ABSTRACT
Background: The Faroese people constitute a geographically isolated population, and research on cancer in this population is sparse. Thus, this study aimed to calculate the age-standardised incidence rate (ASIR) and 5-year survival rates in head and neck cancers (HNC) in the Faroese population from 1985 to 2017.
Materials and methods: All patients registered with HNC in the Faroese Cancer Registry (FCR) from 1985 to 2017 were included. The ASIR per 100,000 (World Standard Population) and 5-year survival rates were calculated. We also calculated the distribution of tobacco, alcohol consumption, cancer stages and various timelines.
Results: 202 patients were included in the study (62% men). The ASIR for all HNC was 10.0/100,000 persons-years and was higher among men than women. Women’s survival rate was significantly higher than men’s (p = 0.026). The results imply that oropharyngeal cancer (OPC) had the best survival rate and was diagnosed at a significantly earlier stage.
Conclusion: This retrospective nation-wide study showed that ASIRs and 5-year survival rates for Faroese HNC patients in general resembled the ones reported for Danish HNC patients. Timelines for Faroese HNC patients were shorter compared with Greenlandic HNC patients, but longer compared with the Danish fast track programme limits.
Introduction
Head and neck cancers (HNC) encompass a heterogeneous group of cancers and covers several anatomical sites in the head and neck region. The vast majority of cancers in the upper aerodigestive tract are squamous cell carcinoma (SCC), accounting for more than 90% of oral and pharyngeal cancers [Citation1,Citation2]. Worldwide, HNC is the sixth most frequent malignancy, accounting for more than 980,000 cases annually and is responsible for considerable morbidity and mortality [Citation3].
Alcohol and tobacco consumption remains the most common risk factor for the development of HNC, although the number of HNC cases due to tobacco consumption varies across the globe [Citation4]. Alcohol and tobacco-related HNC incidences have remained constant or are in decline [Citation5]. On the other hand, the incidence rates for oropharyngeal cancers (OPCs) have increased during recent decades [Citation6]. The increasing rate is recognised as a result of the growing subset of OPCs associated with the infection of human papillomavirus (HPV) [Citation5–8].
HNC is primarily a loco-regional disease and is therefore potentially curable. Early diagnosis of HNC is crucial because diagnostic delay increases risk of mortality [Citation9]. Furthermore, it is known that HNC proliferates rapidly, and a delay in treatment initiation can result in stage progression [Citation10,Citation11] and have an adverse effect on survival [Citation12]. This emphasises the importance of reducing diagnostic delay and treatment delay to ensure a better prognosis for patients with HNC.
The Faroe Islands is an autonomous country in the Kingdom of Denmark, and has an extensive type of self-government [Citation13]. Even though the two countries represent a union, the societies differ in many areas. The population in the Faroe Islands has a high number of hereditary diseases that have been researched, and they play a special part in the Faroese health care system [Citation14–16]. However, the research on cancer in the Faroese population is sparse [Citation17]. To our knowledge, there is no research done on HNC in the Faroe Islands.
This study aimed to evaluate incidences and survival rates for HNCs diagnosed from 1985 to 2017 in the Faroe Islands and to evaluate timelines in the diagnostic and therapeutic process.
The health care system in the Faroe Islands
The Faroe Islands are a group of 18 islands in the North Atlantic Ocean, of which 17 are inhabited. The population counts 51,060 people in total (1st of September 2018) [Citation18]. The National Hospital of the Faroe Islands (NHFI) in Tórshavn is the largest of three hospitals and has the only ear, nose and throat (ENT) department in the country. Two ENT doctor positions are available, but only one position is fully employed.
The Faroe Islands have a shortage of doctors, both at the hospitals and in the general practices [Citation19]. The low population and the isolated society limit the opportunity to hire, train and educate highly specialised doctors.
Since 2006, a pathologist has been fully employed at the NHFI, and it has therefore been possible to receive histology on biopsies within a few days. Before 2006, it was necessary to send all biopsies to Copenhagen University Hospital Rigshospitalet (RH) that resulted in diagnostic delay.
