ABSTRACT
Hepatitis C, caused by the hepatitis C virus (HCV), is a major public health issue in Russia. The aim of our study was to assess the seroprevalence of markers of HCV exposure and factors associated with HCV seropositivity among the general population aged 18–39 years in the city of Arkhangelsk, Northwest Russia. A social research agency applied a quota sampling method to recruit study participants using cell phone numbers. All participants (n = 1243) completed a self-administered questionnaire and provided a blood sample. Sixty-five participants (5.2%, 95% confidence interval [CI] 4.9–5.5) tested positive for HCV IgM+G antibodies, and of these, 55 (84.6%) did not know that they were exposed to HCV. In multivariable logistic regression analysis, HCV seropositivity was significantly associated with older age, a history of injecting drug use, and having ever received a blood transfusion. To reach the goal of the World Health Organisation’s Global Health Sector Strategy on Viral Hepatitis, regional preventive programmes should include measures to reduce injecting drug use as well as scaling up harm-reduction and treatment programs for drug addicts.
Introduction
The prevalence of hepatitis C virus (HCV) infection is high worldwide [Citation1]. HCV is a blood-borne virus which can be transmitted through injecting drug use (IDU), transfusion of infected blood, and reuse or inadequate sterilisation of medical equipment. It can also be transmitted from mother to child and in rare cases during unprotected sex [Citation2]. HCV can cause acute hepatitis, but about 80% of the infected people do not develop symptoms [Citation3]. From 60% to 80% of HCV-infected persons develop chronic hepatitis C, which can be asymptomatic for decades, but may lead to cirrhosis or hepatocellular carcinoma [Citation4]. Globally, more than 71 million people have chronic HCV infection, and about 400,000 people die annually due to HCV infection [Citation3]. Worldwide, the countries with the highest number of HCV cases are China, Pakistan, India, Egypt and Russia [Citation5]. At present, there is no vaccine against HCV [Citation3]. Although new antiviral medicines can cure more than 95% of HCV-infected people, access to diagnostic procedures and treatment is still low on the global scale [Citation6].
In 2016, the World Health Assembly adopted the first Global Health Sector Strategy on Viral Hepatitis, with the global aim of reducing the incidence of HCV infection by 90% and mortality due to HCV infection by 65% by 2030. The World Health Organisation (WHO) proposed 10 core indicators to support the implementation and monitoring of the strategy [Citation7]. The WHO Regional office for Europe assessed the availability of the data for each core indicator in the region and came to the conclusion that current data sources in most European countries are insufficient and that seroprevalence surveys are required to determine the size of the undiagnosed population [Citation8].
HCV infection is an important public health issue in Russia [Citation9]. In 2018, the incidence of reported acute hepatitis C was 1.1 per 100,000 while the incidence of reported chronic hepatitis C was 32.7 per 100,000 () [Citation10]. About 60% of chronic hepatitis C cases are diagnosed among adults aged 20–39 years [Citation11]. Russia has a national strategy for preventing HCV infection. All Russian citizens have the right to treatment for HCV infection free of charge. Moreover, HCV testing is free of charge for blood donors, pregnant women, and recipients of blood transfusions. HCV testing is obligatory for health-care workers and patients who are considered to be at increased risk for viral hepatitis [Citation12]. People who inject drugs are eligible for free of charge treatment in public health-care facilities. The total economic burden of hepatitis C in Russia was estimated to be 48 billion Russian rubles (~0.83 billion US dollars) in 2010 and continues to increase [Citation13].
Figure 1. Incidence of hepatitis C (HC) in Russia and the Arkhangelsk region in 1996–2018 (per 100,000 population)
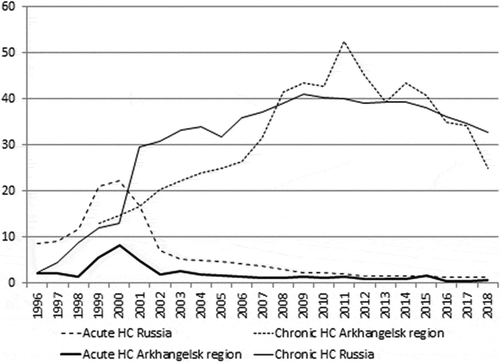
The only study on the seroprevalence of HCV in Russia conducted the last 20 years was published in Russian, and is unavailable to the international audience [Citation14]. This study was conducted in 2008 in five Russian regions (Moscow, Rostov, and Sverdlovsk Regions, as well as the Tyva and Sakha (Yakutia) Republics). All these settings are situated thousands of kilometres away from the Arkhangelsk Region where our study was performed. The overall seroprevalence of HCV among the participants of all ages in the study was 2.6% [Citation14].
