ABSTRACT
Tuberculosis (TB) is a persistent health issue in Greenland. While rapid diagnosis is crucial to reducing transmission of the disease, remote settlements have limited access to healthcare services. We aimed to assess and compare the time intervals from first contact to diagnosis and treatment for patients with active TB in the cities and settlements of Greenland. A total of 153 cases were included and divided according to place of residence and whether the diagnosis was based on symptomatic presentation or contact tracing. The median time from first contact to diagnosis was 19 days for the total population. The symptomatic settlement population waited longer (median = 88.5 days) than the symptomatic city population (median = 19 days) (p = 0.018). The system interval was longer for the symptomatic settlement population than for the symptomatic city population with a median of 49.5 days vs. 3 days for chest imaging (p < 0.001) and 66.5 days vs. 10 days for expectorate sample (p = 0.008). The diagnostic, system, and total intervals were significantly longer for symptomatic patients in settlements than in cities. This may explain a higher TB incidence in the settlements and calls for the development of better diagnostic pathways.
Background
It is estimated that around one-quarter of the world’s population is infected with Mycobacterium Tuberculosis, while 1.5 million people died from tuberculosis (TB) in 2018, which makes it the leading cause of death induced by a single infectious agent [Citation1].
The infection is spread by aerosol transmission originating from the respiratory tract of patients with active pulmonary TB. Accordingly, rapid diagnosis and treatment start is pivotal in limiting the period of potential transmission and reducing the spread of TB in a given population.
While tuberculosis has been known in Greenland for many years, several initiatives were made in the 1950s to improve diagnostics and treatment of the disease. This includes the incorporation of the BCG-vaccine in the children’s vaccination programme in 1955 [Citation2], which led to a 90% reduction in incidence within a 10-year period [Citation3]. The following decade demonstrated a continuous decline with an incidence rate of 9 cases per 100,000 population per year in 1987, in line with other countries in the western world [Citation4]. However, as the efforts in controlling the disease waned, such as pausing systematic BCG vaccination between 1990 to 1996, the numbers began to rise until peaking in 2010, with an incidence rate of 205 cases per 100,000 population per year [Citation2,Citation3,Citation5]. Following several national strategies to counter the increasing spread, the incidence rate is now approx. 100 cases per 100,000 population per year [Citation2,Citation3].
Due to a geographic widespread population living in remote settlements with minimal access to health care services, it is important to know whether this might influence the time to diagnosis and treatment, which may be associated with the spread of TB. Such knowledge can provide important insight on interventions that may improve the health care management of TB in Greenland.
We aimed at estimating the time intervals from first symptomatic contact with the health care system to diagnosis and treatment start, as well as estimating the incidence rate of TB in the settlements and towns in Greenland.
Methods
Study design
A nationwide retrospective cohort study of patients diagnosed with active TB in Greenland.
Large areas of the country are unpopulated and covered by ice. Thus, the population of 56,000 citizens reside mainly in towns (49,000 (87.5%)) and settlements (6,900 (12.3%)), with a vast majority on the west coast [Citation6]. As there are no roads connecting the communities, all transportation is by airplane, helicopter or boat [Citation7]. Healthcare in Greenland is free and divided into five health regions, each with its own regional hospital. Queen Ingrid’s Hospital in Nuuk functions as the central hospital in Greenland and receives patients with advanced illness from all five healthcare regions [Citation7].
While the hospitals and larger healthcare centres of the towns offer consultations with doctors in medical outpatient clinics, the smaller settlements rely on telemedicine and occasional visits by doctors. The expertise of permanent healthcare staff in the settlements is typically low as most have not received any healthcare education, while some function as “settlement healthcare employees”, and have been trained in the use of telemedicine equipment and administration of medicine[Citation8]. However, the settlement populations are entitled to the same services at their corresponding regional hospital as the people residing in towns [Citation9]. Chest x-ray imaging is offered at the regional hospitals and health care centres of towns and settlements with a population of more than 500, while citizens of smaller settlements have to be transported to one of these locations [Citation8,Citation9]. Collection of expectorate samples from possible TB-patients can be performed in the field. Ideally, three samples are collected, of which one is sent to Statens Serum Institute in Denmark for culture and microscopy, while the remaining two are shipped to the central laboratory in Nuuk for PCR analysis [Citation10].
