ABSTRACT
Recent literature has highlighted the importance of transition from paediatric to adult care for children with chronic conditions. Non-cystic fibrosis bronchiectasis is an important cause of respiratory morbidity in low-income countries and in indigenous children from affluent countries; however, there is little information about adult outcomes of childhood bronchiectasis. We reviewed the clinical course of 31 Alaska Native adults 20–40 years of age from Alaska’s Yukon Kuskokwim Delta with childhood bronchiectasis. In patients with chronic suppurative lung disease, a diagnosis of bronchiectasis was made at a median age of 4.5 years by computerised tomography (68%), bronchogram (26%), and radiographs (6%). The patients had a median of 75 lifetime respiratory ambulatory visits and 4.5 hospitalisations. As children, 6 (19%) experienced developmental delay; as adults 9 (29%) experienced mental illness or handicap. Four (13%) patients were deceased, four (13%) had severe pulmonary impairment in adulthood, 17 (54%) had persistent or intermittent respiratory symptoms, and seven (23%) were asymptomatic. In adulthood, only five were seen by adult pulmonologists and most had no documentation of a bronchiectasis diagnosis. Lack of provider continuity, remote location and co-morbidities can contribute to increased adult morbidity. Improving the transition to adult care starting in adolescence and educating adult providers may improve care of adults with childhood bronchiectasis.
Introduction
Bronchiectasis is a chronic lung condition, characterised by chronic productive cough and repeated lung infections [Citation1,Citation2], which can lead to chronic respiratory insufficiency and early death [Citation3]. As pneumonia incidence decreased in the USA, bronchiectasis became a neglected diseaseCitation[4]; however, non-cystic fibrosis (CF) bronchiectasis is increasingly recognised as a cause of respiratory morbidity in low income and affluent countries [Citation5]. Alaska Native children and other Indigenous children from affluent countries, such as Australia and New Zealand, have a high prevalence of bronchiectasis [Citation6–Citation10]. In Alaska’s Yukon Kuskokwim (YK) Delta, one in 63 children born during the 1940s through the 1980s was diagnosed with bronchiectasis [Citation7].
There is little longitudinal information on the course of childhood non-CF bronchiectasis as children progress into adulthood since follow-up studies were published in the 1960s and 1970s [Citation3,Citation11]. These reports described clinical improvement indicated by lack of disease progression and decreased frequency and severity of symptoms into the third or fourth decade for many, but progression of disease in some. Indigenous Australian and New Zealand adults with bronchiectasis have a median age of respiratory-related death (48.8 years, IQR 36.4, 52.9) 20 years lower than for non-Indigenous adults with bronchiectasis (69.6 years, IQR 64.2, 75.1) [Citation12]. Kinghorn et al. recently completed a follow-up evaluation of 34 YK Delta adolescents with childhood bronchiectasis or chronic suppurative lung disease (CSLD) [Citation13]. The authors found that, while respiratory exacerbations decreased in frequency throughout childhood, nearly 80% of adolescents were symptomatic with chronic productive cough and/or recurrent wheeze, and 25% had abnormal pulmonary findings on physical examination.
Recent literature highlights the importance of successful transition to adult care for adolescents with chronic disease [Citation14,Citation15]. Information on the clinical course in transition from adolescent to adult care could guide paediatricians and adult physicians to find ways to reduce disease exacerbations and progression during transition of children with bronchiectasis into adult care. Infants and children in YK Delta have an extremely high incidence of respiratory infections and hospitalisations [Citation16,Citation17], and YK Delta children have a high prevalence of chronic productive cough with and without wheezing [Citation18]. Alaska Native adults have a high incidence of acute lower respiratory tract infection (LRTI), especially among adults with chronic lung disease [Citation19]. Recent guidelines highlight that many adults diagnosed with asthma or chronic obstructive pulmonary disease (COPD) also have unidentified bronchiectasis [Citation20]. We evaluated the clinical course, current diagnoses, morbidity, and mortality in 20–40-year-old YK Delta adults who were diagnosed with childhood bronchiectasis to identify areas for improved management.
