ABSTRACT
Patients with cutaneous leishmaniasis (CL) present an exacerbated inflammatory response associated with tissue damage and ulcer development. In recent years, higher rates of failure to pentavalent antimoniate therapy have been observed, yet the underlying reason remains poorly understood. We hypothesize that the eicosanoid PGE2 favours the establishment of infection by L. braziliensis, which contributes to therapeutic failure. The aim of the present study was to investigate the influence of PGE2 on the survival of L. braziliensis in macrophages and rates of therapeutic failure in CL patients. PGE2, an eicosanoid derived from the metabolism of arachidonic acid by the COX-2 enzyme, plays several roles in immune response. We found that increased PGE2 decreases the microbicidal function of macrophages and is associated with disease severity and therapeutic failure. Additionally, the neutralization of COX-2 by NS398, a selective NSAID, increases the ability of macrophages to kill L. braziliensis and protects against the pathological inflammatory response. Our data suggest that NS398 may serve as an adjunct treatment for CL patients.
Introduction
Cutaneous leishmaniasis (CL), an infectious disease caused by parasites of the genus Leishmania, results in the appearance of one or more skin ulcers, and represents a major public health problem worldwide [Citation1,Citation2]. In Brazil, Corte de Pedra, a town located in the southeastern region of the state of Bahia, is considered an important area of L. braziliensis transmission, with an alarming incidence of CL reaching 1000 cases/year in recent years [Citation3]. In areas endemic for L. braziliensis, Glucantime® is the drug of first choice, yet effectiveness varies as around 70% of patients in the pre-ulcerative phase exhibit therapeutic failure [Citation4,Citation5]. The development of specific and effective treatments require a more robust understanding of the pathogenesis of CL caused by L. braziliensis.
The course of CL is characterized by an intense inflammatory reaction with a marked predominance of lymphocytes and mononuclear phagocytes [Citation1,Citation6]. Although the inflammatory response is extremely necessary to control parasite replication, the exaggerated production of inflammatory cytokines can cause tissue damage and contribute to the emergence of ulcers that typify this disease [Citation7]. Mononuclear phagocytes, one of the main cell types harbouring Leishmania, produce inflammatory cytokines, such as TNF and IL-1β, which participate in tissue damage and the development of ulcers [Citation8–11].
PGE2 is an inflammatory eicosanoid derived from the metabolism of arachidonic acid (AA) by two isoforms of cyclooxygenase, the constitutive isoform COX-1 and COX-2, the induced isoform [Citation12], which are produced by several cell types (e.g. neutrophils and macrophages). The activity of PGE2 in innate immune responses is critically dependent on interaction with one of its four prostanoid receptors which are coupled to G protein (EP1-4). The activation of the EP1 receptor by PGE2 increases intracellular calcium accumulation, while the activation of EP2 and EP4 receptors induces the production of adenylate cyclase and cAMP; in turn, the activation of EP3 decreases cAMP formation [Citation13–15]. Increases in PGE2 and cAMP leads to the activation of protein kinases and decreased microbicidal activity of mononuclear phagocytes [Citation16,Citation17]. The aim of the present study was to investigate the role of PGE2 in L. braziliensis infection and elucidate its participation in the exacerbated inflammatory response observed in CL patients.
Our results demonstrate the increased expression of genes PTGS2, PTGER1, PTGER2 and PTGER4 in active CL lesions. In addition, the expression of PTGS2 and COX-2 protein was further associated with parasite load, disease severity and failure to therapy. Finally, we found high levels of PGE2 in the cells of CL lesions, which positively correlated with higher numbers of lesions. The neutralization of COX-2 in the cells obtained from lesions decreased the levels of TNF, IL-1β and IL-10 in vitro. Surprisingly, the neutralization of COX-2 was found to increase the killing of L. braziliensis by macrophages.
Materials and methods
Ethics statement
The present study was approved by the Institutional Review Board of the Federal University of the State of Bahia (CEP-UFBA, protocol number 2.471.185) and the Brazilian National Commission for Ethics in Research (CONEP protocol number 2.512.434). All included individuals were adult volunteers who provided written informed consent. All procedures described herein were conducted in accordance with the Declaration of Helsinki.
Patient sample
Patients with CL were diagnosed based on the presence of a typical skin ulcer associated with PCR positive for L. braziliensis DNA as well as a positive intradermal skin test for Leishmania. All patients were treated with Glucantime® (Sanofi-Aventis) at the dosage of 20 mg/kg/day intravenously for 20 days. At day 90, patients were reevaluated for lesion resolution. Cure was defined as total re-epithelialization of cutaneous lesions. Patients with lesions that remained active at day 90 were considered to have failed treatment and were submitted to a susbsequent round of Glucantime®. All immunological analyses were performed prior to the initiation of drug therapy.