When a HNC diagnosis is verified in a patient at the NHFI, the patient is referred to the ENT department at RH. After ended treatment, patients will usually have a two-month check-up at RH, followed by regular check-ups at the ENT department at the NHFI. Fast-track programmes introduced in Denmark in 2007 [Citation20] have not been implemented in the Faroese health care system.
Materials and methods
Data included in this study were derived from the Faroese Cancer Registry (FCR) [Citation21]. The FCR was established in 1994 and is directly comparable to the Danish Cancer Registry. The FCR contains data on cancer incidences diagnosed in the Faroe Islands from 1961, but registration first became mandatory in 1995. All patients registered with a HNC in the FCR from 1985 to 2017 were included in this study. Information on sex, birthdate, personal registration number, age at diagnosis, date of diagnosis, cancer location, histology, and possibly death and emigration, was derived from the FCR.
To supplement data from the FCR, the following parameters were registered from the medical records for all patients with a HNC diagnosis code registered in the medical record system Cosmic from January 2006 to December 2017: alcohol consumption, tobacco use, date of first symptom, date of first visit at an ENT doctor’s office, date of histological diagnosis, date of first day of treatment, hospital of treatment and TNM classification. TNM classification was converted to I–IV cancer stages according to the UICCs (Union for International Cancer Control) 8th edition. The medical record system Cosmic was introduced in the Faroe Islands in 2006, and before this, all medical records were paper forms. It was therefore not possible to obtain the mentioned parameters before 2006.
The patients were divided into nine groups according to cancer site: Oral cavity, oropharynx, nasopharynx, hypopharynx, sinonasal cavity, thyroid, major salivary glands, larynx, and others. The histological information was derived based on the ICD-O-3 classification [Citation22] from the FCR. These were categorised into four histology groups: Squamous cell carcinomas, adenocarcinomas, carcinomas unspecified, and others including malignant melanomas, sarcomas and salivary gland malignant tumours.
Patients with a HNC diagnosis code in the medical record system Cosmic were matched with patients with a HNC diagnosis code in the FCR using the personal registration number. The personal registration number is unique to each person and can be used to perfectly match patients who appear in both datasets. The total number of patients included in this study (n = 202) is a combined setting of patients in the FCR (n = 174) and patients registered with a HNC diagnosis code in the medical record system Cosmic and were missing from the FCR (n = 28).
Ethics
The study was approved by the NHFI.
Definition of timelines
The symptomatic timeline was calculated from date of first symptom to first visit at the ENT doctor’s office ().
Figure 3. The median timeline in days for all Faroese head and neck cancer patients diagnosed from 2006 to 2017
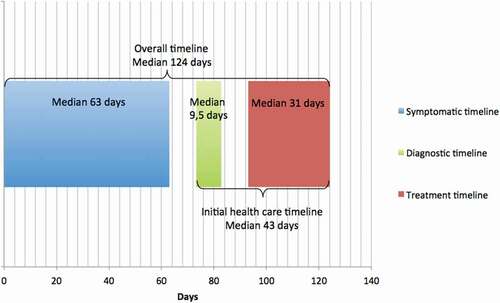
The diagnostic timeline was calculated from date of first visit at the ENT doctor’s office to date of histological diagnosis ().
The time from verified histological diagnosis to first day of treatment was calculated as the treatment timeline ().
The overall timeline was calculated from date of first symptom to first day of treatment. Finally, the initial health care timeline was calculated from date of first visit at the ENT doctor’s office to first day of treatment ().
Statistical analysis
Statistical analysis was performed in R statistics version 3.4.3 [Citation23]. Incidence rates per 100,000 were calculated using the EpiTools package using the Faroese population and the WHO world standard population weighing as reference [Citation24]. Incidence rates were age-standardised according to the 2000 WHO world standard population [Citation25].
Overall survival was calculated from date of diagnosis to death or to the last follow-up date (30 September 2018) and was estimated using the Kaplan–Meier method.
The relationship between two categorical variables was calculated using chi-square test or Fisher’s exact test. The relationship between two independent samples with equal variances was calculated using t-test if the data were normally distributed and Mann–Whitney U test if the data were not normally distributed.
