ABSTRACT
Streptococcus pneumoniae is one of the main pathogens leading to otitis media. In 2010, the 13-valent pneumococcal conjugate vaccine (PCV13) was implemented in the Greenlandic childhood vaccination programme, but the effect of this change is not yet well documented. The objective of this study is to evaluate the effect of the implementation based on the number of episodes of acute otitis media (AOM). Data are obtained from medical records. We included all children born from 1 January 2015 to 30 September 2016, and thus eligible for the three doses of PCV13 including one year of follow-up time. Exclusion criteria were uncertain vaccination status and predefined comorbidities. The children were divided into two groups based on vaccination status: “Vaccinated” or “Incomplete/delayed”. We included 1077 children in total, 742 children were allocated to the ”Vaccinated” group and 335 children were allocated to the “Incomplete/delayed” group. There were significantly fewer episodes of AOM in the “Incomplete/delayed” group (p = 0.01). In conclusion Greenlandic children completely and timely vaccinated with PCV13 and born between January 2015 and September 2016 did not have fewer episodes of AOM compared to children who had incomplete or delayed vaccination status.
Abbreviations: PCV13: 13-valent pneumococcal conjugate vaccine; OM: otitis media; AOM: acute otitis media; CSOM: chronic suppurative otitis media; WHO: World Health Organisation; NTHi: nontypeable Haemophilus influenzae
Introduction
Otitis media (OM) diseases are very frequent among the Inuit population in Greenland, Canada and Alaska as well as Africa and the indigenous people in Australia [Citation1,Citation2]. The term OM covers several diseases ranging from simple and self-limiting disease to complex and chronic[Citation3]. Acute otitis media (AOM) is an OM-subtype which also ranges in severity from self-limiting to needing extensive antibiotic treatment to avoid development into a potentially life-threatening condition [Citation4,Citation5]. AOM is characterised by pus in the middle ear with a bulging tympanic membrane and inflammation or a possible perforation of the tympanic membrane with discharge of pus combined with symptoms such as fever and otalgia [Citation6,Citation7]. Globally AOM is one of the most frequent infections among children and the leading cause for antibiotic prescription to paediatric patients in the USA [Citation4,Citation8]. Three or more AOM episodes in six months or four or more AOM episodes in twelve months are defined as recurrent AOM and may lead to insertion of ear ventilating tubes – an invasive surgical procedure in pre-school children usually requiring general anaesthesia[Citation7].
A tangible consequence of AOM is the risk of permanent hearing impairment which especially occurs if the infection develops into chronic suppurative otitis media (CSOM)[Citation9]. The World Health Organisation (WHO) defines CSOM as the potential outcome of a middle ear infection – most often AOM – and is characterised by chronic inflammation of the middle ear with at least two weeks of otorrhoea through a perforated tympanic membrane[Citation9]. In a study from Greenland it was shown that CSOM during childhood is accompanied by an up to 91% absolute risk of permanent hearing impairment[Citation10]. A Finnish study from 1997 found that hearing impairment in adolescence was associated with a lowered educational yield and a higher risk of unemployment at age 25[Citation11]. According to Monasta et al. 2012 who published data on the world-wide epidemiological pattern of OM and reported that OM leads to 21.000 annual deaths caused by serious complications to OM[Citation12].
The main causative agents of AOM are the bacteria Streptococcus pneumoniae (S. pneumoniae), nontypeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (M. catarrhalis)[Citation13].
The seven-valent pneumococcal conjugate vaccine (PCV7) was first licenced in the USA in 2000 and has since then been part of childhood vaccination programmes around the world [Citation14,Citation15]. In 2010 a 13-valent pneumococcal conjugate vaccine (PCV13) replaced the PCV7 increasing the vaccine coverage with an additional six serotypes of S. pneumoniae[Citation16]. However, this replacement was made without a randomised clinical trial and without data supporting the efficacy against AOM caused by the six extra serotypes [Citation16,Citation17]. Since then different studies have shown different efficacies of the change from PCV7 to PCV13 [Citation14,Citation18]. Ben-Shimol et al. reported a near-elimination of OM episodes caused by PCV13 serotypes in Southern Israel and Lau et al. found a 19% reduction in pneumococcal OM episodes in children younger than 10 months in the UK [Citation14,Citation18].