In silico analysis
Data samples were downloaded from GSE127831 [Citation18] and labelled according to the informed metadata (21 samples infected by L. braziliensis before treatment, and 7 uninfected endemic controls), as well as transcripts of L. braziliensis sequenced by RNA-seq. Heat map and fold change illustrations were constructed using Multi Experiment Viewer (MeV).
In situ analysis
Skin biopsies (11 samples infected by L. braziliensis before treatment and 8 uninfected endemic controls) were fixed in 4% paraformaldehyde and embedded in paraffin. Embedded tissue was cut into 2-μm thick sections, deparaffinized, and rehydrated. Antigen retrieval was performed using 1:100 Trilogy™ solution (Cell Marque) at 96°C. Peroxidase activity was blocked with 3% hydrogen peroxide for 10 min, while nonspecific antibody binding was blocked by the addition of serum-free protein (Dako) for 10 min. Slides were incubated for 2 h with the following antibodies: Rabbit polyclonal to COX2/Cyclooxygenase 2 (1:250) (ABCAM); Peroxidase Kit and Rabbit mouse/horseradish peroxidase KP500 (Diagnostic BioSystems, Pleasanton, CA); Additionally, histological sections were stained with hematoxylin and eosin (H&E) for parasite load quantification. In each field, the number of positive cells and amastigotes were quantified using the semiautomatic counting tool of ImageJ 1.48v software (National Institutes of Health, Bethesda, MD).
Parasite cultures
L. braziliensis (MHOM/BR/LTCP11245) was isolated from a skin lesion of a CL patient, and confirmed as L. braziliensis by multilocus enzyme electrophoresis [Citation19]. The parasites selected for this study were not previously passaged in liquid culture medium. Following selection, parasites were expanded in Schneider's medium (Sigma) supplemented with 20% heat-inactivated fetal bovine serum, penicillin 100 UI/mL, and streptomycin 100 µg/mL (Gibco).
Human macrophage cultures and L. braziliensis infection
Monocyte-derived macrophages from CL patients were prepared following a previously described method [Citation8] to yield macrophages 99% characterized by flow cytometry as CD14-positive, CD3-negative and CD19-negative. After differentiation, cells were infected with stationary phase L. braziliensis promastigotes at a 5:1 ratio for 2 h; uninfected macrophages were used as controls. Following incubation, cells were infected with L. braziliensis and cultured in the presence or absence of NS398 (100 µM) (Sigma, St. Louis, MO), PGE2 10 ng/mL or rIL-10 (0.5, 1, 2 ng/mL) (R&D Systems, Minneapolis, MN USA) for 2, 48 or 72 h. After each time point, the infection rate and parasitic load were evaluated by three different observers using microscopy.
Skin fragment cultures
Biopsies of the active CL lesion and healthy skin were performed using a 4 mm punch. Fragments were dispensed in tubes containing 1 mL of RPMI-1640 (Gibco) supplemented with 10% FBS (Gibco), 100UI /mL penicillin, and 100 µg/mL streptomycin and incubated at 37°C, 5% CO2 for 72 h in the presence or absence of NS398 100 µM (Sigma, St. Louis, MO).
Determination of cytokine and eicosanoid levels
Levels of PGE2, IL-6, IL-1β, TNF and IL-10 (R&D Systems, Minneapolis, MN USA) were evaluated in culture supernatants by competitive or sandwich ELISA technique. All assays were performed following the manufacturer's protocol. For PGE2, a competitive ELISA was performed, and for TNF, IL-6, IL-1β and IL-10, sandwich ELISA was performed.
Statistical analysis
The Mann–Whitney test and/or unpaired t-test were used for comparisons between two independent continuous variables. Wilcoxon's U test and/or the Paired t-test were used for continuous dependent variables, while log-rank (Mantel–Cox) testing was used for survival analysis and Spearman's and/or Pearson's tests were used to establish correlations. All analyses were performed using GraphPad Prism v8, and results presenting a P-value <0.05 were considered statistically significant.