All citizens have a unique personal registration number (CPR) assigned at birth used to link data. Greenland has a nationwide electronic patient record, COSMIC, in which the health care data of each patient is stored and can be accessed by healthcare professionals across the country.
Study population
It is mandatory to register TB cases in the TB database as this serves as a basis for national surveillance. Patients diagnosed with active or latent TB and residing in Greenland are registered in the database. The TB diagnosis, treatment, and surveillance are managed by regional TB doctors and nurses as well as TB key personnel in the smaller towns. This happens in cooperation with a national TB doctor and nurse [Citation3]. It is, however, important to stress that the doctors do not work full-time with TB, as it is only a minor responsibility among many others. Furthermore, while the diagnosis is managed in cooperation with TB-personnel, it is important to note that the diagnostic process of TB is an integrated function of all doctors who must consider TB when initially consulting a patient with relevant symptoms or exposure.
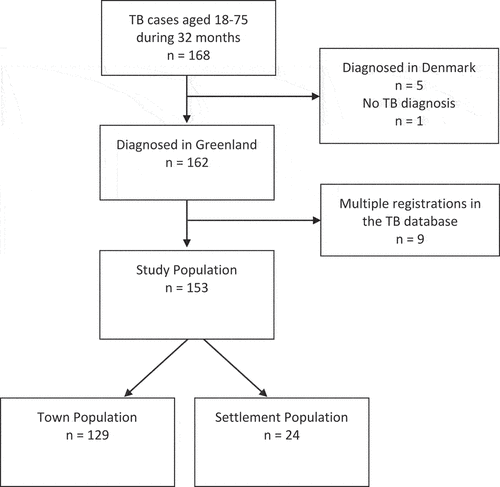
The study population was identified in the TB database. The 168 patients aged 18–75 years diagnosed with active TB between the 1st of January 2018 and the 26th of August 2020 were included. We excluded patients diagnosed in Denmark (5), with no valid TB diagnosis (1), and multiple registrations of the same person (9) due to treatment interruptions (). For the patients with multiple registrations, we included the first pathway only. Towns and settlements were defined according to the division of the national healthcare system and the respective healthcare offers based on population size [Citation8,Citation9], and the populations were categorised according to residency address at the time of diagnosis.
Data
From the TB database, we collected the names, CPR numbers, sex, age, town/settlement of residence, town/settlement of diagnosis, year of diagnosis, whether the diagnosis was confirmed by microbiology (TB positive PCR, microscopy, or bacterial growth) and treatment status. From each patient’s medical record, we collected data on the date of first contact with the healthcare system with relevant symptoms or signs, time of diagnosis, date of treatment start, date of chest imaging and date of expectorate sample collection.
Definition of diagnostic milestones
The study population was divided into two groups based on whether it was a symptomatic presentation or contact tracing among exposed.
For those with symptomatic presentation, time of first contact was defined as the date registered in the medical records at which a patient initially contacted the health care system with relevant symptoms of active tuberculosis. These were defined based on the diagnostic guidelines for pulmonary tuberculosis in Greenland [Citation10] (coughing, shortness of breath, haemoptysis, fatigue, fever, night sweats, loss of appetite, weight loss, unresolved thoracic pain, and recurring pneumonia). The date of first contact was truncated at 24 months before diagnosis.
Time of first contact without symptoms was defined as the date registered in the medical records at which health care staff started suspecting TB by initiating a diagnostic pathway (e.g. contact tracing).
Time of diagnosis was defined as the date of diagnostic entry in the medical records (). Diagnosis of pulmonary TB in Greenland follows the diagnostic guidelines from the Office of the Medical Director of Health and WHO’s definition of TB cases, and is based on definite x-ray changes or the date of positive Mycobacterium Tuberculosis PCR, microscopy, or culture test result [Citation10,Citation11].
Time of treatment start was defined as the entry date for treatment start with TB-antibiotic regimen in medical records or, if unavailable, ordination date in the patient’s prescription list.
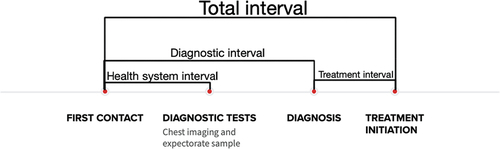
The diagnostic interval was defined as time from first relevant contact with the health care system to date of diagnosis. The system interval was defined as the time from suspecting TB until the investigation (X-ray and expectorate) was done. The treatment interval was defined as time from date of diagnosis to date of TB treatment start. The total interval was defined as time from first relevant contact with the health care system to date of TB treatment start.