The Greenlandic health care system is covering an area of more than two million square kilometres making it a challenging task to distribute an even access to health care professionals. The health care system is divided into five regions, each with a regional hospital, but the only access to a permanent otorhinolaryngologist is in the capital, Nuuk, at Greenland’s single larger secondary health care facility[Citation19]. Each region is visited once a year by a travelling otorhinolaryngologist. The Greenlandic childhood vaccination programme is free for all children with a permanent address in Greenland. In 2010 the PCV13 became the first pneumococcal vaccine implemented in the vaccination programme [Citation20,Citation21]. In a very recent study by Albertsen et al. the overall vaccine coverage for the childhood vaccination programme was close to WHO recommendations[Citation22]. However, not all are timely vaccinated or vaccinated at all. This made it possible to test the efficacy of PCV13 in vaccinated and incomplete/delayed vaccinated Greenlandic children concerning episodes of AOM.
Materials and methods
The study was conducted as a cross-sectional observational register study. The PCV13 was given in three doses when the child was 3, 5 and 12 months old. Data from all children born in Greenland from 1 January 2015 through 30 September 2016 were extracted from the national electronic medical records systems, COSMIC and Aeskulap. 1 January 2015 was chosen as the lower cut-off because COSMIC was implemented in Nuuk in March 2015 and therefore the included children would have had their first dose of PCV13 registered in COSMIC in March. 30 September 2016 was chosen as the higher cut-off because the unvaccinated/delayed cohort was defined as not having received the third and final dose of PCV13 at 15 months of age and allowed for a year of follow-up until the end of the study period in December 2018.
The extracted data consisted of date of birth, sex, town, age at the three doses of PCV13, number of AOM episodes, age at first AOM episode, antibiotic treatment, prematurity, vaginal birth or sectio.
An episode of AOM was defined as having received a prescription for oral antibiotics and that the prescription was based on the following terms: ear pain and/or middle ear infection/otitis media and/or acute discharge (ear discharge within 14 days of oral antibiotics prescription) and/or red/bulging tympanic membrane.
Inclusion criteria were being born between 1 January 2015 and 30 September 2016 and having a Greenlandic address on 17 December 2018. Exclusion criteria were uncertain vaccination status, cranio-facial malformation, Down Syndrome or general immunoincompetence.
The included children were divided into two groups based on vaccination status defined as “Vaccinated”: having received all three doses of PCV13 before the age of 15 months; and “Incomplete/delayed”: not having received all three doses of PCV13 before the age of 15 months.
The study period was chosen to get the most precise registration of vaccinations as the new system, COSMIC, was rolled out in this period. However, there was a risk of missing data in a transition period from the old medical journal system to the new. To avoid children with missing vaccination data being allocated to the wrong group and thereby affect potential results, it was decided to define a child as “uncertain vaccination status” if the date for the first or second dose of the PCV13 could not be found. Children with uncertain vaccination status were excluded.
Study population
The Greenlandic population consists of approximately 56,000 people with a third living in the capital, Nuuk[Citation23]. The rest of the population lives in smaller towns and settlements only connected by sea or air traffic. We did not gather information about ethnicity, but the majority of included children are assumed to be of Greenlandic Inuit descent as 90% of the Greenlandic population are considered Inuit[Citation24].
Statistical analysis
Data from the “Vaccinated” and “Incomplete/delayed” groups were compared. Fisher’s exact test, Kruskal-Wallis H test and chi-squared test were used where appropriate.
Statistical analyses were performed using IBM SPSS Statistics v25 and Statistical Analysis System (SAS) v9.4. A p-value <.05 was considered significant.
Ethical considerations
The study was approved by The Research Ethics Committee for Scientific Health Research in Greenland, no. 8,972,630 (or KVUG 2018–23) as well as the Danish Data Protection Agency, no. REG-113-2018.
Results
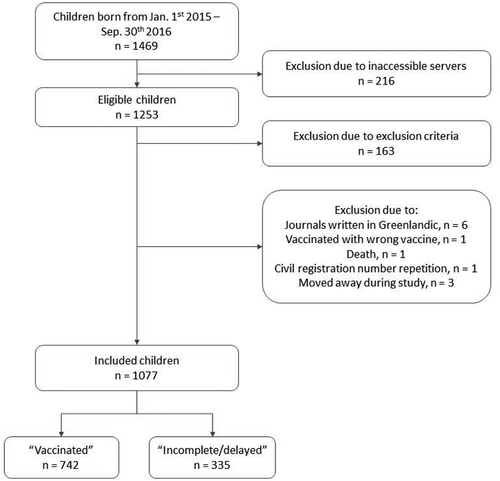
In total, 1077 children were included of which 543 (50.4%) were boys. Of these 742 (68.9%) were allocated to the “Vaccinated” group and 335 (31.1%) to the “Incomplete/delayed” group. See for the enrolment process.