Materials and methods
Subjects and setting – study populations
The subjects for this study were YK Delta adults born 1/1/1977-12/31/1997 (20–40 years of age on 12/31/2017) with a documented history of childhood bronchiectasis. Paediatricians at YK Delta Regional Hospital (YKDRH) have kept a paediatric chronic disease registry since the 1970s. We identified YK Delta adults born 1/1/1977-12/31/1997 from this registry who had a diagnosis of bronchiectasis before 18 years of age. During this time period, there were approximately 150 patients from YK Delta with at least one visit diagnosis of ‘bronchiectasis”[Citation13].
Alaska’s YK Delta region covers 75,000 square miles of coastal wetlands and tundra. The population is approximately 25,000 and comprised primarily of Yup’ik Eskimos who live in 52 villages and the regional town. Healthcare is provided through YKDRH, its ambulatory clinics and community health aides at village clinics. Household crowding, lack of running water in 30% of villages, and indoor air pollution from woodstove use contribute to the high incidence of childhood pneumonia [Citation21,Citation22]. There is no road system in YK Delta and access to care at YKDRH is difficult because travel to the regional centre usually requires aircraft.
Approval
We received approval from the Alaska Area Institutional Review Board and tribal approval from the YK Health Corporation, and Alaska Native Tribal Health Consortium (ANTHC) Executive Boards. We obtained a waiver of informed consent from the YKDRH and ANMC privacy officers.
Definitions
Visit and hospitalisation respiratory diagnoses were defined as diagnoses describing LRTIs or lower airway disease: including pneumonia, bronchiolitis, bronchitis, bronchiectasis, chronic lung disease, cough, asthma, reactive airway disease, wheezing, or COPD.
Evaluations
Clinical evaluation
We reviewed problem lists, ambulatory clinic visit notes and diagnoses, hospitalisation discharge summaries, and radiologist reports during 1977–2018, available in the hard chart, or electronic medical record (EMR) from YKDRH, YK Delta village clinics, and the referral hospital, Alaska Native Medical Centre (ANMC), in Anchorage. The EMR was upgraded from the Resource and Patient Management System (RPMS) to Cerner at YKDRH (2013) and ANMC (2011). We captured clinical exam findings, discharge diagnoses, antibiotic prescriptions, and radiographs. Many respiratory ambulatory visits occurred in village clinics. Those records were not routinely entered into the YKDRH medical chart or EMR until 1990 so some early ambulatory visits are missing for the 18 patients born in the 1970s-1980s.
Radiology
We documented bronchogram and chest high-resolution CT (HRCT) results from radiology reports. Bronchography was the imaging modality used to diagnose bronchiectasis in the 1970s through 1989. One surgeon performed all of the bronchograms at ANMC [Citation6]. HRCT was used for bronchiectasis diagnosis after 1989. In general, HRCT was performed at YKDRH or ANMC in patients evaluated and referred by a paediatric pulmonologist.
Statistical methods
This is an observational study of the clinical findings and outcomes for YK Delta adults, 20–40 years of age who had radiographically confirmed bronchiectasis as young children. We determined the age at which subjects were first treated for a lower respiratory infection, as well as the age at which they first received a bronchiectasis diagnosis. We examined distributions of respiratory visits by age group and tested differences between numbers of visits during ages 0–5, 12–19, and 20–29 using Wilcoxon Rank Sum test. Ambulatory visits were ranked by type of respiratory diagnosis throughout patients’ lifetime, before age 5 years, and 20 years of age and older. We also calculated frequencies of comorbidities.
Results
Early history and bronchiectasis diagnosis
We reviewed the medical records of 31 patients born 1/1/1977-12/31/1997 who met the study criteria and reviewed visits through 12/31/2017. The median age on 12/31/2017 was 30 years of age (range 20–40 years). All patients had been serially evaluated by a paediatric pulmonologist during childhood; 8 (26%) were diagnosed by bronchogram, 21 (69%) were diagnosed by HRCT, and two (6%) by clinical findings and chest radiographs. The median age for first bronchiectasis diagnosis was 4.5 years of age.
All patients had an early childhood pneumonia or recurrent cases of pneumonia and LRTIs preceding bronchiectasis diagnosis. The median age of first ambulatory LRTI was 7.2 months for patients born 1977–1989 (when many village visits were missing from the EMR) and 2.9 months for patients born 1990–1997 (overall median age of 3.9 months). The median age of first respiratory hospitalisation (n = 29) was 9 months of age, range <1 month to 11 years of age. Thirteen (42%) had one or more invasive Haemophilus influenzae or Streptococcus pneumoniae bacterial cases of pneumonia and/or sepsis in early childhood, two had documented respiratory syncytial virus (RSV) pneumonia and one had a severe adenovirus pneumonia prior to bronchiectasis diagnosis.
