ABSTRACT
Seasonal patterns of neonatal mortality and stillbirths have been found around the world. However, little is known about the association between season of birth and infant mortality of pre-industrial societies in a subarctic environment. In this study, we compared how season of birth affected the neonatal and stillbirth risk among the Sami and non-Sami in Swedish Sápmi during the nineteenth century. Using digitised parish records from the Demographic Data Base at Umeå University, we applied logistic regression models for estimating the association of season of birth with stillbirths and neonatal mortality, respectively. Higher neonatal mortality was found among the winter- and autumn-born Sami, compared to summer-born infants. Stillbirth risk was higher during autumn compared to summer among the Sami, whereas we found no seasonal differences in mortality among the non-Sami population. We relate the higher neonatal mortality risk among winter-born Sami to differences in seasonality of living conditions associated with reindeer herding.
Introduction
Higher infant mortality among indigenous populations compared to non-indigenous populations living in the same area has been revealed among historical as well as contemporary populations around the world [Citation1,Citation2]. During the eighteenth and nineteenth centuries, a common belief among contemporary observers was that the Swedish Sami (the indigenous population of northern Sweden) died either very young or very old [Citation3]. From previous research about the demographic transition in Swedish Sápmi, we know that this statement was correct. Infant and child mortality was generally higher among the Sami compared to the non-Sami population living in the same area, especially during the period between 1750 and 1850 [Citation4–Citation6]. Previous research has also revealed large regional differences in infant mortality in Sápmi (northern Sweden) during the nineteenth century, varying from 200 to 500 deaths per 1,000 live births [Citation6,Citation7].
The season of birth affects the risk of infant deaths, and as such is a good proxy for living conditions in utero and during early infancy [Citation8]. Also, seasonal variations in birth outcomes have been observed, such as increased risk of preterm birth, low birth weight, and stillbirths following temperature extremes. The mother’s poor nutritional status and high workload during pregnancy often show seasonal patterns and are predictors for birth outcomes as well [Citation9]. Neonatal mortality (death within the first 28 days of life) also appears to be associated with the seasonality of infectious diseases and extreme temperatures, e.g. low winter temperature [Citation10] or high summer temperature [Citation11]. However, little is known about the seasonal differences in birth outcomes and early infancy conditions, especially concerning stillbirths and neonatal mortality in pre- or industrialising sub-arctic societies. Therefore, in this study we addressed the following questions:
What was the association of seasonality with risk of stillbirth and neonatal mortality in Sápmi during the 19th century?
Did seasonality of stillbirths and neonatal mortality vary by ethnicity?
Living conditions and infant mortality in Sápmi
Swedish Sápmi includes the northern half of Sweden and is characterised by a subarctic climate (long, cold winters and short, mild summers). During the nineteenth century, Sápmi was a rural area inhabited by Sami (semi-nomadic reindeer herders) and non-Sami populations (a majority were sedentary farmers and fishers). Already in 1884, Hellstenius assumed that the harsh living conditions of reindeer herders were the most prominent explanation for their high infant mortality rates [Citation12]. The reindeer-herding year was traditionally divided into eight different seasons characterised the shifting reindeer-herding conditions and work intensity. For the Sami, this traditional nomadic lifestyle played an important part in the high infant mortality, and during migration Sami women only had a couple of days after giving birth before moving on to the next settlement [Citation3,Citation13]. Contemporary observers noted the impression that the Sami did not give birth to many children, and that Sami women preferred an interval of three years between births [Citation14]. Later research, however, revealed that the Sami had higher fertility rates compared to non-Sami living in the same area and to the national population [Citation15].
Breastfeeding is seen as an important factor related to infant mortality because it affects nutritional intake and susceptibility to contaminated water and exposure to diseases [Citation16]. During the mid-nineteenth century, breastfeeding practices diverged widely in Sweden; in some places, it was common to use mixed-feeding practices or not to breastfeed infants at all [Citation17,Citation18]. Instead, a culture of artificial feeding of unboiled cow milk, often sour and of bad quality, replaced breastfeeding [Citation6]. Compared to the general trend, breastfeeding was the only option for Sami mothers (because they did not own cows), and their infant nursing practices were often used as a good example. According to clergy, physicians, and travellers, Sami children were breastfed for 2–4 years [Citation6,Citation19].