Results
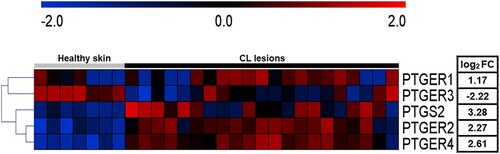
To better understand the components of the PGE2 pathway present at the sites of CL lesions, previously published gene expression data was re-analysed [Citation18]. The new analysis revealed increased expression of PTGS2, PTGER1, PTGER2 and PTGER4, genes involved in the PGE2 pathway, in CL lesions, whereas expression levels of PTGER3 were decreased in comparison to healthy skin (A). These results suggest that elevated expression of COX-2 (PTGS2) and EP1, EP2 and EP4 receptors (PTGER1, PTGER2 and PTGER4) may be a trigger for L. braziliensis survival and increased inflammatory response.
Figure 1. High expression of PGE2 pathway components is observed in active CL lesions. Unbiased RNAseq was performed on skin samples from 7 healthy subjects and lesions from 21 CL patients. Heatmap columns and rows representative of individuals and genes, respectively. Heatmap colour reflects raw z-scores of gene abundance across samples.
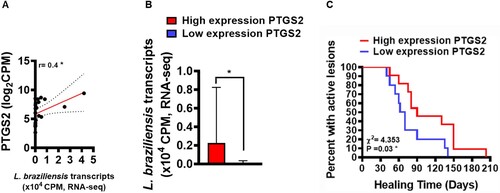
PGE2 has been described as an important eicosanoid related to Leishmania infection susceptibility in murine models [Citation17,Citation20]. To evaluate whether increased PTGS2 gene expression at lesion sites of patients with CL could be involved in parasitic load and clinical outcome, we attempted to establish correlations between the number of L. braziliensis transcripts found in active CL lesions and PTGS2 gene expression. Next, utilizing mean PTGS2 expression as a cut-off point, we distinguished between patients with high or low expression, and then analysed respective parasite loads and patient clinical outcomes. A positive correlation was observed between PTGS2 gene transcripts and parasitic load (A). In addition, we found that patients with high PTGS2 gene expression exhibited higher parasitic load than those with lower gene expression (B). To test whether increases in PTGS2 gene expression could be associated with therapeutic failure, a Kaplan-Meier survival analysis was performed in both high expression and low expression groups, revealing an association between therapeutic failure and increased PTGS2 gene expression (C). These results reinforce the hypothesis that increased PTGS2 gene expression at the lesion site may be an important host factor involved in the establishment of L. braziliensis infection that may consequently lead to therapeutic failure.
Figure 2. Increased PTGS2 gene expression at the lesion site is associated with parasite load and clinical outcome. RNAseq data (from 21 lesions) was used to investigate correlations between gene PTGS2 and number of L. braziliensis transcripts. The mean expression of PTGS2 was used as a cut-off point to determine high or low expression. L. braziliensis transcript number presented as median and interquartile range, with red bar indicating the group with high expression from PTGS2 and blue bar low expression. Statistical analysis performed using Pearson's testing for correlations (A); the Mann–Whitney test was used for comparing parasite load in groups with high and low PTGS2 expression (B); Kaplan-Meier survival analysis of groups with respect to therapeutic failure (C) *P < 0.05.
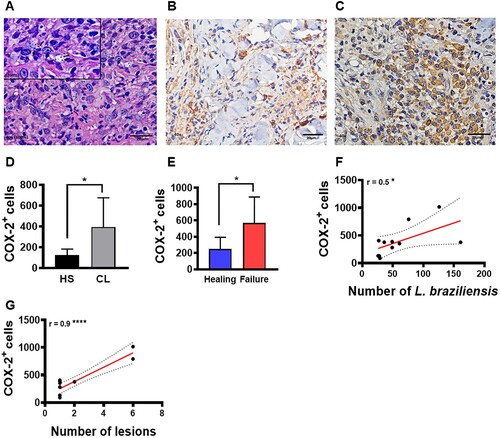
To confirm that increased COX-2 expression at the lesion site may be involved in L. braziliensis survival, we evaluated the relationship between COX-2+ cell frequency at the lesion site and parasite load as well as clinical outcome. A high frequency of COX-2+ cells were found at lesion sites compared to healthy skin (D). In addition, we observed that patients who failed Sbv therapy presented higher frequencies of COX-2+ cells (compared to those who exhibited lesion healing) (E). Finally, we identified that a higher frequency of COX-2+ cells positively correlated with amastigote numbers (F) and quantity of lesions (G).