The aim of our study was to assess the seroprevalence of markers of HCV exposure and factors associated with HCV seropositivity among adults aged 18–39 years in the city of Arkhangelsk, Northwest Russia. Further, to determine the proportion of that population with undiagnosed HCV infection, thus providing key information to health authorities needed to scale up interventions proposed by the WHO’s Global Health Sector Strategy on Viral Hepatitis in this part of Russia [Citation8].
Materials and methods
Study population and study design
This study was a part of a large Russian-Norwegian project [Citation15], and it drew its study sample from the general population aged 18–39 in the Arctic Russian city of Arkhangelsk using a quota sampling method [Citation16]. Arkhangelsk has a population of approximately 350,000 people, and the official data show that its population has an incidence of acute (1.48 per 100,000 in 2018) and chronic (40.8 per 100,000 in 2018) hepatitis C that is close to the national average. A social research agency recruited participants using cell phone numbers. All participants completed a self-administered questionnaire and provided a blood sample between September 2010 and June 2011. Detailed information on enrolment procedures, questionnaire data, and sample collection has been described elsewhere [Citation15]. Altogether, 1265 adults participated in the study. Twenty-two of them had missing blood test results and were excluded from the analyses. Thus, the final study sample consisted of 1243 participants.
Description of the variables
The participants provided information on demographic factors, socioeconomic factors, smoking status, alcohol use, sexual and preventive behaviour, history of sexually transmitted infections (STIs)/HIV, injecting drug use (IDU), blood donation, blood transfusions, operations, tattoos, and whether they knew anyone with hepatitis C. Participants reported their family income on a scale of 1 to 10, based on how they thought their income compared to the average income in Arkhangelsk. We then categorised these responses as low (1–4), medium (5–7), and high (8–10) income. Having tattoos was dichotomised as “yes” and “no”. The variable “knowing someone with hepatitis C” was divided into “no”, “yes”, and “do not know”. The description of other variables used in this study can be found elsewhere [Citation17].
Serological testing and reporting of test results
Serum samples were sent daily to the research laboratory of the Northern State Medical University (NSMU), where they were centrifuged and frozen at −20°C. Once a month, all untested samples were transported to the laboratory of the Regional STI clinic in Arkhangelsk, where serological tests for HCV IgM+G, were done by enzyme-linked immunosorbent assay, according to the manufacturer’s instructions. A confirmation test was performed in case of positive results on IgM+G. The sample was tested for antibodies to HCV antigens corresponding to the parts of proteins encoded by the structural (core) and non-structural (NS3, NS4, NS5) genome parts. If a participant had positive results for one or both tests (IgM+G or IgM), he/she was considered to have been exposed to HCV and was categorised as seropositive. All reagents were produced by the Vector-Best company, Novosibirsk, Russia.
All participants were asked to provide a contact e-mail address or telephone number so they could be informed on their test results and the results were reported via e-mail or cell phones. The participants with positive test results were referred to a specialist in infectious diseases who reported back that 17 of them complied. Further follow-up and RNA testing of positive participants was not part of this study as these participants were referred to the health service.
Statistical analyses
The direct standardisation technique was applied to provide the age-standardised seroprevalence of markers of HCV infection to the 2010 Arkhangelsk population, based on the 2010 census. Associations between HCV seropositivity and independent variables were studied using crude logistic regression. All associations with p < 0.15 or lower in crude analyses were included in the multivariable model [Citation18]. Crude and adjusted odds ratios with 95% confidence intervals (CIs) were estimated. All analyses were performed using Statistical Package for Social Sciences version 20 (SPSS, Inc., Chicago, IL, USA).