There is a lack of research regarding determinants of neonatal mortality and stillbirths in Sápmi during the nineteenth century. In Jokkmokk between 1805 and 1894, Brändström found a higher neonatal mortality among the Sami population compared to the non-Sami [Citation7]. Between 1861 and 1870, the stillbirth rates were around 27/1,000 live births among the entire population in coastal northern Sweden (Skellefteå and Sundsvall) and the overall perinatal rate between 1831 and 1899 was 42/1,000 live births [Citation20]. A history of previous stillbirths, advanced age, unmarried status, and low socioeconomic position of the mothers were associated with a higher risk of mortality [Citation20].
Season of birth and infant mortality
Research shows that the season of birth affects the risk of infant deaths. In Italy and Spain of the nineteenth century, mortality was highest among infants born in winter, which was explained by the cold winter climate and the high risk of respiratory infections during the particularly sensitive first weeks of life [Citation21]. The mortality of babies born in winter was three times higher than that of those born in summer [Citation22]. In 19th-century Russia and Poland, however, mortality was higher among infants born in summer, explained as a combination of hot temperatures and higher risk of contracting infections of the digestive tract [Citation23,Citation24]. The higher risk among summer-born infants in Russia and Poland was also related to socioeconomic factors, where the mothers were expected to participate in farm work, thus leaving home and taking less care of the infant [Citation23,Citation24]. In the south Sápmi area of Föllinge, Sköld et al. [Citation6] found a small effect of season of birth on infant mortality, where higher mortality rates appeared among infants born during the winter months of January–March.
Seasonality, neonatal mortality, and stillbirths
Previous research of neonatal mortality in Swedish Sápmi has revealed that Sami infants born during the summer months (July and August) had the highest neonatal mortality risk, whereas the non-Sami infants showed no seasonal differences [Citation7]. We previously showed that the season of birth indicated differential effects depending on age at death among Sami populations in the parishes of Jokkmokk and Jukkasjärvi in Swedish Sápmi [Citation25]. Being born during winter was related to a higher risk of neonatal mortality, while being born during summer was related to a higher risk of mortality after 6 months of age [Citation25]. These results implied that infant mortality risks are generally higher during winter and are also related to sensitive stages during the first year of life, both as new-borns during the neonatal period and around the age of six months when food was introduced in combination with the waning effect of maternal antibodies. In southern Sweden (Scania), however, no excess neonatal mortality risk among winter-born children was found [Citation16]. In Sweden, during the period 1831–1860 the highest rate of stillbirths was during the first three months of January–March, and the lowest rate was during June–August [Citation26]. International research in historical populations has revealed a major pattern showing high stillbirth rates during the winter and spring and low rates during the summer months, for example, in Switzerland [Citation27], UK [Citation28–Citation30] and the US [Citation31].
A model of seasonality, stillbirths, and neonatal mortality
We expected that the Sami had a higher neonatal mortality than the non-Sami, but that neonatal mortality rates within each population varied by season, based on the mother’s seasonal workload. Following previous research on seasonality and infant mortality in Sápmi [Citation25], we hypothesised that winter-born Sami infants had the greatest risk of neonatal mortality, whereas infants born in summer experienced the lowest levels. The cold and harsh climate during winter, in combination with movements between settlements, are likely to be factors related to an excess in neonatal mortality among the Sami. The mild weather during the stay in the summer settlement (June–August), and the least work-intense period for Sami women with infants or who were pregnant [Citation32], are likely to be factors associated to a lower neonatal mortality among Sami. For non-Sami women (mostly farmers), the harvest season (July and August) was the most work-intensive period. Among non-Sami, summer-born infants were hypothesised to be at a higher risk than the winter-born due to women’s participation in agriculture, which meant less breastfeeding and higher risks of food contamination.
Further, during the neonatal period, this study also expected the risk factor “constitution at birth” and endogenous factors, here measured by sex and parity, to be vital for infant survival. According to previous studies, boys are at a higher risk of death during the very beginning of life [Citation28]. Regarding parity, studies have shown a J-shaped curve, where the mortality risk decreases after the first child and then increases again for four or more children [Citation33]. This higher vulnerability among first-born infants is especially clear in terms of neonatal mortality [Citation28].