Figure 3. High frequency of COX-2+ cells at the lesion site is associated with parasite load, disease severity and therapeutic failure. Lesion biopsies from CL patients (n = 11) and skin samples from healthy subjects (n = 8) were obtained using a 4 mm punch. Black arrows indicate the presence of L. braziliensis amastigotes in CL lesions (A), cells immunostained with polyclonal antibody anti-COX2 in healthy skin (B) and CL lesions (C), respectively. COX-2+ cell frequency presented as mean and standard deviation: black bar indicates healthy skin while grey bar represents CL lesions (D). Blue bar indicates patients that evolved to cure, while the red bar patients those that failed therapy (E) Correlation between number of amastigotes and frequency of COX-2+ cells (F), and correlation between number of lesions and frequency of COX-2+ cells (G). Statistical analysis performed using the Unpaired t-test to compare COX-2+ frequency, with Pearson's testing used for correlation analysis; *P < 0.05, ****P < 0.0001.
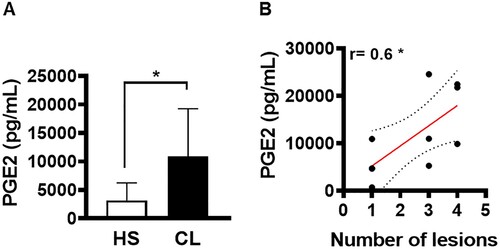
It was previously shown that patients who fail therapy present high parasitic load [Citation18]. In an attempt to identify the mechanisms that favour the establishment of infection, we evaluated the ability of cells residing in active CL lesions to produce PGE2. First, we investigated whether PGE2 was produced at active lesion sites of CL patients. Compared to healthy skin, we observed high levels of PGE2 production by cells residing in CL lesions (A). Next, we endeavored to determine whether the high production of PGE2 was associated with disease severity, and found a positive correlation between PGE2 levels and the number of lesions (B).
Figure 4. PGE2 production in active lesions correlates with disease severity. Lesion biopsies from CL patients (n = 11) and skin samples from healthy subjects (n = 8) were obtained using a 4 mm punch and cultured for 72 h. PGE2 levels were determined in culture supernatants by ELISA. (A) Levels of PGE2 presented as mean and standard deviation. (B) Correlation between number of lesions and PGE2 levels. Statistical analysis performed using the Unpaired t-test to compare PGE2 levels, while Pearson's testing was used for correlation analysis; *P < 0.05.
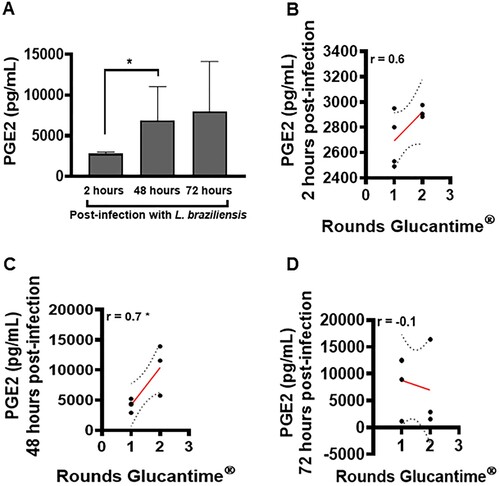
An elevated frequency of macrophages has been identified in the inflammatory infiltrate of CL lesions [Citation6]. These cells are extremely important for the effective control of parasitic load [Citation8]. We investigated the ability of these cells to produce PGE2 following L. braziliensis infection, as well as the role of this eicosanoid in the pathogenesis of CL. We identified low PGE2 production by macrophages at 2 h after L. braziliensis infection, which increased significantly at 48 h post-infection; however, the difference observed at 72 h post-infection was not statistically significant compared to 48 h (A). In addition, at 48 h post-infection, increased levels of PGE2 were found to positively correlate with the number of rounds of Glucantime® (C).
Figure 5. High levels of PGE2 in macrophages infected with L. braziliensis correlate with treatment duration. Monocyte-derived macrophages obtained from CL patients (n = 7) were infected with stationary-phase L. braziliensis (5:1) and cultured for 2, 48 or 72 h. PGE2 levels were determined in culture supernatants by ELISA. (A) PGE2 levels presented as mean and standard deviation. (B) Correlation between rounds of Glucantime® and PGE2 levels. Statistical analysis performed using the Unpaired t-test to compare the PGE2 levels, while Pearson's testing was used for correlation analysis between lesion number and PGE2 levels; *P < 0.05.