Ethical approval
This study was conducted in accordance with the Helsinki Declaration and approved by the Ethical Committee at the NSMU in the city of Arkhangelsk (Protocol 04/03 from 15 March 2010). The participants were given an invitation letter with information about the study aims, procedures, and a telephone number they could call to obtain their test results. All participants signed a written informed consent form.
Results
The final study sample consisted of 543 men and 700 women (mean age 27.6, standard deviation [SD] = 5.6 years and 27.0, SD = 5.7 years, respectively). Most of the participants had high education (58.9%) and medium income (72.0%). Ever smokers comprised 60.5% of the study sample, and 80.5% of participants reported at least one episode of binge drinking (at least 6 units of alcohol in one occasion) (). Previous HIV testing was reported by 55.4% of men and 73.9% of women. No one reported being diagnosed with HIV infection (data not shown). A history of IDU was reported by 2.7% (n = 34) of the sample, and of those, 14 were seropositive (10 men and 4 women). More than one-fifth of the sample had ever donated blood; 4.7% had ever received a blood transfusion. Among the participants, 12.1% knew someone with hepatitis C, and 16.9% did not know if they knew someone with hepatitis C ().
Table 1. Crude odds ratios (OR) and 95% confidence intervals (CI) for the association between seroprevalence of markers of hepatitis C virus (HCV) exposure and its correlates among 1243 participants aged 18–39 years, 2010–2011, Arkhangelsk, Russia
Laboratory results revealed that 65 participants (5.2%, 95% CI 4.9–5.5) tested positive for HCV IgM+G antibodies, among whom six tested positive for IgM, only. The seroprevalence of markers of HCV exposure was 6.4% (95% CI 4.5–8.9) among men and 4.3% (95% CI 2.9–6.1) among women. Fifty-five of the 65 seropositive participants (84.6%) did not know that they were exposed to HCV; eight (12.3% of seropositive participants) had been previously told by medical workers that they had HCV infection, and two persons (3.1%) did not know whether or not they had ever had HCV infection.
Compared to the group-level census data from Arkhangelsk in the same period, women and younger individuals were slightly overrepresented in our study sample. After direct standardisation by age, the overall seroprevalence of markers of HCV exposure increased to 5.6% (95% CI 5.3–5.9). It increased to 7.1% (95% CI 6.7–7.5) among men, but decreased to 4.2% (95% CI 3.8–4.6) among women (). So, the sex difference in HCV seropositivity became significant after direct age standardisation.
In crude logistic regression, HCV seropositivity was significantly associated with older age, age at sexual debut <18 years, having two or more sexual partners in the last 6 months, having ever received a blood transfusion, having a tattoo, and knowing someone with hepatitis C (). The highest seroprevalence of markers of HCV infection and the strongest association with HCV seropositivity were found among participants with a history of IDU and those with sexual partners who had a history of IDU. In total, 45 persons reported having had a sexual partner with a history of IDU, 10 (22%) of whom had a history of IDU themselves. One hundred four participants reported that they did not know the HCV serostatus of their partners. Marital status, education, income, smoking status, binge drinking, self-reported STIs, and ever having donated blood were not associated with HCV seropositivity. Women had lower odds of being seropositive for HCV than men. In the multivariable logistic regression analysis, HCV seropositivity was significantly associated with older age, history of IDU and history of blood transfusions ().
Table 2. Hepatitis C virus (HCV) seropositivity and correlates by multivariable logistic regression analysis among 1243 persons in a population-based seroepidemiological study of adults aged 18–39 years, 2010–2011, Arkhangelsk (Russia)
Discussion
The seroprevalence of markers of HCV infection among adults aged 18–39 years in the general population in this study was 5.2%. This is twice as high as the seroprevalence observed in Eastern Europe for the general adult population (3.2% in Romania, 2.4% in Latvia, and 2.0% in Slovakia). These countries did not experience economic collapse to the same degree as Russia with a dramatic increase in IDU and cuts in health-care expenditures. At the same time, a seroprevalence of HCV of 5.9% has been reported in Italy which is similar to our findings. Considerable variations in the prevalence of HCV across Europe can be explained by different transmission routes, risk factors and prevention strategies used in different countries [Citation19].