Materials and methods
Registration of the Swedish population in church registers began in the eighteenth century, covering the total population [Citation34,Citation35]. The source material for this study was a set of data files from the Demographic Data Base at Umeå University [Citation36], including digitised parish records from the Sápmi area from the eighteenth and nineteenth centuries. The dataset is a result of a combination of sources, including records of births, deaths, marriages, and migration as well as catechetical examination registers. The database contains all individuals born in, or migrating into, the parishes.
The parishes included in the study and belonging to the North Sápmi area are Jokkmokk, Gällivare, Jukkasjärvi, and Karesuando. South Sápmi includes the parishes in Jämtland of Undersåker, Föllinge, Hotagen, Frostviken, and Hede ().
The total study sample (1800–1899) comprises 32,617 births, 16,917 for Sami and 15,700 for non-Sami (), which provides a sufficient statistical power for the planned analyses. Even though the data provide opportunities to study the association between season of birth, stillbirths and neonatal mortality, it should also be mentioned that the sources have their shortcomings. Because it is important to know the exact date of birth, only infants born within the study area and with a known date of birth were included when calculating mortality rates by month of birth. Around 92% of the population have both a known date of birth and of death in the data. A higher proportion of Sami than non-Sami ended their registration without a known reason, mainly due to unreported out-migration or unreported death [Citation5]. The higher proportion of underreported causes of ending the registers among the Sami population may lead to an underestimation of death rates (neonatal and stillbirths). However, the distribution of unreported causes of ending the register is consistent over time, which makes it possible to analyse trends in neonatal and stillbirth mortality for both population.
Table 1. Total number of births, neonatal deaths, and stillbirths in Sápmi by ethnicity, 1800–1899
Variables
Ethnicity
The parish registers did not include information about ethnicity, but it could be indirectly inferred based on a number of ethnic indicators developed by Nordin [Citation37], such as occupation, mortality, geographical information, name, and family relations. Inclusion of the word “Lapp”, “Lappish”, or “Nomad” is the most prominent indicator of Sami ethnicity found in the different sources [Citation37,Citation38]. The non-Sami group includes the settlers who began moving to the Sami area in the mid-eighteenth century, mostly from other parts of Sweden and from Norway and Finland [Citation6].
Stillbirths and neonatal mortality
Only infants born within the study area and with known dates of birth and dates of death have been included when calculating infant mortality rates. Neonatal mortality rates were calculated as the number of deaths within the first 28 days of life per 1,000 live births (i.e. stillbirths were excluded) [Citation39].
Previous research on estimating stillbirths in historical populations follows two main paths and has either used calculations of recorded stillbirths, where they exist, or estimations of the stillbirth rate based on the neonatal mortality rate because the two are closely related [Citation29]. In this data set, we have information on stillbirths, but the accuracy of the registration might be problematic. For example, there might be some under-registration of stillbirths due to the ways in which stillbirths were defined and handled in practice [Citation29,Citation40]. During the early nineteenth century, legislation required that infants be baptised within eight days afterbirth [Citation35], and the baptism was perceived as the “salvation” of the individual [Citation40]. In such cases, this would have caused some stillbirths to be reported as “live at birth” (i.e. there might be an over-registration of neonatal deaths). In Sweden, Woods found an increased number of stillbirths by the mid-nineteenth century, which would confirm an improvement in the system of registration [Citation29]. Therefore, we would expect greater accuracy for stillbirths in the latter half of the nineteenth century.
Statistical methods
Due to the small numbers of stillborn children per month, we categorised months into season of birth as Winter: December–February, Spring: March–May, Summer: June–August, and Autumn: September–November.
The association of seasonality with stillbirths and neonatal mortality was calculated using logistic regression models, with season of birth, sex, and parity as the explanatory variables. Parity was categorised as “1”, “2 or 3”, or “4 or more”. All analyses were conducted stratified by ethnicity. Odds ratios were calculated with 95% confidence intervals.
Results
Stillbirth and neonatal mortality rates, 1800–1899
In , births counts are presented by ethnicity and month of birth. Generally, for both populations, births were over-represented during January–March.