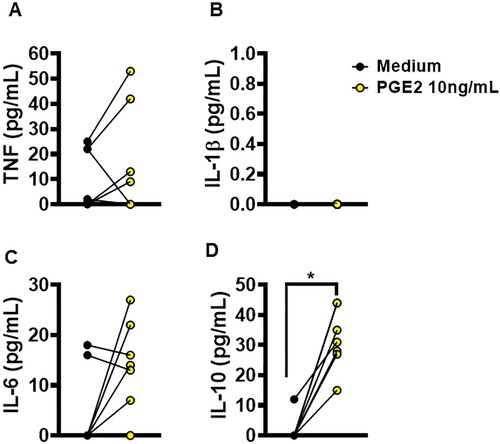
Previous studies have reported that increased PGE2 induces the production of IL-1β in macrophages [Citation21,Citation22]. To test whether PGE2 influences the production of these cytokines, we enriched cultures of uninfected macrophages from CL patients with PGE2 to evaluate levels of TNF, IL-1β, IL-6 and IL-10. Interestingly, we observed that the enrichment of macrophage cultures with PGE2 did not affect inflammatory cytokine production (A–C); however, by way of an unclear mechanism, PGE2 did induce the production of IL-10 (D). To investigate whether the amount of IL-10 induced by PGE2 had an effect on the ability of macrophages to kill Leishmania, we then infected macrophages from CL patients and enriched the cultures with increasing doses of rIL-10. We found that increases in IL-10 impeded the ability of macrophages to kill L. braziliensis, thereby allowing parasites to proliferate (supplementary Figure 1). It follows that the inhibition of PGE2 synthesis may represent a promising strategy for in the control of inflammatory response and infection by L. braziliensis in vitro.
Figure 6. Ability of PGE2 to alter macrophages cytokine production. Monocyte-derived macrophages from HS (n = 5) were cultured in the presence or absence of PGE2 (10 ng/mL) for 72 h. The levels of (A) TNF, (B) IL-1β, (C) IL-6 and (D) IL-10 were determined in culture supernatants by ELISA. Statistical analysis performed using the Wilcoxon test; *P < 0.05.
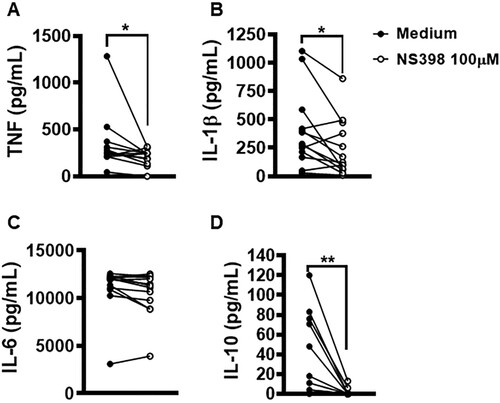
Lesions with high parasitic load exhibit a more intense inflammatory response observed to contribute to tissue damage and therapeutic failure [Citation18]. NS398 is a COX-2 selective NSAID that was shown to be effective in controlling L. infantum infection in vivo [Citation23]. Thus, we investigated whether the inhibition of COX-2 would exert any effect on macrophage infection by L. braziliensis. We found that COX-2 inhibition via NS398 decreased both the number of infected cells and the number of amastigotes at both 2 and 48 h after infection (A,B). Regarding immune response, we observed that the treatment of cells residing in CL lesion sites with NS398 resulted in decreased TNF, IL-1β and IL-10 levels, yet no change in IL-6 was observed after 72 h of culture (A–D), which suggests that other cells, i.e. not macrophages, are the producers of this cytokine. These results serve to indicate the benefits of neutralizing COX-2 in cells infected with L. braziliensis.
Figure 7. Neutralization of COX-2 increases L. braziliensis killing by macrophages obtained from CL patients. Monocyte-derived macrophages from CL patients (n = 7) were infected with stationary-phase L. braziliensis (5:1) and cultured in the presence or absence of NS398 (100 µM), a selective COX-2 inhibitor, for 2, 48 or 72 h. (A) Frequency of infected cells. (B) Number of amastigotes per 100 macrophages. Results presented as mean and standard deviation, with black bars indicating macrophages infected with L. braziliensis, and white bars macrophages infected with L. braziliensis treated with NS398. Statistical analysis performed using the Paired t-test; *P < 0.05, **P < 0.005, ***P < 0.001.
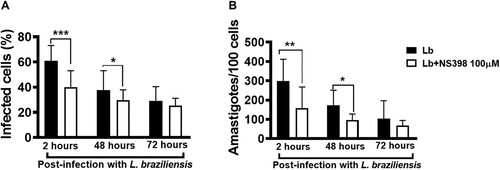
Figure 8. Neutralization of COX-2 in lesion cells decreases production of IL-1β, TNF and IL-10. Skin lesion biopsies from CL patients (n = 13) were obtained using a 4 mm punch and cultured for 72 h. Levels of (A) TNF, (B) IL-1β, (C) IL-6 and (D) IL-10 were determined in culture supernatants by ELISA. Statistical analysis performed using Wilcoxon's test; *P < 0.05 and **P < 0.005.