Our data are in a disagreement with data on the seroprevalence of markers of HCV infection among pregnant women in the Arkhangelsk region (0.6% in 2012) [Citation20]. We propose two explanations for this. First, according to the official statistics the incidence of HCV infection is higher in the city of Arkhangelsk than in the rural areas and small towns in the region [Citation10]. Second, data from Europe suggest that pregnant women are not a reliable population to use when trying to estimate the seroprevalence HCV in the general population. Indeed, a systematic review from 2017 reported that the seroprevalence of HCV among pregnant women was several times lower than that in the general population in European countries [Citation19]. Data from our study are close to data from another Russian study published in 2001, which reported a seroprevalence of markers HCV infection of 5.3% among adults aged 25–64 years in Siberia [Citation21]. However, our 30–39-year-old participants had a higher seroprevalence of markers of HCV exposure compared to those aged 20–29 years in Siberia (7.8% versus 3.8%). Data from another Russian study showed a seroprevalence of HCV of 3.1% among 20–29 year-olds and 4.9% in 30–39 year-olds [Citation14]. This pattern may be explained by the high incidence of acute hepatitis C among 15–29 year-olds in 1997–2002 [Citation10]. Our participants were aged 30–39 years in 2010–2011, thus they were 15–29 old in 1997–2002, when the incidence of HCV infection was high due to the IDU epidemic in the country [Citation9]. The highest prevalence of HCV infection have been reported from Central Asia (3.6%) and Eastern Europe (3.3%) followed by Central Sub-Saharan Africa (2.1%) and North Africa/Middle East (1.7%). The prevalence of HCV in Central- and Western Europe, North- and Latin America does not exceed 1% [Citation5].
Globally, the prevalence of anti-HCV antibody is greater among men, but the findings vary between countries. A study among blood donors in China did not find gender differences in the prevalence of anti-HCV antibody [Citation22]. However, the prevalence of HCV infection among men was about twice as high as among women in the USA and Iran [Citation23–Citation25]. In our study, the prevalence of anti-HCV antibody was higher among men compared to women (6.4% vs. 4.3%). This may partly be explained by more IDU among men than among women. Different susceptibilities to the infection, different biological responses on HCV infection in men and women as well as social and behavioural factors were proposed to explain gender differences [Citation26]. The dominant rote of transmission in the community can also partly explain existing gender differences. Further investigation of factors explaining gender differences in the seroprevalence of HCV markers may contribute to the development of gender-specific preventive strategies.
In our study, a history of IDU showed the strongest association with HCV seropositivity. This is in line with the findings from the USA and Western Europe. People with a recent history of IDU comprise 8.5% of all HCV infections globally with the greatest proportions in North America (30.5%), Latin America (22.0%) and Eastern Europe (17.9%). The prevalence of HCV infection among people with a recent history of IDU is 39.2% globally being the highest in Eastern Europe (48.6%), the Caribbean (47.6%) and Latin America (46.4%). Proportion of people with a recent history of IDU among the total population with HCV infection is the highest in North America (30.5%), Latin America (22.0%), Western- (17.9%) and Eastern (17.2%) Europe [Citation27]. In our study, people who reported a history of ever IDU had a HCV seroprevalence of 41.2%. Several studies have shown a seroprevalence of markers of HCV infection up to 80% among individuals with a history of IDU in Russia [Citation28–Citation31]. According to the official data, IDU was responsible for 40% of acute hepatitis C cases in 1997 and for 21.5% of all new cases of acute hepatitis C in 2010 in Russia [Citation32]. In the Arkhangelsk Region, 41.7% (5 out of 12) of registered acute hepatitis C cases were attributed to IDU in 2010 [Citation20].
Drug possession is a crime in Russia; therefore some HCV-infected participants could provide false negative answers on their history of IDU leading to an underestimation of this route of transmission and potentially biasing our associations toward the null. There is no national programme to promote safe IDU in Russia, and despite the effectiveness of opioid substitution therapy and syringe exchange programmes in some countries [Citation33,Citation34], such programmes are neither implemented in the Arkhangelsk region nor in Russia in general. The number of beds in rehabilitation facilities for people with substance abuse disorders has increased in the last decade; however, there is still a lack of specialists in the field of addiction as well as equipment and facilities for preventive and harm-reduction programmes in the region [Citation35].