Table 2. Number of live births according to month of birth, 1800–1899
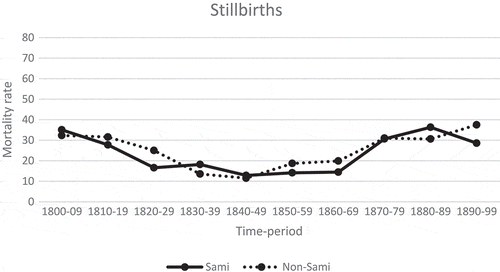
At the beginning of the century, the stillbirth rate among the Sami was 35 deaths per 1,000 live births, and for non-Sami 32 deaths per 1,000 live births (). The stillbirths for both populations followed a U-shaped pattern, with a minimum between 1820 and 1869, thereafter an increasing trend. Overall, the stillbirth rates for the Sami and the non-Sami population were similar to each other during the entire century. At the end of the nineteenth century, the stillbirth rate was similar to that at the beginning of the century (38 and 32, respectively).
Figure 2. Stillbirth mortality rate per 1,000 total births by time-period, Sami and non-Sami population, 1800–1899
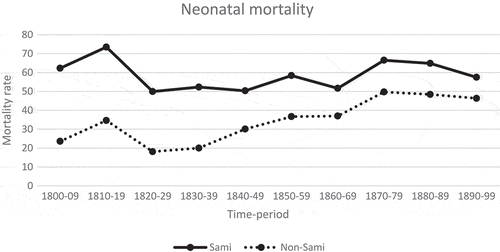
At the beginning of the nineteenth century, neonatal mortality rate among the Sami population was high, with 61 deaths per 1,000 live births (). Sami neonatal mortality was lowest between 1820 and 1870, and at the end of the century the neonatal rate ending at 57 deaths per 1,000 live births. For the non-Sami population, the neonatal mortality was 20/1,000 at the beginning of the nineteenth century followed by an increase during the second decade (31/1,000), and then reaching its lowest rate between 1820 and 1829 (18/1,000). From 1830 until 1870, the non-Sami neonatal mortality rate increased, reaching its highest level during the 1870s (50/1,000) followed by a minor decrease until the end of the century (46/1,000). During the entire century, the Sami had higher neonatal mortality rates compared to the non-Sami population. The higher Sami neonatal mortality was especially seen at the beginning of the century; however, from the mid of the century the differences decreased and at the end of the century, the gap in mortality between the two populations had almost closed.
Season of birth, stillbirth, and neonatal mortality, 1800–1899
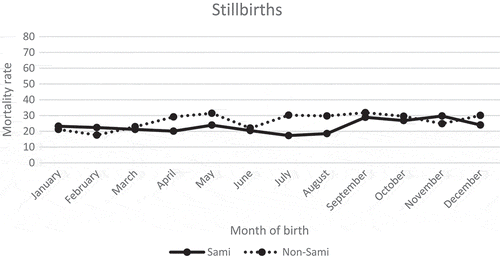
The Sami stillbirth rate was lowest during summer and highest during September–November at about 29/1,000 total births (). For non-Sami stillbirths, the highest risk was found in May (31/1,100) and September (32/1,000), and the lowest risk was among infants born in February (18/1,000). Overall, the stillbirth rate among the Sami and the non-Sami did not reveal a distinct seasonal pattern.
Figure 4. Stillbirth mortality rate per 1,000 total births by month of birth, Sami and non-Sami population, 1800–1899
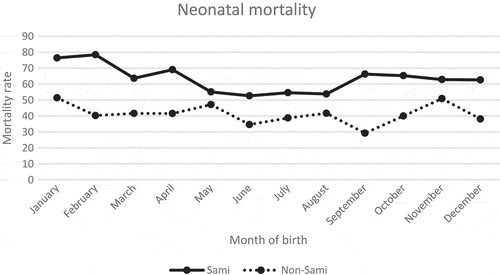
Compared to the stillbirth rate, the Sami population revealed differences in neonatal mortality according to month of birth, where children born in January and February had the highest risk (76 and 77 deaths per 1,000 live births, respectively) (). The lowest neonatal mortality rates were found among children born during the spring and summer months of May–August (between 51 and 53 deaths per 1,000 live births). In more general terms, infants born during the warmer period were at a more advantaged position. For the non-Sami, the highest neonatal mortality risk was among infants born in January and November (51 and 50/1,000, respectively), and there was a decreased risk among those born in September (29/1,000), revealing a flatter pattern compared to the Sami.
