areas rather than resulting from ongoing local transmission. Further phylogenetic analysis was conducted to compare the genetic relationships among the strains in Beijing. The results revealed that the local strains segregate into eight subclusters. Since the Mpox virus is a DNA virus with a relatively low evolution rate, the presence of multiple subclusters of Mpox viruses in Beijing suggests that the MPXV was introduced into Beijing from several sources and disseminated through multiple local transmission chains. Two strains imported from Thailand were identified, and the phylogenetic analysis results showed that both strains were highly homologous to the strain isolated in Japan rather than Thailand. This suggests that the B.1.3 lineage might be prevalent and highly genetically related in the region. Since the evolution of recent global prevalent MPXV is still ongoing and data sharing is insufficient; therefore, genomic surveillance of the MPXV still needs to be strengthened. This highlights that proper prevention and control measures are urgently needed to contain the importation and local transmission of the MPXV. The epidemiological investigation revealed that, with the exception of a few cases, there was no discernible epidemiological association among the majority of reported cases. Furthermore, most of the cases had no documented history of international travel. The MSM population in Beijing is among the largest of all large cities across China, and the prevalence of Mpox in Beijing may serve as a snapshot of the situation among MSM populations in large cities nationwide.
Although the Mpox virus was discovered in 1958, there had been limited research on it prior to 2022 [Citation19]. The global outbreak also lasted for only one year, and the clinical, epidemiological, and virological features of Mpox are not yet fully understood. Our study has identified unique clinical and epidemiological features specific to Mpox cases in China. Understanding the underlying patterns of Mpox quickly and developing targeted prevention and control measures across different countries and cultural backgrounds is highly challenging. However, integrating bibliometrics [Citation20] and big data analysis [Citation21], along with the use of artificial intelligence [Citation22], can greatly aid in early case diagnosis, exploring the patterns of spread, and expanding research perspectives. Currently, there is relatively limited information available regarding Mpox in China, and this study provides a solid foundation for researching Mpox in China.
This study has several limitations. First, this study only analyzed the early clinical symptoms of the Mpox cases and did not further track the disease progression and outcomes. Second, the data of Mpox cases were derived from self-reports, which may be subject to information bias. Third, the Mpox cases were identified through outpatient clinics and contact tracing, which may introduce detection bias and cannot exclude the possibility of overlooked cases.
In conclusion, this study presents a comprehensive description of the clinical, epidemiological, and virological features, clinical diagnosis, and reporting challenges associated with the initially reported cases of Mpox in the Chinese mainland. The findings contribute valuable insights to the understanding of mpox and have important implications for prevention, control, and research in the near future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Petersen E, Kantele A, Koopmans M, et al. Human Monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019 Dec;33(4):1027–1043. doi:10.1016/j.idc.2019.03.001
- Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022 Nov 10;387(19):1783–1793.
- Sukhdeo S, Mishra S, Walmsley S. Human monkeypox: a comparison of the characteristics of the new epidemic to the endemic disease. BMC Infect Dis. 2022 Dec 12;22(1):928, doi:10.1186/s12879-022-07900-7
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries – April–June 2022. N Engl J Med. 2022 Aug 25;387(8):679–691. doi:10.1056/NEJMoa2207323
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases – United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022 Aug 12;71(32):1018–1022. doi:10.15585/mmwr.mm7132e3
- World Health Organization. 2022-23 Mpox (Monkeypox) outbreak: global trends 2013 [updated 12 July 2023]. Available from: https://worldhealthorg.shinyapps.io/mpx_global/.
- Laurenson-Schafer H, Sklenovska N, Hoxha A, et al. Description of the first global outbreak of Mpox: an analysis of global surveillance data. Lancet Glob Health. 2023 Jul;11(7):e1012–e1023. doi:10.1016/S2214-109X(23)00198-5
- Zhao H, Wang W, Zhao L, et al. The first imported case of monkeypox in the mainland of China - Chongqing Municipality, People’s Republic of China, September 16, 2022. China CDC Wkly. 2022 Sep 23;4(38):853–854.
- Zhang D, Qi X, Li F, et al. The first local case of Mpox caused by an imported case in the Chinese mainland. Biosafety and Health. 2023.
- Hoang DT, Chernomor O, von Haeseler A, et al. Ufboot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018 Feb 1;35(2):518–522. doi:10.1093/molbev/msx281
- Tarin-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022 Aug 27;400(10353):661–669. doi:10.1016/S0140-6736(22)01436-2
- McCollum AM, Damon IK. Human monkeypox. Clin infect Dis. 2014 Jan;58(2):260–267.
- Candela C, Raccagni AR, Bruzzesi E, et al. Human monkeypox experience in a tertiary level hospital in milan, Italy, between May and October 2022: epidemiological features and clinical characteristics. Viruses. 2023 Mar 2;15(3), doi:10.3390/v15030667
- Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022 Sep;22(9):1321–1328. doi:10.1016/S1473-3099(22)00411-X
- Thornhill JP, Palich R, Ghosn J, et al. Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: a global case series. Lancet. 2022 Dec 3;400(10367):1953–1965. doi:10.1016/S0140-6736(22)02187-0
- Beeson A, Styczynski A, Hutson CL, et al. Mpox respiratory transmission: the state of the evidence. Lancet Microbe. 2023 Apr;4(4):e277–e283. doi:10.1016/S2666-5247(23)00034-4
- Zhang R, Liu Z, Chen Z. Research progress on the clades of monkeypox virus. Guoji Bing Du Xue Za Zhi. 2022;29:334–339.
- Wang L, Shang J, Weng S, et al. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J Med Virol. 2023 Jan;95(1):e28036), doi:10.1002/jmv.28036
- Cheng K, Guo Q, Zhou Y, et al. Concern over monkeypox outbreak: What can we learn from the top 100 highly cited articles in monkeypox research? Travel Med Infect Dis. 2022 Sep-Oct;49:102371.
- Cheng K, Zhou Y, Wu H. Bibliometric analysis of global research trends on monkeypox: are we ready to face this challenge? J Med Virol. 2023 Jan;95(1):e27892), doi:10.1002/jmv.27892
- Cheng K, He Y, Li C, et al. Talk with ChatGPT about the outbreak of mpox in 2022: reflections and suggestions from AI dimensions. Ann Biomed Eng. 2023 May;51(5):870–874. doi:10.1007/s10439-023-03196-z
- He Y, Wu H, Chen Y, et al. Can ChatGPT/GPT-4 assist surgeons in confronting patients with Mpox and handling future epidemics? Int J Surg. 2023 Aug 1;109(8):2544–2548. doi:10.1097/JS9.0000000000000453