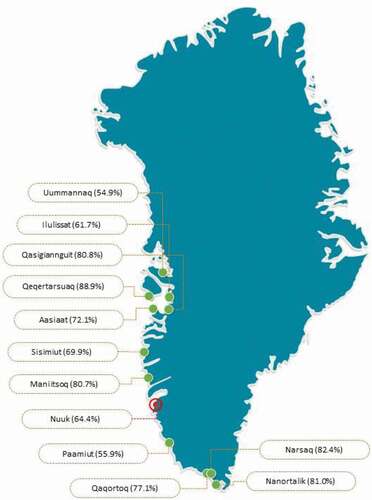
shows PCV13 vaccination status in towns around Greenland in the study period. The respective vaccination rates ranged from 55% to 89%.
Figure 2. Vaccination coverage in investigated towns in Greenland (From: www.yourfreetemplates.com)
Baseline characteristics for the included children are shown in . The child’s age at time of vaccination was calculated in the two groups and significant differences were found at all three vaccination doses between the groups (p < 0.0001) (see ).
Table 1. Baseline data for fully vaccinated vs. incomplete/delayed vaccinated Greenlandic children
A significant difference was found in number of AOM episodes per child with 0.5 episodes in the “Incomplete/delayed” group and 0.7 episodes in the “Vaccinated” group (p = 0.01) (see ). In the “Incomplete/delayed”-vaccinated group 34.9% of the children had one or more episodes of AOM compared 41.5% in the “Vaccinated” group (p = 0.04). Using only data from the children with one or more AOM episodes a significant difference remained as there were 1.5 episodes in the “Incomplete/delayed” group and 1.8 episodes in the “Vaccinated” group (p = 0.02).
Table 2. AOM episodes during follow-up according to PCV-13 vaccination status in Greenlandic children
In order to investigate if the number of PCV13 doses was associated with number of AOM episodes the children from the “Incomplete/delayed” group was divided into four groups based on how many doses of PCV13 they had received (see ). The two groups with the highest frequencies of AOM throughout the follow-up were “Complete” and “0 vacc.”. Also, it can be seen from that the number of children with ≥5 AOM was too small for meaningful comparison.
Table 3. Expanded PCV-13 vaccination status and AOM episodes in Greenlandic children
shows the distribution of children in the two groups regarding having an AOM episode before the first PCV13 vaccination. A significant difference was found with more children in the “Incomplete/delayed” group having had AOM before first vaccination.
Table 4. Numbers and frequency of first AOM episode before first PCV-13 vaccination in Greenlandic children according to vaccination status. P-value is calculated by Fisher’s exact test
shows a re-analysis of where we excluded the children that had an AOM episode before their first PCV13 vaccination. The same significant differences were found as in except for “Children with ≥1 AOM episodes” which now has a p-value of 0.07.
Table 5. AOM episodes during follow-up according to PCV-13 vaccination status, but with the exclusion of children who had an AOM episode before 1st PCV13 vaccination
Discussion
This register-based cohort study is the first to investigate the effect of the implementation of the PCV13 on episodes of AOM in Greenland. The “Incomplete/delayed” group had significantly fewer episodes of AOM compared to children receiving full and timely vaccination with the PCV13. This finding persisted when we adjusted for children with no AOM episodes. To determine the effectiveness of the PCV13 it is important to remember that this study focused solely on the possible reducing effect on AOM episodes among Greenlandic children and not on invasive pneumococcal diseases where the vaccine is highly effective [Citation16,Citation17]. Also, it is important to mention that the PCV13 has little to no effect on the AOM episodes caused by the other main otopathogens NTHi and M. catarrhalis[Citation25]. Furthermore, serotype-shifting from vaccine types to non-vaccine types may be of importance. Such a serotype-shift has been documented in Greenlandic children by Navne et al. in 2017 who compared nasopharyngeal carriage from 2011 to 2013 with carriage three years after the implementation of the PCV13[Citation26]. They also found a reduction of vaccine types in non-vaccinated children suggesting a herd immunity[Citation26]. A serotype-shift and herd immunity against the vaccine serotypes may dilute the effect in this study, but the examined medical records in this study did not have information regarding what serotype or otopathogen that caused the registered AOM episode.
The difference in age could be attributed to the “Incomplete/delayed” group being defined as having received the third and final dose of PCV13 after 15 months of age and one could therefore hypothesise that the “Incomplete/delayed” group mainly consisted of those children whose parents were behind schedule of the children vaccination programme from the beginning.