A model of season of birth and stillbirth
shows higher stillbirth risk among autumn-born Sami infants compared to summer-born infants (OR 1.51 [95% confidence interval 1.10–2.10]). Among the non-Sami infants, there was no seasonal difference in stillbirth risk. Further, males had a higher risk of stillbirth, and this was true for both the Sami and the non-Sami populations. Regarding parity, as expected, the highest risk was among the firstborn. Among the Sami, the risk of being stillborn was lowest among the high-parity infants (4+), whose odds ratio was 0.5 compared to firstborns.
Table 3. Logistic regression of stillbirths in Sápmi, 1800–1899, presented as odds ratios with 95% confidence intervals
Season of birth and neonatal mortality
Winter- and autumn-born Sami infants had a significant higher neonatal mortality risk, compared to summer-born infants (OR 1.41 and OR 1.24, respectively) (). Like for stillbirths, the Sami males had a greater neonatal mortality risk, and for parity the lowest risk was found among a parity of 2 or 3. For the non-Sami population, reveals no seasonal differences in neonatal mortality risks or differences between the genders. Regarding parity, the highest risk of neonatal mortality was found among the firstborn non-Sami infants.
Table 4. Logistic regression of neonatal mortality in Sápmi, 1800–1899, presented as odds ratios with 95% confidence intervals
Discussion
This paper showed higher neonatal rates among the Sami population compared to the non-Sami population during the entire period (1800–1899). For total Sweden of this entire period, there are data for infant mortality, but not specifically for neonatal mortality. The official statistics from 1860/1866 and 1891/1900 reveal that the neonatal mortality rates (counted as death within in the first 30 days) were 47 and 34 out of 1,000 live births, respectively [Citation41]. The corresponding rates presented here are rather consonant with the national rates; in 1860–1869, neonatal were 52/1,000 for Sami, and 37/1,000 for non-Sami, whereas the rates in 1890–1899 were, 57 and 46 out of 1,000 live births, respectively.
Compared to neonatal mortality, the stillbirth rates for the Sami and non-Sami were closer to each other during the entire century. Both populations had an increasing stillbirth trend from the second half of the nineteenth century onwards. Corresponding rates for rural Sweden during the 1860s were 31,6/1,000 whereas the rates in towns generally were higher 40,4/1,0000 [Citation42]. Compared to Sweden overall, the two northern populations did not follow the decreasing trend in neonatal mortality and the positive shift in health transitions in this northern part were delayed.
This study revealed similar patterns of neonatal and stillbirth rates over time but differences in magnitude, where neonatal mortality rates generally were higher than stillbirth rates. Stillbirth and neonatal mortality rates among non-Sami were closer to each other, while they diverged more among the Sami. The excess in neonatal rate among the Sami indicates that there might have been an under-registration and thus underestimation of stillbirths, while underreporting was presumably lower among non-Sami. In Sweden, stricter reporting of demographic data was introduced in 1860 through the newly formed state authority of Statistic Sweden [Citation42]. Therefore, an underreporting of deaths before 1860 is possible and might explain the increase in neonatal and stillbirth rates after 1860.
This study showed that neonatal mortality among Sami was higher during the winter season, with a peak among infants born in February. A couple of explanations for the seasonal variability in Sami neonatal mortality can be given. First, the traditional nomadic lifestyle should have been especially dangerous for neonates during winter with hazardous conditions both outdoors (extreme cold) and inside the hut (with wood smoke from open fires) [Citation6]. Second, during the nineteenth century, the delivery of infants was still a private matter, especially for Sami, which might have increased the risk of hypothermia, an important contributor to seasonal differences in neonatal mortality [Citation43,Citation44]. Third, winter was a period of high workload for Sami, including pregnant women and mothers with neonates. The months of June–August (with the lowest neonatal mortality), on the other hand, corresponded to the less work-intensive period for these women. For the non-Sami, the neonatal mortality did not reveal as clear a seasonal pattern, although slightly higher mortality rates were observed in January, May, and November. Stillbirths were not associated with season of birth among the non-Sami, whereas autumn-born (September–November) Sami had higher stillbirth risk compared to summer-born infants. In late August, the Sami moved to the autumn settlement where the slaughter of reindeer occurred in September–October, followed by moving to the winter settlement in November. We believe that workload and movements between settlements might have increased stillbirths and neonatal mortality rates among Sami.