We considered a p-value < 0.05 as statistically significant.
Results
A total of 202 patients were included in this study of which 62% (n = 126) were men (). The median age at diagnosis was 63.8 years (range 19.6–93.8 years) with no significant difference between men and women. The highest median age based on anatomical site was among laryngeal cancer (65.9 years; range 40.3–80.8 years) and sinonasal cavity cancer (65.7 years; range 46.9–77.2 years) while the lowest median age was among major salivary gland cancer (56.3 years; range 23.8–84.8 years) and thyroid cancer (56.8 years; range 22.2–89.1 years). Cancer of oral cavity and larynx were the most frequent of all HNCs, followed by thyroid cancer (). The most common histology was SCC followed by carcinomas unspecified.
Table 1. Numbers and age-standardised incidence rate of head and neck cancer per 100,000 persons-years in the Faroe Islands in the period 1985–2017
Incidence
We calculated the age-standardised incidence rate (ASIR) for all HNCs from 1985 to 2017, which was 10.0/100,000 persons-years (). The ASIR for all HNCs was 12.2/100,000 persons-years for men and 7.9/100,000 persons-years for women (). Laryngeal cancer had the highest ASIR among men of 4.2/100,000 persons-years while thyroid gland cancer had the highest ASIR among women of 2.9/100,000 persons-years (). The ASIR for all HNCs was also calculated for two separate periods, 1985–1999 and 2000–2017. The same trends were also seen for these two periods with the highest ASIR in laryngeal cancer among men and the highest ASIR for thyroid cancer among women (Supplementary Table 1a and 1b).
Overall survival rate
Patients were followed for a median period of 4.4 years (range 0–36.9 years) with a significant difference between men and women, where women were followed for a longer period (p = 0.03). We observed a total HNC 5-year survival rate of 59.1% (95% CI; 52.5; 66.6). We found that women had a significantly better survival rate than men (p = 0.026), with 5-year survival rates of 62.8% (95% CI 52.5; 75.1) and 56.9% (95% CI 48.6; 66.8), respectively.
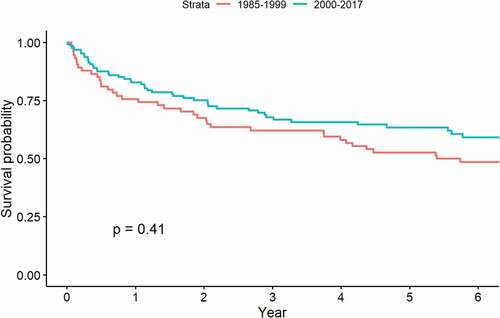
The HNC 5-year survival rate rose from 52.7% (95% CI 42.5; 65.4) in 1985–1999 to 63.5% (95% CI 55.2; 73.0) in 2000–2017, but this result was not significant (p = 0.41) ().
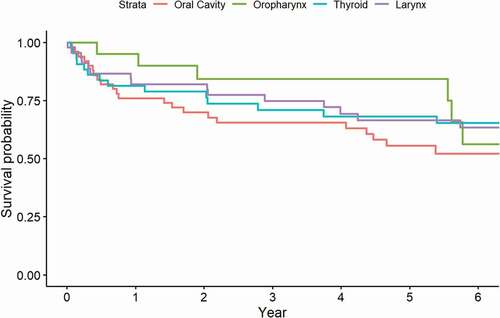
Based on anatomical site, we observed that OPC had a 5-year survival rate of 84.4% (95% CI 69.5; 100.0) (). Thyroid cancer had a 5-year survival rate of 68.2% (95% CI; 55.2; 84.2), closely followed by laryngeal cancer [66.5% (95% CI 53.4; 82.7)] (). The grouping of various cancers others and hypopharyngeal cancer had a 5-year survival rate of 20.0% (95% CI; 3.5; 100.0) and 27.3% (95% CI; 10.4; 71.6) respectively, closely followed by major salivary glands [40.0% (95% CI 18.7; 85.5)].