From 2001 all donated blood has been screened for HCV as a part of the national infection control policy for blood banks [Citation36]. In our analyses, participants with self-reported blood transfusions had a higher risk of HCV seropositivity. According to Order 193 07.05.2003, “About implementation of quarantine of fresh frozen plasma in practice of work of blood service in the Russian Federation”, all blood from donors had to be quarantined for 6 months and could only be used after the donor was tested for a second time. Obviously, it took time to reorganise the blood donation programme. We did not ask our participants to give information on when they received blood transfusions. Unsafe medical procedures have been reported as the most common risk factor for HCV infection in India and China [Citation37,Citation38]. Having a blood transfusion before 1991 is the second most common risk factor for HCV infection in Europe [Citation39]. In the USA, the risk of HCV infection after blood transfusion decreased from 33% in the 1960s to 0.3% in the mid-1990s after the universal screening of blood and donors was implemented [Citation40].
The proportion of cases of acute Hepatitis C attributed to medical procedures decreased from 12.8% in 1997 to 2.8 in 2010 in Russia. The corresponding numbers for Northwest Russia were 24.4% and 2.1% ([Citation32], ).Taking into account that approximately 25% of people naturally clear the infection and 75% remain chronically infected after the exposure to HCV [Citation41] the estimates for the prevalence of chronic HCV infection among our participants would be 5.3% (95% CI 5.0–5.6) among men and 3.2% (95% CI 2.9–3.5) among women. Our results suggest that almost 85% of the participants with markers of exposure to HCV infection had not previously received a formal diagnosis from medical personnel. Taking into account the same routes of transmission, our participants were asked about HIV testing, and no one reported HIV infection. Nevertheless, the HIV epidemic in the Arkhangelsk region is scaling up; the incidence rate has risen from 10.6 to 27.7 per 100,000 during the last 5 years, and IDU is considered to be responsible for at least 40% of all new HIV cases in the region [Citation42]. Many researchers support the idea that Russia urgently needs to implement harm-reduction programmes to prevent blood-borne infections among people who practice IDU [Citation43–Citation45]. Data on needle/syringe distribution is one of the core indicators of the WHO monitoring and evaluation framework for HCV elimination in 2016–2021, but this indicator is not available in most regions of Russia.
Sexual transmission of HCV is possible but occurs seldom while the sexual transmission is a common route of transmission of hepatitis B virus and HIV [Citation46,Citation47]. Our study shows a low risk of sexual transmission of HCV. According to our data, the risk of being HCV seropositive among participants with two or more sexual partners during 6 months prior to the study was the same as for those who knew someone with hepatitis C, although neither of these results were significant. To our opinion, a relatively high rate of HIV testing in the study sample (55.4% of men and 73.9% of women) does not mean a selection of risk groups for our study. According to Russian Sanitation Rules 3.1.5.2826–10 “Prevention of HIV infection” testing for HIV infection is free of charge for all citizens. Moreover all blood donors, pregnant women and their sexual partners are tested for HIV during antenatal screening while military personnel, hospitalized patients before operations, people with Hepatitis B and C and other groups during routine medical check-ups. About 22% of the Russian population is tested for HIV every year [Citation48].
Strengths and weaknesses of the study
Due to the cross-sectional design, a temporal relationship between high-risk behaviour and HCV seropositivity could not be established. Our sampling method cannot rule out selection bias associated with recruitment via cell phone owners. Even though the number of cell phones in Arkhangelsk region was high in 2011 (1890/1000 population, according to information from the Territorial Body of the Federal State Statistics Service of the Arkhangelsk region), generalisation of our study results should be done with caution. Moreover, as we investigated the adult population aged 18–39 years, we cannot generalise our results to the whole adult population of Arkhangelsk. In addition, the study questionnaire contained sensitive questions, which may have caused social desirability bias due to underreporting of high-risk behaviour [Citation49]. However, more people at risk for HCV infection may have chosen to attend our study to avoid the stigmatisation they may have faced at the ordinary clinics. Blood sampling, seen as unpleasant by some, may have been a deterrent to participation [Citation50]. The main strength of our study is its population-based design, including both sexes and presentation of sex-specific prevalence estimates. Other advantages of this study are its sufficient sample size, the use of multivariable statistics and adjustment for the most common potential confounders. We chose to study adults aged 18–39 years because that group is at highest risk for HCV infection [Citation32]. To our knowledge, this study provides the most updated information of HCV seroprevalence and associated factors among young adults in an Arctic Russian urban setting in the international peer-reviewed literature.