Some of the results presented in this study are consistent with previous findings regarding infant mortality in historical populations, although we also found some dissimilarities. Likewise, the studies of Breschi and Livi-Bacci in Italy [Citation23], this study found that the neonatal mortality of Sami was highest among winter-born and lowest among infants born during summer. Several studies on infant mortality in northern and central Italy [Citation23,Citation45] showed U-shaped profiles of neonatal mortality by month (or season of birth), explained as a relationship with low winter temperatures [Citation23,Citation45]. A similar but less pronounced pattern was found among the Sami in this study, with odds ratios of 1.41 for winter-born compared to summer-born infants, whereas the corresponding risk among winter-born infants in some Italian communities was about 4 times the risk of summer-born [Citation16]. From a comparative perspective thus, the neonatal mortality among Sami winter born seems exceptionally low. Contemporary research has found countries with a milder winter climate having the highest effect in winter mortality [Citation46]. One factor that can affect the low winter neonatal mortality among populations in a northern Sweden is the adaptation over time to living in a harsh environment where temperature below 0°C is common from October to April.
In this paper, neonatal mortality was shown to vary by season of birth, but the contribution of weather variations in addition to cultural practices that vary over the year is not known. Factors linking climate and weather conditions to the seasonality of neonatal mortality and stillbirths include the availability of food and the mother’s health and nutritional status during pregnancy [Citation16]. For instance, a poor diet during pregnancy can affect the immune system of the foetus, exposing the newborn to infections of the digestive tract after weaning, especially during warm summers. On the other hand, infants of poorly nourished mothers are prone to be of low birth weight and at a risk of dying from hypothermia, especially during cold winters [Citation47,Citation48]. In Swedish rural communities, food supplies were scarcer in spring and early summer, especially after poor harvests in the previous year, causing a diet that was inferior compared to that of other seasons [Citation16,Citation49]. The years 1867–1869 were associated with extraordinary weather conditions (extreme cold, heavy snowfall and dry weather) that culminated in severe crop failures, followed by increased overall mortality and lower fertility [Citation50]. In this study, we did not observe an increase in stillbirth or neonatal mortality rates during these three years.
Neonatal mortality and stillbirths were major challenges to overcome in this region, and the most significant changes occurred after 1900. Knowledge about mortality patterns among the Sami from 1900 onwards is limited (due to the absence of ethnic information in population registers). In their study of mortality patterns 1961–2000, Hassler et. al [Citation51] revealed similar life expectancies among the Sami and non-Sami population, but we still lack knowledge of when the turning point in Sami infant health and neonatal survival occurred. Forthcoming research will investigate weather and seasonal effects on neonatal mortality over a longer time period as well as geographical differences of vulnerability in this subarctic environment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Moffat T, Herring A. The historical roots of high rates of infant death in aboriginal communities in Canada in the early twentieth century: the case of Fisher River, Manitoba. Soc Sci Med. 1999 Jun;48(12):1821–10.
- MacMillan HL, MacMillan AB, Offord DR, et al. Aboriginal health. CMAJ. 1996 Dec 1;155(11):1569–1578.
- Düben G. Om Lappland och lapparne, företrädesvis de svenske: ethnografiska studier. Stockholm: Norstedt; 1873.
- Karlsson L. Demography of colonisation and the ageing population: population profiles and mortality in Swedish Sapmi, 1750–1900. Ageing Soc. 2012 Jul;32:812–832.
- Karlsson L. Indigenous life expectancy in Sweden 1850–1899: towards a long and healthy life? Demographic Res. 2013 Mar 8;28:433–456.
- Sköld P, Axelsson P, Karlsson L, et al. Infant mortality of Sami and settlers in Northern Sweden: the era of colonization 1750–1900. Glob Health Action. 2011;4:8441.
- Brändström A. Från förebild till motbild. Spädbarnsvård och spädbarnsdödlighet i Jokkmokk. In: Åkerman S, Lundholm K, editors. Älvdal i norr. Människor och resurser i Luledalen 1300–1800. Stockholm: Almqvist & Wiksell; 1990. p. 307–351.