The significant difference in prematurely born children in the two groups may be due to the fact that we used the children’s actual age at vaccination dates and not their adjusted age. Some of the premature children did not receive their vaccinations timely because their adjusted age was too low and therefore, they were older than 15 months of age before completing the PCV13 vaccination schedule.
Potential differences in the socio-economic status of people living in the capital Nuuk and people living in towns and settlements may have contributed to our findings. Our study showed large regional differences in vaccine coverage, with the health region containing the capital, Nuuk, as having one of the lowest coverages[Citation22]. Besides having a low vaccine coverage Nuuk also has better living conditions than the settlements[Citation27]. As poorer living conditions such as crowding, indoor smoking and poor dietary supply – is a known risk factor for AOM, our results may have been affected by children in good living conditions with an incomplete or delayed vaccination programme having fewer AOM episodes than children in poor living conditions with a complete vaccination programme[Citation28].
Contrary to our study, other studies have found the PCV13 to be effective. In 2018 Pichichero et al. found that the PCV13 reduced the number of AOM episodes caused by the six additional serotypes of S. pneumoniae that was added from the 7-valent to the 13-valent PCV[Citation17]. In the UK in 2015 Lau et al. found a decline in OM-related health care visits and a decrease in OM-associated prescriptions for antibiotics following the implementation of both the 7-valent and 13-valent PCV[Citation14]. More in line with the findings of this study is the Cochrane systematic review “Pneumococcal conjugate vaccines for preventing otitis media” that concludes that “promoting PCV solely to reduce AOM for the individual does not seem justified.”[Citation13]
Surprisingly, we found that more children had had their first AOM episode before vaccination in the group of “Vaccinated” children. This may be due to parents to children in the “Vaccinated” group were more prone to seek the health care system than parents of children in the “Incomplete/delayed” group.
We also found that when we excluded the children with a pre-PCV13 vaccination AOM episode there were still significantly more AOM episodes per child in the “Vaccinated” group. This analysis was done to investigate if a pre-PCV13 vaccination AOM episode would make the children more prone to AOM episodes later in life despite having a full and timely PCV13 vaccination programme.
Due to the design of this study and the PCV13 being already implemented in the Greenlandic childhood vaccination programme, it is difficult to accept our null hypothesis of no difference in AOM episodes between “Vaccinated” and “Incomplete/delayed”-vaccinated children mainly because we largely compare fully vaccinated children to semi-vaccinated children and only to very few not vaccinated at all. The ideal situation for studying this subject would be comparing two large identical groups, but with one of the groups being fully and timely vaccinated with PCV13 and the other group having received no doses of PCV13. However, there were only eight children in this study who had received no doses of PCV13 vaccination.
Strengths and limitations
The main strength of this study was that it included nationwide registry data from two years in Greenland.
Despite the nationwide registration of the population in Greenland a limitation of this study is the small population size which affects the statistical power of our findings. This also hampers the possibility of using multivariate statistics.
The observational cross-sectional cohort design does not rule out the risk of selection bias, although we only excluded children with inaccessible data. A risk of differential bias is that the parents of the children from the “Incomplete/delayed” group would seek medical professionals more rarely compared to the parents of the “Vaccinated” group – maybe due to lower health literacy in the parents of the “Incomplete/delayed” group. This would result in a lower probability to be diagnosed with AOM for the children in the “Incomplete/delayed” group. However, we do not have data on this. The diagnosis of AOM is difficult, but this bias is supposedly the same in the “Vaccinated”- and “Incomplete/delayed” group. To avoid the risk of confirmation bias and lower the risk of faulty registration, it would have been optimal to have had two persons to scrutinise the data and compare their data sheets.
An important limitation was the lack of access to information about confounders of AOM such as socioeconomic status, parental smoking, number of siblings, breastfeeding, family history, day care attendance and crowding. Should a new study be performed to further understand AOM in Greenlandic children access to this information is highly recommendable. Limitations regarding the study design include that some of the AOM-prone children had had two AOM episodes within 14 days and therefore only counted as one episode. The definition of an AOM episode was based on prescription of oral antibiotics in addition to clinical findings. However, it seemed that the willingness to prescribe antibiotics for AOM decreased during the study period, reflecting a gradual change in clinical practice over time. In addition, there may have been a difference in willingness to prescribe antibiotics depending on what type of medical professional who was consulted. This is the main limitation as this study was based on medical records and not in-person observations. The difference in how readily the diagnosis AOM was given may vary in the health care system and could also impact the findings, but it is probably difficult to overcome in a setting such as the Greenlandic with many remote areas where access to medical professionals is sparse. Finally, as this study was conducted in the transition period from the old data system, Aeskulap, to COSMIC, quite many children had missing data on vaccinations leading to exclusion.