Ambulatory visits and hospitalisations
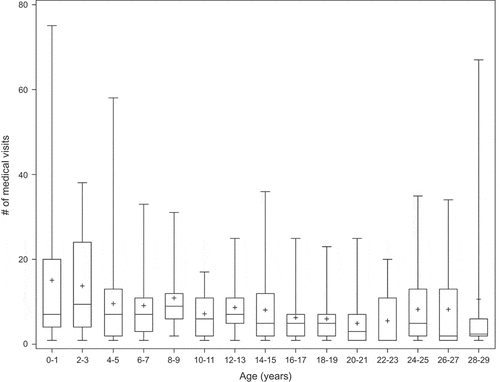
We identified 3168 ambulatory visits with respiratory diagnoses among the 31 patients (median 75 visits/patient, range 18–325) () during a median of 23.6 years (min. 14.3, max 35.5). These included 719 ambulatory visits in adults 20 years and older (median eight visits during 22–29 years of age). There were 242 respiratory hospitalisations among the 31 patients (median 4.5 hospitalisations/patient, range 0–39) (), including 51 hospitalisations in adults 20 years and older.
Table 1. Top five ambulatory clinic diagnoses in respiratory visits in childhood (<5 years old) and adulthood (≥20 years old) for Yukon Kuskokwim Delta adults 20–40 years of age with childhood bronchiectasis
Table 2. Top hospital discharge diagnoses in respiratory hospitalisations in childhood (<5 years old) and adulthood (≥20 years old) for Yukon Kuskokwim Delta adults with childhood bronchiectasis
During early childhood, <5 years of age, there was a median of 3 ambulatory respiratory visits in patients born 1997–1989 and 9 ambulatory respiratory visits in patients born during 1990–1997. The most common diagnoses in ambulatory visits at <5 years of age were asthma/wheezing (22%), pneumonia (17%) and chronic lung disease (12%) (). The most common respiratory discharge diagnoses in children hospitalised <5 years were pneumonia (27%), asthma (13%), and bronchiolitis (10%) ().
In contrast, during adulthood at ages 20 years and older, the most common ambulatory diagnoses were bronchiectasis (23%), asthma (21%), cough (10%), and bronchitis (8%); ). Although bronchiectasis was the most common ambulatory diagnosis in adulthood, one patient accounted for 168/238 (70%) of the bronchiectasis codes. Among respiratory hospitalisations in adults 20 yrs and older, the most common diagnoses were bronchiectasis (40%), pneumonia (26%); asthma (9%) (). One patient accounted for 43% of adult respiratory hospitalisations. Removing this patient, there were 56 total hospital diagnosis codes. Seventeen (30%) of diagnosis codes were bronchiectasis, 16 (29% of codes) were pneumonia, and 8 (14% of codes) were asthma.
Early childhood ambulatory visits are under-reported for our cohort since medical records for subjects born in the 1970s through the early 1980s do not include village clinic visits. Despite this, patients in our cohort had a significantly higher number of respiratory ambulatory visits at <5 years of age compared to ages 12 through 19 years (p = 0.03) or 20 through 29 years (p = 0.01). However, visit rates by year were similar for teen (12–19 years) and adult (20–29 years) age groups ().
Contributing childhood medical conditions
Underlying childhood medical conditions were identified in 16 (52%) children (). Six (19%) children had congenital heart disease, including 3 who required surgery for ventricular septal defects. Four (13%) had childhood scoliosis; two had congenital scoliosis associated with congenital anomalies, two developed scoliosis during later childhood. Four (13%) were born prematurely (<37 weeks). Six (19%) were diagnosed with developmental delay including foetal alcohol syndrome (FAS), and three had growth failure or failure to thrive during childhood. Children were routinely screened for tuberculosis, cystic fibrosis, and for immune deficiencies when appropriate. The most common childhood co-morbidity was lower airway disease – 27 (87%) had asthma or “reactive airway disease” during childhood initially diagnosed at a median age of 2.5 years.