- McEniry M. Infant mortality, season of birth and the health of older Puerto Rican adults. Soc Sci Med. 2011 Mar;72(6):1004–1015.
- Strand LB, Barnett AG, Tong S. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res. 2011 Apr;111(3):451–462.
- Hare EH, Moran PA, Macfarlane A. The changing seasonality of infant deaths in England and Wales 1912–78 and its relation to seasonal temperature. J Epidemiol Community Health. 1981 Jun;35(2):77–82.
- Victora CG, Vaughan JP, Barros FC. The seasonality of infant deaths due to diarrheal and respiratory diseases in southern Brazil, 1974–1978. Bull Pan Am Health Organ. 1985;19(1):29–39.
- Hellstenius J. Barnadödligheten i Vesternorrlands och Jemtlands län. Stat Tidskr. 1884;73:153–168.
- Serning I. Lappbarnen, Deras Vård och Uppfostran i Spädbarnsåldern och Lekåldern. Vol. 1949. Luleå, Sweden: Norrbottens-Kurirens Tryckeri; 1950. p. 55–109.
- Turdfjäll J. Utdrag af Juckasjervi församlings kyrkoböcker och folktabeller, för 50 år, ifrån 1725 till och med 1774, med anmärkningar. In: Kungliga Vetenskaps Academiens Handlingar För År 1779. Stockholm: Johan Georg Lange, XL; 1779. p. 55–62.
- Nordin G, Sköld P. True or false? Nineteenth-century Sápmi fertility in qualitative vs. demographic sources. Hist Family. 2012 Jun 1;17(2):157–177.
- Oris M, Derosas R, Breschi M. Infant and child mortality. Life under pressure: mortality and living standards in Europe and Asia, 1700–1900. Cambridge, Massachusset: MIT Press; 2004. p. 359–398.
- Thorvaldsen G. Was there a European breastfeeding pattern? Hist Fam. 2008;13(3):283–295.
- Rogers J, Brändström A, Edvinsson S. Illegitimacy, infant feeding practices and infant survival in Sweden 1750–1950. Hygiea Internationalis Interdiscip J Hist Public Health. 2002;3(1):13–52.
- Ellmin J. Annual report. District physician of Jämtland. Stockholm: National Archives; 1851.
- Andersson T, Hogberg U, Bergstrom S. Community-based prevention of perinatal deaths: lessons from nineteenth-century Sweden. Int J Epidemiol. 2000 Jun;29(3):542–548.
- Dalla-Zuanna G, Rosina A. An analysis of extremely high nineteenth-century winter neonatal mortality in a local context of Northeastern Italy. Eur J Popul. 2011 Feb;27(1):33–55.
- Breschi M, Derosas R, Manfredini M. Mortality and environment in three Emilian, Tuscan, and Venetian Communities, 1800–1883. In: Bengtsson T, Campbell C, Lee J, editors. Life under pressure: morality and living standards in Europe and Asia, 1700–1900. Cambridge (MA): MIT Press; 2004. p. 209–251.
- Breschi M, Livi-Bacci M. Month of birth as a factor in children’s survival. In: Desjardins B, editor. Infant and child mortality in the past. Oxford: Clarendon Press; 1997. p. 157–173.
- Tymicki K. Correlates of infant and childhood mortality: A theoretical overview and new evidence from the analysis of longitudinal data of the Bejsce (Poland) parish register reconstitution study of the 18th-20th centuries. Demographic Res. 2009 May 26;20:559–594.
- Karlsson L. Indigenous infant mortality by age and season of birth, 1800–1899: did season of birth affect children’s chances for survival? Int J Environ Res Public Health. 2017 Dec 23;15(1):18. doi:10.3390/ijerph15010018.
- Fåhraeus E. Om förhållandet mellan de särskilda månaderna i fråga om befolkningens årliga förändringar. Stockholm: Kongl. Statistiska Centralbyrån; 1865. p. 223–243.
- Eriksson AW, Fellman J. Seasonal variation of livebirths, stillbirths, extramarital births and twin maternities in Switzerland. Twin Res. 2000 Dec;3(4):189–201.