Discussion
Increasing rates of failure to drug therapy have been observed in patients with CL over the years [Citation4,Citation5,Citation24,Citation25]. Host factors and parasite burden have been associated with the development of skin ulcers and failure to Glucantime® therapy in areas of high L. braziliensis transmission [Citation18]. It follows that the identification of factors and mechanisms responsible for the disproportionate immune response observed in Leishmania infection, as well as those favouring the establishment of parasite persistence, remain extremely important. Lipid mediators are molecules with suspected involvement in resistance or susceptibility to pathogens in a range of infections [Citation16,Citation26–29]. PGE2, an inflammatory eicosanoid derived from the metabolism of arachidonic acid by COX enzymes, is known to contribute to the survival of various species of Leishmania, including L. amazonensis, L. donovani and L. infantum [Citation17,Citation20,Citation23]. However, the roles played by COX-2 and PGE2 in CL immunopathogenesis arising from L. braziliensis infection have yet to be elucidated. The present study represents the first attempt to determine associations between COX-2 and PGE2 with respect to L. braziliensis survival, treatment failure and disease severity.
The activation of the EP2 receptor by PGE2 induces the production of cAMP, a molecule that decreases the production of oxygen derivatives [Citation16,Citation30,Citation31], molecules relevant to the control of Leishmania proliferation [Citation32]. It has been documented that increased COX-2 and EP2 gene expression in lesions from patients with cutaneous and diffuse leishmaniasis caused by L. amazonensis was associated with parasite survival [Citation33]. Concordantly, we also identified increased expression of the genes encoding COX-2 and the EP2 receptor in the lesions of CL patients in comparison to healthy skin, which suggests that the COX-2/EP2 axis may be a crucial element in the survival and replication of L. braziliensis, as well as in therapeutic failure. Here, our results also show that, during the course of L. braziliensis infection, increased levels of COX-2 correlated positively with parasite load in the lesions of patients with CL. It is known that increased parasite load at the lesion site is associated with the uncontrolled production of a variety of inflammatory molecules (e.g. IL-1β) that contribute to tissue damage and, consequently, lead to therapeutic failure [Citation6,Citation18]. Our results additionally show that patients with high expression of the COX-2 gene failed therapy more frequently and presented greater numbers of lesions than those with low COX-2 gene expression. Moreover, we identified a strong correlation between the number of lesions and COX-2/PGE2 production, suggesting that increases in these molecules may be a contributing factor in disease severity. It is important to emphasize that patients with disseminated leishmaniasis, an emerging form of American Tegumentary Leishmaniasis (ATL), present multiple skin lesions and produce high levels of PGE2 [Citation34]. Consequently, the data reported herein support the notion that exaggerated PGE2 production at lesion sites may be a key factor in parasite dissemination as well as the severity of disease.
The innate host immune response mediated by macrophages and neutrophils represents an important line of defence against parasites, due to the ability of these cells to produce ROS, molecules harmful to Leishmania spp. [Citation8,Citation27,Citation32,Citation35]. However, at the lesion sites of CL patients, low numbers of neutrophils in addition to a dense infiltrate containing macrophages have been documented [Citation1,Citation6]. Accordingly, we investigated the ability of macrophages to produce PGE2, as well as the role of this inflammatory eicosanoid in the context of L. braziliensis infection. It was previously demonstrated that macrophages from CL patients are capable of producing high levels of PGE2 in the first hours of infection by L. braziliensis [Citation36]. Here we found that, following the internalization of L. braziliensis in macrophages, PGE production increased, reaching a peak at 48 h post-infection. We also observed that the levels of PGE2 at this timepoint positively correlated with the number of rounds of Glucantime®, which reinforces the involvement of this eicosanoid in therapeutic failure.