Conclusions
We observed a rather high seroprevalence of HCV among the general population aged 18–39 years in Arkhangelsk. Factors associated with HCV seropositivity were included increasing age, IDU, and having received a blood transfusion. HCV screening programmes in population groups considered to be at risk for HCV infection should be expanded, i.e. among persons practicing IDU, persons acquiring tattoos or being subject to medical procedures/blood transfusions abroad in places with unsafe hygienic standard and testing routines. To reach the goal of the World Health Organisation’s Global Health Sector Strategy on Viral Hepatitis in reducing the incidence of and mortality due to HCV infection in the next decade, regional preventive programmes should include measures to reduce IDU as well as harm-reduction and treatment programmes for drug addicts.
Conflict of interest
The authors report no conflicts of interest.
Authors’ contributions
EK, TB, and AMG created the study design, all authors contributed to the research methodology. TB carried out data collection and data entry. EK, AMG, OS, and AS supervised data collection and data entry. TB analysed the data, and EK, AMG, and OS supervised the interpretation of the data. TB drafted the first version of the manuscript, all authors critically reviewed, commented on, and revised the manuscript, as well as approved the final version of the manuscript for submission.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgments
This study is part of the Norwegian-Russian collaboration project “The Governance of HIV/AIDS Prevention in North-West Russia” coordinated by the Norwegian Institute for Urban and Regional Research (NIBR). We thank the responding women and men in Arkhangelsk for their participation in this study. We further thank the research assistant Anna Bogdanova who supervised the data collection. Finally, we thank Trudy Perdrix-Thoma for English language review.
Data availability statement
Due to Russian legal regulation, the study data cannot be made available for public use. However, anonymized original data can be provided on request to experts if there are questions about statistical analyses. Public availability of the data was not among the issues that were cleared with the ethical committee at Northern State Medical University in Arkhangelsk. The authors are not authorized to make the data freely available because there is no informed consent for this from the participants (Federal Law No. 149-FZ on Information, Information Technologies and Data Protection 2006 (Data Protection Act)).
Additional information
Funding
References
- Hanafiah KM, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age–specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–9.
- EASL Clinical Practice. Guidelines. Management of hepatitis C virus infection. J Hepatol. 2014;60:392–420.
- World Health Organization [Internet]. Hepatitis C. Key facts. Geneva (Switzerland): World Health Organization; 2018 July 18 [cited 2018 Dec 10]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c
- Heymann DL. Control of communicable diseases manual. In: An official report of the American public health association. 19th ed. Washington (DC): American Public Health Association; 2008. p. 293–295.
- Blach S, Zeuzem S, Manns M, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. Available from: https://www.thelancet.com/action/showPdf?pii=S2468-1253%2816%2930181-9
- World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017.
- World Health Organization. Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework. Geneva: World Health Organization; 2016.
- Duffell EF, Hedrich D, Mardh O, et al. Towards elimination of hepatitis B and C in European Union and European economic area countries: monitoring the World Health Organization’s global health sector strategy core indicators and scaling up key interventions. Euro Surveill. 2017;22(9):30476.
- Onischenko GG, Zhebrun AB. Viral hepatitis in the Russian Federation. Saint Petersburg: NIIEM Pasteur; 2009. Russian.