Table 3. Underlying childhood medical conditions and adult co-morbidities in Yukon Kuskokwim Delta adults 20–40 years of age with childhood bronchiectasis
Adult comorbidities
Nineteen of the subjects alive as adults, 19/29 (66%) had an asthma diagnosis at a medical visit during adulthood, most without accompanying pulmonary function testing. All but one of these had their first asthma diagnosis in childhood. Thirteen percent of adults (n = 4) were diagnosed with COPD (median age 21.5 years, range 11–31 years). Two were initially diagnosed with COPD before age 18 years and retained the diagnosis during adulthood. Three adults were diagnosed with cor pulmonale (median age 15 years). Twenty-four (83%) of adult 20 years and older used tobacco, 18 (62%) had alcohol abuse or misuse documented in medical visits, and 9 (31% were treated for mental illness or intellectual disability ().
Outcomes
Four of the 31 patients (13%) were deceased (). Two died in childhood (accident and suicide), while two died in adulthood related to pulmonary decompensation. Four of the 31 patients (13%) had severe pulmonary impairment in adulthood, dependent on family or in assisted living and requiring frequent ambulatory visits and/or hospitalisations. Seventeen (54%) had persistent or intermittent respiratory symptoms, and seven (23%) were asymptomatic.
Table 4. Outcome of Yukon Kuskokwim Delta adults 20–40 years of age with childhood bronchiectasis
One case illustrates the course of a patient with severe progressive bronchiectasis. This patient was born with limb anomalies, scoliosis and a ventricular septal defect. She experienced her first pneumonia at age 2 months and had recurrent cases of pneumonia (including pneumococcal pneumonia) and persistent infiltrates during childhood. She was diagnosed with multi-lobar bilateral bronchiectasis on bronchogram in childhood. She had progressive diffuse bronchiectasis throughout adolescence and early adulthood. During early adulthood, she experienced worsening cough, wheeze and dyspnoea and was diagnosed with mixed obstructive and restrictive lung disease, cor pulmonale and pseudomonas endobronchial colonisation. She died of respiratory complications in her early 30s.
Linkage to adult care
Of the 29 patients surviving to adulthood, 18 (62%) patients had no mention of bronchiectasis in the medical visits or hospitalisations at or after 20 years of age although 21 (72%) remained symptomatic, and only four patients had mention of bronchiectasis at a visit after 12/31/15. The four patients with bronchiectasis diagnosis throughout adulthood had severe disease. Only five patients were seen by an adult pulmonologist, and most patients had no ongoing regular medical follow-up.
One case illustrates difficulties in transition from adolescent to adult medical care. Starting in infancy, this patient was hospitalised with recurrent bacterial cases of pneumonia. Her childhood was complicated by documented FAS, mental handicap, parental neglect and poor adherence to medications. She was diagnosed with bronchiectasis by HRCT in childhood and was treated for multiple bronchiectasis exacerbations in adolescence; however, after age 21 years, bronchiectasis was not mentioned at visits, and she was diagnosed with COPD and asthma. In early adulthood, she had a HRCT which showed bronchiectasis and underwent a new workup for bronchiectasis aetiology.
Discussion
Our retrospective study confirms the high incidence of morbidity and mortality in young Alaska Native adults with non-CF childhood bronchiectasis and the precarious nature of the transition from adolescent to adult care. Of the original 31 patients, 13% were deceased, 13% had severe pulmonary impairment and were dependent on family or assisted living, and an additional 35% had persistent pulmonary symptoms. Our study extends the findings by Kinghorn et al. Citation[13] who found persistent respiratory symptoms, especially wheezing, in a majority of Alaska Native adolescents with a history of childhood bronchiectasis.
Lower airway conditions such as wheezing and bronchitis were the most common respiratory presentations in our adult populations. Asthma, and recurrent wheezing that does not meet asthma criteria, have both been associated with idiopathic bronchiectasis in children and adults [Citation3,Citation4,Citation23]. However, diagnosis of asthma without comprehensive evaluation may obscure the underlying diagnosis of bronchiectasis [Citation20,Citation24]. Patients 20 years and older in our cohort who presented with wheezing were frequently diagnosed with asthma without mention of the patient’s history of bronchiectasis. Persistent asthma symptoms may be associated with higher morbidity in adults. In a 1961 follow-up of adults with childhood bronchiectasis, Field identified a small group in which diffuse bronchiectasis with persistent moderately severe asthmatic symptoms which were associated with more severe disease, cor pulmonale and death [Citation3].