The inhibition of PGE2 synthesis through the use of NSAIDs, both those selective and non-selective for COX enzymes, has been shown to be effective in controlling infection by several species of Leishmania in vitro and in vivo [Citation17,Citation20,Citation23]. However, it is important to emphasize that non-selective NSAIDs can interfere with pathways other than those involving COXs, and may provoke alterations in different physiological environments. Thus, our protocol employed the first selective NSAID described for COX-2 [Citation37], which was applied to macrophages infected with L. braziliensis and cells from CL lesions. Our results indicate that the inhibition of COX-2 via NS398 decreased the numbers of amastigotes per macrophage, which stands in agreement with the findings from other studies involving other species of Leishmania and different NSAIDs [Citation17,Citation23]. An important result obtained in the current study was that although the neutralization of COX-2 in cells residing in CL lesions led to decreases in the secretion of cytokines TNF, IL-1β and IL-10, the addition of PGE2 in cultures of non-infected macrophages did not exert any effects on TNF, IL-1β and IL-6 production. To our surprise, we also found that exogenous PGE2 induced IL-10 production by non-infected macrophages. IL-10 is a well-described cytokine known to down modulate inflammatory responses [Citation24,Citation38], thus favouring parasite multiplication within macrophages [Citation39]. However, the low concentration of IL-10 produced by infected macrophages is not capable of regulating the inflammatory response trigged during L. braziliensis infection. Even though PGE2 was able to increase IL-10 production in uninfected macrophages, the levels produced were apparently insufficient to regulate the inflammatory response observed during infection. Finally, we found that even at low concentrations IL-10 is capable of disabling the microbicidal functions of macrophages, thus favouring parasite proliferation. Together, these data show suggest that COX-2 enzyme inhibition using selective NSAIDs increases the ability of macrophages to kill Leishmania, thus attenuating inflammatory response.
In conclusion, the present study documents the advantages of inhibiting COX-2 with a selective NSAID, suggesting the role of NS398 as a promising candidate for adjuvant CL therapy.
Acknowledgements
We thank Andris K. Walter for English language revision and manuscript copyediting assistance, and Cristiano Franco for secretarial assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bittencourt AL, Barral A. Evaluation of the histopathological classifications of American cutaneous and mucocutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1991;86(1):51–56. doi:10.1590/S0074-02761991000100009
- WHO. Expert Committee on the Control of the Leishmaniases & World Health Organization. Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases. World Health Organization. Geneva, 22–26 March 2010.
- Jirmanus L, Glesby MJ, Guimarães LH, et al. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg. 2012;86(3):426–433. doi:10.4269/ajtmh.2012.11-0378
- Unger A, O’Neal S, Machado PR, et al. Association of treatment of American cutaneous leishmaniasis prior to ulcer development with high rate of failure in northeastern Brazil. Am J Trop Med Hyg. 2009;80(4):574–579. doi:10.4269/ajtmh.2009.80.574
- Machado PR, Ampuero J, Guimarães LH, et al. Miltefosine in the treatment of cutaneous leishmaniasis caused by leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4(12):e912. doi:10.1371/journal.pntd.0000912
- Saldanha MG, Queiroz A, Machado PRL, et al. Characterization of the histopathologic features in patients in the early and late phases of cutaneous leishmaniasis. Am J Trop Med Hyg. 2017;96(3):645–652.
- Ribeiro-de-Jesus A, Almeida RP, Lessa H, et al. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res = Revista Brasileira de Pesquisas Medicas e Biologicas. 1998;31(1):143–148. doi:10.1590/S0100-879X1998000100020
- Giudice A, Vendrame C, Bezerra C, et al. Macrophages participate in host protection and the disease pathology associated with leishmania braziliensis infection. BMC Infect Dis. 2012;12:75.
- Passos S, Carvalho LP, Costa RS, et al. Intermediate monocytes contribute to pathologic immune response in leishmania braziliensis infections. J Infect Dis. 2015;211(2):274–282. doi:10.1093/infdis/jiu439
- Santos D, Campos TM, Saldanha M, et al. IL-1β Production by intermediate monocytes Is associated with immunopathology in cutaneous leishmaniasis. J Invest Dermatol. 2018;138(5):1107–1115. doi:10.1016/j.jid.2017.11.029
- Carvalho AM, Novais FO, Paixão CS, et al. Glyburide, a NLRP3 inhibitor, decreases inflammatory response and Is a candidate to reduce pathology in leishmania braziliensis infection. J Invest Dermatol. 2020;140(1):246-9.e2. doi:10.1016/j.jid.2019.05.025
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. science (New York. NY. 2001;294(5548):1871–1875. doi:10.1126/science.294.5548.1871
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–166. doi:10.1016/j.pharmthera.2004.06.003
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. doi:10.1074/jbc.R600038200
- Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. doi:10.4049/jimmunol.1101029
- Serezani CH, Chung J, Ballinger MN, et al. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37(5):562–570. doi:10.1165/rcmb.2007-0153OC
- Saha A, Biswas A, Srivastav S, et al. Prostaglandin E2 negatively regulates the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis. J Immunol. 2014;193(5):2330–2339. doi:10.4049/jimmunol.1400399
- Amorim CF, Novais FO, Nguyen BT, et al. Variable gene expression and parasite load predict treatment outcome in cutaneous leishmaniasis. Sci Transl Med. 2019;11(519). doi:10.1126/scitranslmed.aax4204
- Cupolillo E, Grimaldi G Jr, Momen H. A general classification of New world leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50(3):296–311. doi:10.4269/ajtmh.1994.50.296
- Lonardoni MV, Barbieri CL, Russo M, et al. Modulation of leishmania (L.) amazonensis growth in cultured mouse macrophages by prostaglandins and platelet activating factor. Mediat Inflamm. 1994;3(2):137–141. doi:10.1155/S0962935194000177
- Sheppe AEF, Kummari E, Walker A, et al. PGE2 augments inflammasome activation and M1 polarization in macrophages infected with salmonella typhimurium and yersinia enterocolitica. Front Microbiol. 2018;9:2447. doi:10.3389/fmicb.2018.02447
- Zoccal KF, Sorgi CA, Hori JI, et al. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat Commun. 2016;7:10760. doi:10.1038/ncomms10760
- Araújo-Santos T, Prates DB, França-Costa J, et al. Prostaglandin E2/leukotriene B4 balance induced by lutzomyia longipalpis saliva favors leishmania infantum infection. Parasit Vectors. 2014;7(1):1–8.