Regarding histology, carcinomas unspecified and adenocarcinomas had a 5-year survival rate of 70.1% (95% CI 57.6; 85.4) and 66.7% (95% CI 37.9; 100), respectively, while the grouping of various histology types others encompassing malignant melanomas, sarcomas and salivary gland malignant tumours had a 5-year survival rate of 40.0% (95% CI 25.8; 62.0).
Timelines
We were capable of finding specific timeline dates in the medical records regarding 88 patients who were included from 2006 to 2017. 65% (n = 57) were men. For all HNC patients, the median symptomatic time was 63 days (range 0–1461 days), the median diagnostic time was 9.5 days (range 0–141 days), and the median treatment time was 31 days (range 0–133 days) ( and ). For all HNC patients, the median overall timeline was 124 days (range 25–1232 days), while the median initial health care timeline was 43 days (range 0–176 days) ( and ). There was no significant difference between men and women’s various timelines.
Table 2. Timeline in days for all Faroese head and neck cancer patients diagnosed from 2006 to 2017
Six patients did not receive any treatment. Furthermore, it was not possible to obtain information regarding the various timelines for four patients.
Alcohol and tobacco consumption and treatment hospital
We were capable of finding alcohol consumption, tobacco consumption and treatment hospital in the medical records regarding 80, 85 and 91 patients, respectively, which were included form 2006 to 2017. The total percentage of HNC patients diagnosed from 2006 to 2017 consuming over the recommended, low-risk, alcohol limit per week (i.e. alcohol consumption > 7♀/14♂ units of alcohol per week) was 29% (n = 23) with a significant (p < 0.05) preponderance of men (42% vs 4%). The total percentage of HNC patients that currently were, or formerly had been, consumers of tobacco was 69% (n = 59), again with a significant preponderance of men (82% vs 45%).
In total, 90% of the Faroese HNC patients diagnosed from 2006 to 2017 received treatment in Denmark, 6% received treatment at the NHFI, and 4% received treatment at other hospitals.
It was not possible to obtain information regarding alcohol consumption for 12 patients, tobacco consumption for seven patients and treating hospital for one patient.
Cancer stage distribution
We were capable of finding the TNM classification in the medical records regarding 68 patients who were included form 2006 to 2017. According to UICCs 8th edition, we found that 37% (n = 34) of the Faroese HNC patients diagnosed from 2006 to 2017 had cancer stage I–II, 37% (n = 34) had cancer stages III–IV, and for 26% (n = 24) the cancer stage was not specified in the medical record. There was no significant difference between men and women’s I–II and III–IV cancer stages. Hypopharyngeal cancer was discovered at a significantly later stage, stages III–IV (78%) (p = 0.05), while OPC was discovered at a significantly earlier stage, stage I–II (60%) (p = 0.02). In our study, half of the patients with OPC had HPV-positive tumours (n = 5) and the other half was HPV-negative or unknown (n = 5) within 2006–2017.
Discussion
In this study, we have retrospectively investigated a total number of 202 patients diagnosed with HNC in the Faroe Islands in the period 1985–2017.
Overall the ASIR for Faroese HNC patients were quite similar for those reported in Denmark [Citation26]. Our study showed that the ASIR for all HNC was higher among men than women. Similar trends are seen in other Nordic studies [Citation26–30]. Laryngeal cancer had the highest ASIR among Faroese men while thyroid gland cancer had the highest ASIR among Faroese women. Similar findings are also confirmed in other studies [Citation3,Citation26,Citation30]. The biggest difference between Faroese and Danish HNC patients was for men with oral cavity cancer and OPC with a noticeably higher ASIR for Danish male HNC patients. The cause of this has not been researched in this study, but a hypothesis could be, that there is a higher number of men in Denmark diagnosed with HPV-positive cancer than in the Faroese Islands, but this needs to be investigated further.