Our study highlights challenges with successful transition to adult care in children with chronic medical conditions. Two-thirds of patients did not have any documentation of bronchiectasis in their medical record after the transition to adult care, only five patients were seen by an adult pulmonologist, and only four patients had ongoing regular medical care. Childhood diagnoses of bronchiectasis were often not incorporated into adult Problem Lists in the new EMRs. A new provider seeing an adult patient would frequently find no bronchiectasis diagnosis in recent EMR visit records, and the adult patient may not have recalled his/her childhood diagnosis of bronchiectasis. This resulted in inadequate treatment of bronchiectasis exacerbations, such as analgesics without antibiotics for “bronchitis” with chest pain. In one patient a new work-up for presumed “adult-onset” bronchiectasis was initiated.
The individual health and financial burdens of bronchiectasis particularly in minority and populations with low-income have been well documented [Citation12,Citation25]. In addition to new workups, the frequent and recurrent ambulatory visits and hospitalisations for unresolved symptoms and recurrent exacerbations led us to explore the financial burden experienced by this group of patients. The medical costs for respiratory illness experienced by this small group of 31 patients were very high and highlight the importance of optimising care. We estimated the cost of medical and transport for the 3168 ambulatory visits and 242 hospitalisations documented in this cohort to be $8,630,000 at 2018 health care rates.
As with other chronic diseases [Citation15,Citation26], the morbidity of over half our patients was strongly influenced by mental illness, intellectual disability, child neglect or abuse, and adult tobacco use and substance abuse, as well as a high rate of medical co-morbidities such as congenital heart disease, prematurity and scoliosis. In our study, seven children suffered neglect and six children had developmental delay (including three with FAS). This data is consistent with other studies of childhood bronchiectasis [Citation7], and comorbidities such as mental disorders can affect patient wellbeing and affect adult outcomes of childhood chronic lung disease and tuberculosis [Citation27–Citation29]. In their cohort of 65 Australian children with bronchiectasis, Chang et al. found that 66-74% had failure to thrive and another 17% of children had growth failure [Citation30].
In low-income countries, as well as Indigenous populations in Alaska, Australia and New Zealand the risk factors for childhood bronchiectasis are primarily pneumonia and related to environmental factors (i.e. household crowding, limited access to a running water, indoor wood smoke) [Citation8,Citation21,Citation31,Citation32]. These sociodemographic conditions are experienced daily by a sizeable portion of the developing world, where 2017 World Health Organisation data indicate that pneumonia accounted for 15% of deaths of children less than 5 years of age [Citation33]. Edwards et al used a deprivation score in a New Zealand study to quantify the role of socio-economic status (SES) in outcomes of bronchiectasis and found that lower SES in combination with other factors contributes to limitations in access to care [Citation10]. Similarly, in an Australian study by Chang et al. [Citation30] environmental factors including limited access to healthcare, organic fuel and poor living conditions were a common theme within that group. Household crowding, lack of running water, difficult access to care due to isolation of villages, wood fuel-based heating in small inadequately ventilated and crowded homes, food insecurity posed by remoteness and isolation of the villages and poverty have been associated with a higher incidence of acute and chronic respiratory conditions [Citation7,Citation22,Citation30,Citation34]. In our patient cohort born prior to Hib (1991) and pneumococcal conjugate vaccines (2001), bacterial pneumonia caused by Hib or Streptococcus pneumoniae often preceded bronchiectasis. Adenovirus pneumonia was also identified in one patient. While control of vaccine-preventable disease has contributed to an apparent decrease in bronchiectasis in this population[Citation13], disparities in environmental conditions and prematurity contribute to ongoing morbidity from pneumonia and non-CF bronchiectasis among indigenous children from Alaska, Australia and New Zealand [Citation8,Citation12,Citation13,Citation30,Citation31].