Conclusion
In conclusion, based on medical records, Greenlandic children born between January 2015 and September 2016 and completely and timely vaccinated with the PCV13 did not have a reduced number of AOM episodes compared to not timely or not vaccinated children.
To further the understanding of the PCV13 and its effect on AOM episodes in Greenlandic children more research is needed. In such research it would be interesting to gather information regarding AOM confounders and pneumococcal serotypes or other otopathogens causing the AOM. Furthermore, a concise national guideline to AOM diagnosis would help reducing the diagnostic variability between different types of medical professionals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Homøe P, Christensen RB, Bretlau P. Prevalence of otitis media in a survey of 591 unselected Greenlandic children. Int J Pediatr Otorhinolaryngol. 1996;36:215–8.
- Schilder AGM, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Prim. 2016;2:16063.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63:611–618.
- Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964–e999.
- Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203.
- Avnstorp MB, Homøe P, Bjerregaard P, et al. Chronic suppurative otitis media, middle ear pathology and corresponding hearing loss in a cohort of Greenlandic children. Int J Pediatr Otorhinolaryngol. 2016;83:148–153.
- Heidemann CH, Lous J, Berg J, et al. Danish guidelines on management of otitis media in preschool children. Int J Pediatr Otorhinolaryngol. 2016;87:154–163.
- Vojtek I, Nordgren M, Hoet B. Impact of pneumococcal conjugate vaccines on otitis media: a review of measurement and interpretation challenges. Int J Pediatr Otorhinolaryngol. 2017;100:174–182.
- World Health Organization. Chronic suppurative otitis media, burden of illness and management options. 2004. https://apps.who.int/iris/handle/10665/42941.
- Jensen RG, Koch A, Homøe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2013;77:1530–1535.
- Järvelin MR, Mäki-Torkko E, Sorri MJ, et al. Effect of hearing impairment on educational outcomes and employment up to the age of 25 years in northern Finland. Br J Audiol. 1997;31:165–175.
- Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226.
- Fortanier AC, Venekamp RP, Boonacker CW, et al. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst. Rev. 2014. DOI:https://doi.org/10.1002/14651858.CD001480.pub4.
- Lau WCY, Murray M, El-Turki A, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the UK. Vaccine. 2015;33:5072–5079.
- Mokuno E, Morozumi M, Ubukata K, et al. Epidemiology of acute otitis media in children after introduction of the 13-valent pneumococcal conjugate vaccine. J Otolaryngol Japan. 2018;121:887–898.
- Moore MR, Link-Gelles R, Schaffner W, et al. Impact of 13-valent pneumococcal conjugate vaccine used in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance HHS public access. Lancet Infect Dis. 2015;15:301–309.
- Pichichero M, Kaur R, Scott DA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Heal. 2018;2:561–568.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014;59:1724–1732.
- Bhutta MF. Models of service delivery for ear and hearing care in remote or resource-constrained environments. J Laryngol Otol. 2019;133:39–48.
- Stenz FK. USI - Landslægeembedets Nyhedsbrev. USI - Landslægeembedets Nyhedsbrev. 2010;1:2.
- Albertsen N, Fencker IM, Noasen HE, et al. Immunisation rates among children in Nuuk. Int J Circumpolar Health. 2018;77:1426948.
- Albertsen N, Lynge AR, Skovgaard N, et al. Coverage rates of the children vaccination programme in Greenland. Int J Circumpolar Health. 2020;79:1721983.
- Statistics Greenland. Grønlands befolkning. Vol. 2019. Grønlands Statistik; 2019
- Young TK, Bjerregaard P. Towards estimating the indigenous population in circumpolar regions. Int J Circumpolar Health. 2019;78:1653749.
- Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics. 2017;140:e20170181.
- Navne JE, Koch A, Slotved H-C, et al. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal carriage by respiratory pathogens among greenlandic children. Int J Circumpolar Health. 2017;76:1309504.
- Niclasen B, Mulvad G. Health care and health care delivery in Greenland. Int J Circumpolar Health. 2010;69:437–447.
- Jervis-Bardy J, Sanchez L, Carney AS. Otitis media in indigenous australian children: review of epidemiology and risk factors. J Laryngol Otol. 2014;128:S16–S27.