Our study showed that the HNC survival rate among Faroese women was significantly higher than that of men. Similar trends are seen in Denmark [Citation28]. In 2017, 27% of the Faroese adult population were smokers (defined as smoking ≥ two times per month), and the percentage of smokers was higher among men [Citation31]. This is in accordance with our results, where a significantly higher percentage of men currently, or formerly, consumed tobacco on a daily basis, and consumed over the recommended, low-risk, alcohol limit per week. This could, in part, explain the higher survival rate and the lower ASIR of HNC among Faroese women compared with men. Furthermore, our study found a significantly increased survival among women compared with men. These results could also be due to the fact that men seek medical counselling less often than women and underutilise medical and mental health services [Citation32].
Our results showed a tendency towards a better 5-year HNC survival rate in 2000–2017 than in 1985–1999. This is not unexpected, as the general life expectancy also rose from 76.0 years in 1985 to 82.2 years in 2017 [Citation18]. The Danish fast-track programme, which was introduced in Denmark in 2007 to reduce waiting time for diagnosis and treatment [Citation20], was implemented in the later period and this could have impacted the survival rate even though the fast-track programme is not implemented in the Faroese health care system. Contributing factors could also be the general development in diagnostic tools and treatment advances (both surgical and oncological).
Based on anatomical site our results showed a tendency towards that a mixed group of various HNCs and hypopharyngeal cancer had the worst survival rate. Hypopharyngeal cancer was also diagnosed at a significantly later stage (stages III–IV). Low survival rates among hypopharyngeal cancer are seen in other studies, and the reason has been argued to be due to an advanced stage at time of diagnosis [Citation28,Citation33]. Our results suggest that thyroid cancer and laryngeal cancer had one of the highest survival rates. Thyroid cancer is often diagnosed at an early stage [Citation34]. So is glottis cancer, the most abundant form of laryngeal cancer, due to an early presentation of symptoms, together with the minimal risk of regional metastasis due to the lack of lymphoid drainage from the vocal cords [Citation28,Citation33,Citation35]. According to our study, OPC had the best survival rate. This does not match other studies, where it has been reported that OPCs have a relatively poor prognosis, as they are often diagnosed at an advanced stage [Citation1]. However, studies have found that the survival for OPCs is increasing [Citation36] and that patients with HPV-positive OPC have better survival and a lower risk of recurrence than HPV-negative OPC [Citation37]. Our results also showed that OPC was diagnosed at a significantly earlier stage (stage I–II). These findings could, in part, explain the higher survival rate of OPC in the Faroese population, but has to be researched further. Our survival rates based on histology were in accordance with other studies [Citation28]. In this study, there is a difference in the estimates according to the subgroups overall survival rates as descripted, but the confidence intervals could suggest that there are no statistical significant differences between them.
The Faroe Islands and Greenland are both autonomous self-governing countries in the Kingdom of Denmark. The vast majority of both countries’ HNC patients receive treatment at RH. However, our results showed that diagnostic and treatment timelines were shorter for the Faroese HNC patients compared with the Greenlandic HNC patients [Citation29,Citation30]. More advanced cancer stages for Greenlandic HNC patients compared with Faroese HNC patients are also seen [Citation30]. One reason could be that frequent follow-up of HNC patients at ENT doctors or oncologists is an even greater challenge in Greenland due to the demographic structure with scattered communities far from the regional health centres [Citation30]. Also, the three countries have different health care procedures and health legislation; the Danish fast-track programme is, e.g. not implemented in the Faroese or Greenlandic health care system. Nevertheless, our study showed that 67% of the Faroese HNC patients stayed within the fast-track programme’s diagnostic timeline limit, which is 15 calendar days () [Citation38]. However, 89% of the Faroese HNC patients did not stay within the fast-track programme’s treatment timeline limits and 71% of the Faroese HNC patients did not stay within the fast-track programme’s initial health care timeline limits (). It has not been possible to refer HNC patients to an MRI scan in the Faroe Islands, which may facilitate early diagnosis, but, more importantly, the waiting time for a mandatory CT scan in the Faroe Islands can be up to 3 weeks. Obviously, the fact that Faroese and Greenlandic HNC patients have to go abroad to receive treatment requires time. There is also only one ENT doctor at the department at the NHFI, which results in less time for each patient, and the possibility of overlooking something may, therefore, be higher.