Our cohort highlights the challenges that occur when paediatric patients with chronic medical conditions transition to adult care. The American Academy of Paediatrics recommends that discussion of transition to adult care begins as early as 12 years of age with the goal of completing integration into adult care by 26 years of age [Citation35]. The outcomes of an effective paediatric transition to care include consistent follow-up and decreased hospitalisations[Citation14]. Unlike non-CF bronchiectasis, CF is a well-recognised chronic clinical condition, and patients with CF receive multidisciplinary specialist care from an early age and transition within this framework. This approach has contributed to the increased survival, to nearly 40 years and longer for half of the patients with CF; although, even for CF there is an information gap with respect to psychological models that can help guide the transition processes [Citation26]. In contrast, non-CF bronchiectasis in childhood remains a neglected disease in adulthood despite evidence of continued respiratory morbidity [Citation36].
Adolescence is a vulnerable period particularly for those with chronic health conditions. Sadun et al. recently highlighted how transition issues contributed to the death of a young adult with sarcoidosis[Citation15]. In our cohort, these difficulties with transition contributed to lack of recognition by adult physicians of an underlying bronchiectasis condition leading to inadequate treatment and deviation from the standard bronchiectasis care in our study patients [Citation20]. The unique challenges of access to primary and tertiary care amplify the inherent issues that exist when patients, especially those with chronic disease go between practices. The experience in Alaska highlights the need and opportunity that exists for the development of a multidisciplinary approach to transitioning care.
Limitations
There are several limitations in our study. The study is a retrospective review with a sample that was obtained from a registry and may not be representative of the population as a whole. The sample is small which creates a challenge in generalisability. The registry was created and maintained by paediatricians at YKDRH and they did not have access to early records or records from other facilities where patients were treated. Many village respiratory visits before 1990 were unavailable.
Conclusion
Our study evaluated adult outcomes of childhood bronchiectasis. Lack of provider continuity, remote location of patients and co-morbidities all contribute to poor transition into adult care and increased adult morbidity. A transition of care during adolescence with a gradual increase in patient responsibility, identification of patients in a bronchiectasis registry, education of adult primary care providers and adherence to adult bronchiectasis guidelines [Citation20] may improve medical care and outcomes of adults with non-CF childhood bronchiectasis.
Disclaimer
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the tribal organizations.
Acknowledgments
The authors wish to thank the people of the Yukon Kuskokwim Delta and the Yukon Kuskokwim Health Corporation for supporting this study. We dedicate this work to Alaska Native persons with bronchiectasis and their families. We would like to thank the dozens of people who donated time and collaboration to help make this study possible, including Leslie Herrmann, Mien Chyi, Jane McClure, Cynthia Mondesir, Jennifer Hampton, Tammy Alderdice, Lori Chikoyak, Bessie Francis, Elizabeth Sanseau and others from Yukon Kuskokwim Health Corporation; Matthew Hirschfeld, Amy Schumacher, Christine Tan Cadogan, and others from Southcentral Foundation; Daniela Lammers, Amy Wilson, Timothy Thomas from Alaska Native Tribal Health Consortium, and Jason Debley, BreAnna Kinghorn, Lucas Hoffman and others from Seattle Children’s Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barker AF, Bardana EJ Jr. Bronchiectasis: update of an orphan disease. Am Rev Respir Dis. 1988;137(4):969–8.
- Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383–1393.
- Field CE. Bronchiectasis: A long-term follow-up of medical and surgical cases from childhood. Arch Dis Child. 1961;36:587–603.
- Callahan CW, Redding GJ. Bronchiectasis in children: orphan disease or persistent problem? Pediatr Pulmonol. 2002;33(6):492–496.
- Goyal V, Grimwood K, Marchant J, et al. Pediatric bronchiectasis: no longer an orphan disease. Pediatr Pulmonol. 2016;51(5):450–469.
- Fleshman KJ, Wilson JF, Cohen JJ. Bronchiectasis in Alaska Native children. Arch Environ Health. 1968;17(4):517–524.
- Singleton R, Morris A, Redding G, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatr Pulmonol. 2000;29(3):182–187.
- Singleton RJ, Valery PC, Morris P, et al. Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol. 2014;49(2):189–200. ePub Date 2013/02/13.
- Chang AB, Grimwood K, Mulholland EK, et al. Working group on indigenous paediatric Respiratory H. Bronchiectasis in indigenous children in remote Australian communities. Med J Aust. 2002;177(4):200–204.