- Reid A. Neonatal mortality and stillbirths in early twentieth century Derbyshire, England. Pop Stud J Demog. 2001 Nov;55(3):213–232.
- Woods R. Death before birth: fetal health and mortality in historical perspective. Oxford: Oxford University Press; 2009.
- Wrigley EA. Poverty, progress, and population. Cambridge: Cambridge University Press; 2004.
- Keller CA, Nugent RP. Seasonal patterns in perinatal mortality and preterm delivery. Am J Epidemiol. 1983 Nov;118(5):689–698.
- Amft A. Sápmi i förändringens tid: en studie av svenska samers levnadsvillkor under 1900-talet ur ett genus- och etnicitetsperspektiv. Umeå: Kulturgräns norr; 2000. (Kulturens frontlinjer, 1402–8506; 20).
- Knodel JE. Demographic behavior in the past: a study of fourteen German village populations in the eighteenth and nineteenth centuries. Cambridge: Cambridge University Press; 1988. (Cambridge studies in population, economy and society in past time, 99-0510337-6; 6).
- Westberg A, Engberg E, Edvinsson S. A unique source for innovative longitudinal research: the POPLINK database. Hist Life Course Stud. 2016;3:20–31.
- Nilsdotter Jeub U. Parish records: 19th century ecclesiastical registers. Umeå: Demografiska databasen, Univ.; 1993. (Robbins J, Umeå universitet. Demografiska d, editors).
- Demographic Data Base [Internet]. Sweden: Umeå Univeristy; [cited 2019 June 17]. Available from: http://umu.se/en/centre-for-demographic-and-ageing-research/databases/.
- Nordin G. Äktenskap i Sápmi: giftermålsmönster och etnisk komplexitet i kolonisationens tidevarv, 1722–1895. Umeå: Institutionen för idé- och samhällsstudier, Umeå universitet; 2009.
- Sköld P, Axelsson P. The northern population development; colonization and mortality in Swedish Sapmi, 1776–1895. Int J Circumpolar Health. 2008 Feb;67(1):27–42.
- Rowland DT. Demographic methods and concepts. Oxford: Oxford University Press; 2003.
- Garðarsdóttir Ó. Saving the child: regional, cultural and social aspects of the infant mortality decline in Iceland, 1770–1920. Umeå: Umeå: Univ.; 2002.
- Statistics Sweden S. Historisk statistik för Sverige.: [Population 1720–1967] D. 1, Befolkning 1720–1967. 2. uppl ed. Örebro; Stockholm: Statistiska centralbyrån SCB; 1969. (Population).
- Woods R. Lying-in and laying-out: fetal health and the contribution of midwifery. B Hist Med. 2007 Win;81(4):730–759.
- Mullany LC. Neonatal hypothermia in low-resource settings. Semin Perinatol. 2010;34(6):426–433.
- Goldsmith JR, Arbeli Y, Stone D. Preventability of neonatal cold injury and its contribution to neonatal mortality. Environ Health Perspect. 1991;94:55–59.
- Breschi M, Derosas R, Manfredini M. Infant mortality in nineteenth-century Italy: interactions between ecology and society. Population and economy. From hunger to modern economic growth. Oxford (UK): Oxford University Press; 2000. p. 457–489.
- Healy JD. Excess winter mortality in Europe: a cross country analysis identifying key risk factors. J Epidemiol Commun H. 2003 Oct;57(10):784–789.
- Derosas R. The joint effect of maternal malnutrition and cold weather on neonatal mortality in nineteenth-century Venice: an assessment of the hypothermia hypothesis. Pop Stud J Demog. 2009;63(3):233–251.
- Scalone F, Samoggia A. Neonatal mortality, cold weather, and socioeconomic status in two northern Italian rural parishes, 1820–1900. Demographic Res. 2018 Sep 19;39:525–560.
- Eastman PR. Infant mortality in relation to month of birth. Am J Public Health Nations Health. 1945 Sep;35(9):913–922.
- Västerbro M. Svälten: hungeråren som formade Sverige. Stockholm: Albert Bonniers förlag; 2018.
- Hassler S, Johansson R, Sjolander P, et al. Causes of death in the Sami population of Sweden, 1961–2000. Int J Epidemiol. 2005 Jun;34(3):623–629.