- Federal Service for Surveillance on Consumer Right Protection and Human Wellbeing. State report “About sanitary and epidemiological wellbeing of population in the Arkhangelsk region in 2018”. Arkhangelsk (Russian Federation): Federal Service for Surveillance on Consumer Right Protection and Human Wellbeing; 2019. Available from: http://29.rospotrebnadzor.ru/dokumenty/doklad/-/asset_publisher/ZdP5/content/государственныи-доклад-«о-состоянии-санитарно-эпидемиологического-благополучия-населения-в-архангельскои-области-в-2018-году»?redirect=http%3A%2F%2F29.rospotrebnadzor.ru%2Fdokumenty%2Fdoklad%3Fp_p_id%3D101_INSTANCE_ZdP5%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn-1%26p_p_col_count%3D1. Russian.
- Trifonova GF, Levakova IA, Bolsun DD, et al. Acute and chronic hepatitis C in the Russian Federation in 1994–2013. Infect Immun. 2014;4(3):267–274. Russian.
- World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO member states. Geneva (Switzerland); 2013. Available from: http://apps.who.int/iris/bitstream/handle/10665/85397/9789241564632_eng.pdf
- Yakusheckina NA. Define the burden of hepatitis C in the Russian Federation and pharmacoeconomic evaluation of new methods to antiviral therapy [dissertation]. Moscow (Russian Federation): Russian national research medical University named after N. And. Pirogov; 2016. Russian.
- Soboleva NV, Karlsen AA, Kozhanova TV, et al. The prevalence of the hepatitis C virus among the conditionally healthy population of the Russian population. Zhurnal Infectologii. 2017;9(2):56–64. Russian.
- Aasland A, Grønningssæter AB, Mylakhs P, et al. The governance of HIV/AIDS prevention in Northwest Russia: a description of the Norwegian–Russian project. Hum Ecol. 2011;12:49–54. Russian.
- Study.com [Internet]. Study.com; c2003–2019. Quota Sampling: Definition, Method & Examples; [cited 2017 Aug 9]; [about 7 screens]. Available from: http://study.com/academy/lesson/quota-sampling-definition-method-examples.html
- Balaeva T, Grjibovski AM, Sidorenkov O, et al. Seroprevalence and correlates of herpes simplex virus type 2 infection among young adults in Arkhangelsk, Northwest Russia: a population–based cross–sectional study. BMC Infect Dis. 2016;16(1):616.
- Katz MH. Multivariable analysis. A practical guide for clinicians. Cambridge (UK): Cambridge University Press; 1999. Chapter 3, Setting up a multivariable analysis: subjects; p. 60–83.
- Hofstraat SHI, Falla AM, Duffell EF, et al. Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EAA: a systematic review. Epidemiol Infect. 2017;145:2873–2885.
- Annual Report of the Arkhangelsk Office of Federal Service for Surveillance on Consumer Right Protection and Human Wellbeing to the Reference Viral Hepatitis Monitoring Centre of Rospotrebnadzor, 2010–2011. Arkhangelsk (Russian Federation): Arkhangelsk; Arkhangelsk office of the Federal Service for Surveillance of Consumer Rights Protection and Human Wellbeing; 2011. Available at: http://29.rospotrebnadzor.ru/dokumenty/doklad
- Reshetnikov OV, Khryanin AA, Teinina TR, et al. Hepatitis B and C seroprevalence in Novosibirsk, western Siberia. Sex Transm Infect. 2001;77:463.
- Yang S, Jiao D, Liu C, et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses, and Treponem pallidum infections among blood donors at Shiyan, Central China. BMC Infect Dis. 2016;16:531.
- Moezzi M, Imani R, Karimi A, et al. Hepatitis C seroprevalence and risk factors in adult population of Chaharmahal and Bakhtiari province of Iran in 2013. J Clin Diagn Res. 2015;9(10):Lc13–17.
- Hall EW, Rosenberg ES, Sullivan PS. Estimates of state-level chronic hepatitis C virus infection, stratified by race and sex, USA, 2010. BMC Infect Dis. 2018;18:224.
- Facente SN, Grebe E, Burk K, et al. Estimated hepatitis C prevalence and key population sizes in San Francisco: a foundation for elimination. PLoS ONE. 2018;13(4):e0195575.
- Esmaeili A, Mirzazadeh A, Carter GM, et al. Higher incidence of HCV in females compared to males who inject drugs: a systematic review and meta-analysis. J Viral Hepatitis. 2017;24(2):117–127.