Table 3. Comparison between the Danish fast track program limits and the Faroese head and neck cancer patients diagnosed from 2006 to 2017 various timelines
The data included in this study was derived from the FCR, which covers the Faroese population and gives an excellent opportunity to investigate national cancer trends. Furthermore, the Faroe Islands provides universal, tax-financed healthcare to all citizens with uniform standards of treatment, hereby diminishing referral- and selection-bias. The strength of this study is thus the non-selected group of patients in a definite geographical area.
Our study has some limitations. Firstly, we realised that not all HNC patients were registered in the FCR, but, by double-checking with diagnosis codes, we believe that we are as close to the actual number of Faroese HNC patients. Secondly, the overall survival rate was not calculated in relation to the expected survival rate in the Faroe Islands matched by age, sex and calendar year, as this was not possible to obtain. If this were possible, we would have calculated the relative survival rate. Thirdly, the time of first symptom is subject to recall bias since it is based on retrospective, self-reported data. Fourthly, as the Faroese population is relatively small, there is a risk of coincidental changes.
In conclusion, to our knowledge, we present the first study on HNC in the Faroe Islands. Our study provides a presentation of trends in the ASIRs and 5-year survival rates of HNC, the division of tobacco, alcohol consumption and cancer stage, and it determines various timelines for these patients. Even though these findings are satisfying and in general resembled the ones reported for Danish HNC patients, the results indicate that there is room for improvements regarding the time from first visit at the ENT doctor’s office and verified histological diagnosis to first day of treatment (treatment timeline and initial health care timeline, respectively).
Supplemental Material
Download MS Word (24.2 KB)Acknowledgments
We would like to thank Ári Brend Bech, Bachelor in Food Science and Technology, for his help with statistical analysis.
Disclosure statement
The authors declare no conflict of interest.
Supplemental data
The Supplemental data for this article is accessed here.
Additional information
Funding
References
- Lambert R, De Camargo SC, Cancela M, et al. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:(8):633–9. .
- Franchi A, M. L, Palomba A, et al. Sinonasal carcinomas: recent advances in molecular and phenotypic characterization and their clinical implications. Crit Rev Oncol Hematol. 2011;79:(3):265–277. .
- Ferlay J, S. I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. .
- Hashibe M, B. P, Chuang S, et al. Interaction between tobacco and alcohol use and the risk of Head and neck cancer: pooled analysis in the international Head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. .
- Simard E, Jemal TL, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50(5):387–403.
- Carlander A-LF, C. GL, DH J, et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: a Danish population-based study from 2011 to 2014. Eur J Cancer. 2017;70:75–82.
- Garnaes E, K. K, Andersen L, et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: the largest registry-based study to date. Int J Cancer. 2015;136(9):2196–2203. .
- Garnaes E, K K, Andersen L, et al. Increasing incidence of base of tongue cancers from 2000 to 2010 due to HPV: the largest demographic study of 210 Danish patients. Br J Cancer. 2015;113(1):131–134. .
- Seoane J, Varela-Centelles TB, Varela-Centelles P, et al. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clinical Otolaryngology. 2012;37(2):99–106. .
- Jensen AR, Overgaard NH, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5–10.
- Waaijer A, Dehnad TC, Dehnad H. Waiting times for radiotherapy: consequences of volume increase for the TCP in oropharyngeal carcinoma. Radiother Oncol. 2003;66(3):271–276.
- Murphy CT, G. T, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with Head and neck cancer in the USA. J Clin Oncol. 2016;34(2):169–178. .
- The unity of the Realm [Internet]. Statsministeriet. [ Cited 2018 December 13]. Available from: http://www.stm.dk.
- Poulsen SD, A. L, Christensen E, et al. Carnitine transporter deficiency is a hereditary disease with a high incidence in the Faroe Islands. Ugeskr Laeger. 2012;174(18):1217–1219.
- Schwartz M, N. S, Brandt NJ, et al. High incidence of cystic fibrosis on the Faroe Islands: a molecular and genealogical study. Hum Genet. 1995;95(6):703–706. .