- Lago ASD, Nascimento M, Carvalho AM, et al. The elderly respond to antimony therapy for cutaneous leishmaniasis similarly to young patients but have severe adverse reactions. Am J Trop Med Hyg. 2018;98(5):1317–1324. doi:10.4269/ajtmh.17-0736
- Ponte-Sucre A, Gamarro F, Dujardin JC, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. doi:10.1371/journal.pntd.0006052
- Secatto A, Rodrigues LC, Serezani CH, et al. 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PloS one. 2012;7(3):e31701. doi:10.1371/journal.pone.0031701
- Tavares N, Afonso L, Suarez M, et al. Degranulating neutrophils promote leukotriene B4 production by infected macrophages To kill leishmania amazonensis parasites. J Immunol. 2016;196(4):1865–1873. doi:10.4049/jimmunol.1502224
- Morato CI, da Silva IA, Borges AF, et al. Essential role of leukotriene B4 on leishmania (viannia) braziliensis killing by human macrophages. Microbes Infect. 2014;16(11):945–953. doi:10.1016/j.micinf.2014.08.015
- Harbour DA, Blyth WA, Hill TJ. Prostanglandins enhance spread of herpes simplex virus in cell cultures. J Gen Virol. 1978;41(1):87–95. doi:10.1099/0022-1317-41-1-87
- Ottonello L, Morone MP, Dapino P, et al. Cyclic AMP-elevating agents down-regulate the oxidative burst induced by granulocyte-macrophage colony-stimulating factor (GM-CSF) in adherent neutrophils. Clin Exp Immunol. 1995;101(3):502–506. doi:10.1111/j.1365-2249.1995.tb03141.x
- Lin P, Welch EJ, Gao XP, et al. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174(5):2981–2989. doi:10.4049/jimmunol.174.5.2981
- Carneiro PP, Conceição J, Macedo M, et al. The role of nitric oxide and reactive oxygen species in the killing of leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PloS one. 2016;11(2):e0148084. doi:10.1371/journal.pone.0148084
- França-Costa J, Van Weyenbergh J, Boaventura VS, et al. Arginase I, polyamine, and prostaglandin E2 pathways suppress the inflammatory response and contribute to diffuse cutaneous leishmaniasis. J Infect Dis. 2015;211(3):426–435. doi:10.1093/infdis/jiu455
- Oliveira WN, Dórea AS, Carneiro PP, et al. The influence of infection by different leishmania (viannia) braziliensis isolates on the pathogenesis of disseminated leishmaniasis. Front Cell Infect Microbiol. 2021;11:740278. doi:10.3389/fcimb.2021.740278
- Conceição J, Davis R, Carneiro PP, et al. Characterization of neutrophil function in human cutaneous leishmaniasis caused by leishmania braziliensis. PLoS Negl Trop Dis. 2016;10(5):e0004715. doi:10.1371/journal.pntd.0004715
- Bonyek-Silva I, Nunes S, Santos RL, et al. Unbalanced production of LTB(4)/PGE(2) driven by diabetes increases susceptibility to cutaneous leishmaniasis. Emerg Microbes Infect. 2020;9(1):1275–1286. doi:10.1080/22221751.2020.1773744
- Futaki N, Takahashi S, Yokoyama M, et al. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47(1):55–59. doi:10.1016/0090-6980(94)90074-4
- Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70(12):6734–6740. doi:10.1128/IAI.70.12.6734-6740.2002
- Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166(2):1141–1147. doi:10.4049/jimmunol.166.2.1141