- Grebely J, Larney S, Peacock A, et al. Global, regional, and country‐level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114(1):150–166. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/add.14393
- Rhodes T, Platt L, Judd A, et al. Hepatitis C virus infection, HIV co-infection, and associated risk among injecting drug users in Togliatti, Russia. Int J STD AIDS. 2005;16(11):749–753.
- Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–98.
- Rhodes T, Platt L, Maximova S, et al. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101:252–266.
- Abdala N, Carney JM, Durante AJ, et al. Estimating the prevalence of syringe–borne and sexually transmitted diseases among injection drug users in St Petersburg, Russia. Int J STD AIDS. 2003;14:697–703.
- Onischenko GG, Zhebrun AB. Viral hepatitis in the Russian Federation. Saint Petersburg: NIIEM Pasteur; 2011. Russian.
- Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–1988.
- Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction. 2018;113(3):545–563.
- Ministry of Health of the Arkhangelsk Region. State report “About the public health and health organization in the Arkhangelsk region based on the results of activities during 2017”. Arkhangelsk (Russian Federation): Ministry of Health of the Arkhangelsk Region; 2017. Available from: https://www.minzdrav29.ru/ministry/Open_data/Госдоклад%2027.03.2018%20%20для%20размещения.pdf. Russian.
- Ministry of Health of the Russian Federation. Order of the Ministry of Health of the Russian Federation number 364. About Approval of the Procedure of Medical Examination of Donor’s Blood and its Components. Moscow (Russian Federation): Ministry of Health of the Russian Federation; 2001. Russian.
- Jindal N, Bansal R, Grover P, et al. Risk factors and genotypes of HCV infected patients attending tertiary care hospital in North India. Indian J Med Microbiol. 2015;33:189–190.
- Huang Y, Guo N, Yu Q, et al. Risk factors for hepatitis B and C infection among blood donors in five Chinese blood centers. Transfusion. 2015;55(2):388–394.
- Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162.
- HCV epidemiology in the USA [Internet]. Seattlwe (WA): University of Washington; [cited 2018 Dec 11]. Available from: https://www.hepatitisc.uw.edu/go/screening–diagnosis/epidemiology–us/core–concept/all
- Chen SL, Morgan TR. The natural history of Hepatitis C Virus (HCV) infection. Int J Med Sci. 2006;3(2):47–52.
- Belyakov NA, Konovalova NV, Ogurtsova SV, et al. Is a new wave of HIV spread in the northwest of the Russian Federation a threat or the fact? VICH–infekciya I Immunosupressii. 2016;8(1):73–82. Russian.
- Meylakhs P, Aasland A, Gronningseter A. “Until people start dying in droves, no actions will be taken”: perception and experience of HIV-preventive measures among people who inject drugs in Northwestern Russia. Harm Reduct J. 2017;14:33.
- Rhodes T, Sarang A, Vickerman P, et al. Policy resistance to harm reduction for drug users and potential effect of change. BMJ. 2010;341:c3439.
- Cohen J. Late for the epidemic: HIV/AIDS in Eastern Europe. Science. 2010;329(5988):160:162–164.
- Tor J, Llibre JM, Carbonell M, et al. Sexual transmission of hepatitis C virus and its relation with hepatitis B virus and HIV. Br Med J. 1990;301:1130–1133.
- Pala CK, Baggio S, Toan TN, et al. Blood–borne and sexually transmitted infections: a cross–sectional study in a Swiss prison. BMC Infect Dis. 2018;18:539.
- Rospotrebnadzor of Russian Federation. Sanitation rules 3.1.5.2826-10 [Prevention of HIV infection]. Moscow (Russian Federation): Rospotrebnadzor; 2010. Russian.
- Kholmatova KK, Gorbatova MA, Kharkova OA, et al. Cross-sectional studies: planning, sample size, data analysis. Ekologiya Cheloveka (Human Ecology). 2016;2:49–56. Russian.
- Mott DA, Pedersen CA, Doucette WR, et al. A national survey of U.S. pharmacists in 2000: assessing nonresponse bias of a survey methodology. AAPS Pharm Sci. 2001;3:E33.