- Ostergaard E, F. H, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130(3):853–861. .
- Hjalgrim H, Tulinius H, Dalberg J, et al. High incidence of classical Kaposi’s sarcoma in Iceland and the Faroe Islands. Br J Cancer. 1998;77(7):1190–1193. .
- Nýggjastu hagtølini, fólkatalið 1. september 2018 [ Internet]. Hagstova Føroya. 2018, [ cited 2018 Sept 24]. Available from: http://www.hagstova.fo.
- Um tíggju ár mangla 100 læknar í Føroyum [Internet]. http://www.In.fo. 2017, [ cited 2018 Oct 3]. Available from: http://www.in.fo.
- Historisk overblik på kræftområdet [Internet]. Sundhedsstyrelsen. 2018, [ cited 2018 Dec 30]. Available from: https://www.sst.dk.
- Dalberg J, Jacobsen O, Storm HH, et al. Cancer registration in the Faeroe Islands. Ugeskr Laeger. 1998;160(21):3058–3062.
- International classification of diseases for Oncology third edition [Internet]. World Health Organization. [ Cited 2018 December 30]. Available from: http://www.iacr.com.fr.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Internet. Available from https://www.r-project.org
- Aragon TJ, Fay MP, Wollschlaeger D, et al. Epidemiology tools [R package epitools version 0.5-9]. 2017, [ cited 2018 Dec 5]. Available from: https://www.r-project.org.
- World (WHO 2000-2025) standard [Internet]. http://www.seer.cancer.gov. 2020, [ cited 2020 Jan 22]. Available from: https://seer.cancer.gov.
- Incidence/mortality data [Internet]. NORDCAN. 2019, [ cited 2020 Oct 31]. Available from: https://www-dep.iarc.fr/NORDCAN.htm.
- DAHANCA Årsrapport 2017 [Internet]. DAHANCA. 2017, [ cited 2018 Dec 12]. Available from: https://www.dahanca.oncology.dk.
- Jakobsen KK, Grønhøj C, Jensen DH, et al. Increasing incidence and survival of head and neck cancers in Denmark: a nation-wide study from 1980 to 2014. Acta Oncol. 2018;57(9):1143–1151. .
- Jensen RG, Friborg J, Rosborg J, et al. Survival of head and neck cancer in Greenland. Int J Circumpolar Health. 2010;69(4):373–382. .
- Lawaetz M, J. R, Friborg J, et al. Improved survival of head and neck cancer patients in Greenland. Int J Circumpolar Health. 2018;77(1):1536252. .
- Gallup kanningin 2017 [Internet]. Fólkaheilsuráðið. 2017, [ cited 2018 Dec 12]. Available from: http://www.folkaheilsa.fo.
- Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003;58(1):5–14.
- Sewnaik A, Hoorweg JJ, Knegt PP, et al. Treatment of hypopharyngeal carcinoma: analysis of nationwide study in the Netherlands over a 10-year period. Clinical Otolaryngology. 2005;30(1):52–57.
- Nguyen QT, Lee EJ, Huang MG, et al. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015;8(1):30–40.
- Peller M, Katalinic A, Wollenberg B, et al. Epidemiology of laryngeal carcinoma in Germany, 1998-2011. Eur Arch Otorhinolaryngol. 2016;273(6):1481–1487. .
- Jensen JS, Jensen DH, Grønhøj C, et al. Incidence and survival of oropharyngeal cancer in Denmark: a nation-wide, population-based study from 1980 to 2014. Acta Oncol. 2018;57(2):269–275. .
- Larsen CG, Jensen DH, Carlander A-LF, et al. Novel nomograms for survival and progression in HPV+ and HPV- oropharyngeal cancer: a population-based study of 1,542 consecutive patients. Oncotarget. 2016;7(44):71761–71772. .
- Pakkeforløb for hoved- og halskræft [Internet]. Sundhedsstyrelsen. 2016, [ cited 2018 Oct 30]. Available from: https://www.sst.dk.