- Edwards EA, Asher MI, Byrnes CA. Paediatric bronchiectasis in the twenty-first century: experience of a tertiary children’s hospital in New Zealand. J Paediatr Child Health. 2003;39(2):111–117.
- Landau LI, Phelan PD, Williams HE. Ventilatory mechanics in patients with bronchiectasis starting in childhood. Thorax. 1974;29(3):304–312.
- Blackall SR, Hong JB, King P, et al. Bronchiectasis in indigenous and non-indigenous residents of Australia and New Zealand. Respirology. 2018;23(8):743–749.
- Kinghorn B, Singleton R, McCallum GB, et al. Clinical course of chronic suppurative lung disease and bronchiectasis in Alaska Native children. Pediatr Pulmonol. 2018;53(12):1662–1669.
- White PH, Cooley WC. Transitions Clinical Report Authoring G, American Academy of P, American Academy of Family P, American College of P. Supporting the Health Care Transition From Adolescence to Adulthood in the Medical Home. Pediatrics. 2018;142(5):e20182587.
- Sadun RE, Chung RJ, Maslow GR. Transition to adult health care and the need for a Pregnant Pause. Pediatrics. 2019;143(5):e20190541.
- Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska Native children. J Infect Dis. 1999;180(1):41–49.
- Foote EM, Singleton RJ, Holman RC, et al. Lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general USA child population. Int J Circumpolar Health. 2015;74:29256.
- Lewis TC, Stout JW, Martinez P, et al. Prevalence of asthma and chronic respiratory symptoms among Alaska Native children. Chest. 2004;125(5):1665–1673.
- Gounder PP, Holman RC, Seeman SM, et al. Infectious disease hospitalizations among American Indian/Alaska Native and Non-American Indian/Alaska native persons in Alaska, 2010-2011. Public Health Rep. 2017;132(1):65–75.
- Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1–69.
- Bulkow LR, Singleton RJ, Debyle C, et al. Risk factors for hospitalization with lower respiratory tract infections in children in rural alaska. Pediatrics. 2012;129(5):e1220–1227.
- Hennessy TW, Ritter T, Holman RC, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health. 2008;98(11):2072–2078.
- Karadag B, Karakoc F, Ersu R, et al. Non-cystic-fibrosis bronchiectasis in children: a persisting problem in developing countries. Respiration. 2005;72(3):233–238.
- LindenSmith J, Morrison D, Deveau C, et al. Overdiagnosis of asthma in the community. Can Respir J. 2004;11(2):111–116.
- Goeminne PC, Scheers H, Decraene A, et al. Risk factors for morbidity and death in non-cystic fibrosis bronchiectasis: a retrospective cross-sectional analysis of CT diagnosed bronchiectatic patients. Respir Res. 2012;13:21.
- Kreindler JL, Miller VA. Cystic fibrosis: addressing the transition from pediatric to adult-oriented health care. Patient Prefer Adherence. 2013;7:1221–1226.
- Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE. Functional status and well-being of patients with chronic conditions. Results from the medical outcomes study. JAMA. 1989;262(7):907–913.
- Turkel S, Pao M. Late consequences of chronic pediatric illness. Psychiatr Clin North Am. 2007;30(4):819–835.
- Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(10):1129–1137.
- Chang AB, Masel JP, Boyce NC, et al. Non-CF bronchiectasis: clinical and HRCT evaluation. Pediatr Pulmonol. 2003;35(6):477–483.
- Munro KA, Singleton RJ, Edwards EA, et al. Burden of bronchiectasis in indigenous peoples - How can it be improved? Curr Pediatr Rev. 2009;5(4):198–206.
- Robin LF, Less PJ, Winget M, et al. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr Infect Dis J. 1996;15(10):859–865.
- Organization WH. Pneumonia Fact Sheet. 2019; [updated 2019 Mar 10] Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia
- Singleton R, Salkoski AJ, Bulkow L, et al. Housing characteristics and indoor air quality in households of Alaska Native children with chronic lung conditions. Indoor Air. 2017;27(2):478–486.
- The National Alliance to Advance Adolescent Health. Got transition: health care providers. Available from: https://www.gottransition.org/providers/index.cfm
- Prentice BJ, Wales S, Doumit M, et al. Children with bronchiectasis have poorer lung function than those with cystic fibrosis and do not receive the same standard of care. Pediatr Pulmonol. 2019;54(12):1921–1926.