Analysis
The data were entered in Excel and anonymised and then transferred to Stata 13. The time intervals were calculated as median days with interquartile ranges and minimum and maximum. Differences between groups were tested with non-parametric statistics (Mann-Whitney) due to the right-skewed distribution of time intervals.
Estimates of the incidence rate of active tuberculosis diagnosed in towns and settlements were calculated as the number of TB cases per the population numbers of people aged 18–75 years residing in the specific areas in 2020, from the statistics bank of Greenland [Citation12]. This yielded a total town population of 35,933 (94.3%) and a total settlement population of 2183 (5.7%) people. The incidence rates were calculated under the assumption of stable population numbers from 2018 to 2020. A p-value of 5% or less was considered statistically significant.
Ethics
The study was approved by the Scientific Ethics Committee of Greenland. All data were anonymised at data entry.
Results
Characteristics of the study population
A total of 153 patients were included in the analyses of which 129 (84.3%) lived in towns and
24 (15.7%) in settlements. Men constituted 64.7%. The median age of the total population was 48.0 years, with 49.0 years in the towns and 40.5 years in the settlements. Cases discovered by contact tracing were younger in the settlements, with a median of 25.0 years, compared to the town median of 49.0 years ().
Table 1. The characteristics of the 153 included patients with active TB
Of all, 126 (82.4%) presented symptoms at first contact with 66.7% in the settlement population and 85.3% in the town population.
Geographic distribution of cases
Of the 17 towns and 56 settlements in Greenland [Citation12], cases of people aged 18–75 years and diagnosed with active TB within the study period were found in 13 towns and 15 settlements. The population number of each settlement included in this study was less than 500. Of the 129 cases in the towns, 16 were diagnosed in two towns on the east coast, while three of the 24 settlement cases were diagnosed in three separate settlements on the east coast. Of the 24 settlement cases included, eight were residing in one settlement on the west coast at the time of diagnosis. Of these, three cases were symptomatic, while five were asymptomatic.
Incidence rate of active TB
The incidence rate of active TB diagnosed in Greenland for people aged 18–75 years during the 32 months was 141.0 per 100,000 per year for the total population with 135.2 per 100,000 per year in the towns, and 182.9 per 100,000 per year in the settlements.
Total interval
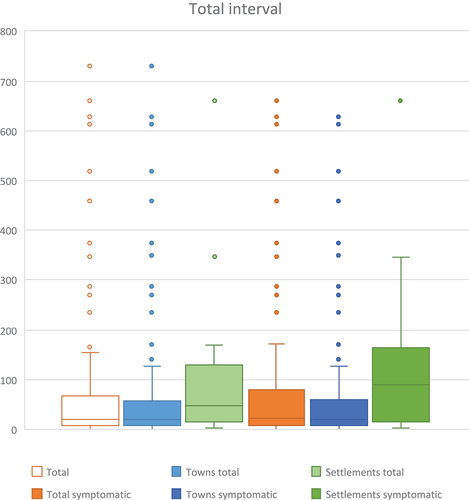
The median time from first contact to treatment start was 20 days (IQI = 8–65) for the total population (, ), with 20.0 days (IQI = 8–56) for the total town population and 47.5 days (IQI = 14.5–105) for the total settlement population, respectively (p = 0.041).
Table 2. The diagnostic intervals for the 153 TB patients shown for all and divided into patients in towns and settlements
Diagnostic interval
For all, the median time from first contact to diagnosis was 19 days (IQI = 7–65) with 21 days (IQI = 7–76.5) for the symptomatic population vs. 14 days (IQI = 6.5–24) for the asymptomatic population () (p = 0.599). The median diagnostic interval for the town population was 17 days (IQI = 7–54) vs. 45 days (IQI = 13–103.5) for the settlement population (p = 0.049).
Table 3. The total intervals for the 153 TB patients shown for all and divided into patients in towns and settlements
The diagnostic interval was longer for the symptomatic settlement population with a median of 88.5 (IQI = 16.5–156.5) compared to the 16 days (IQI = 8–29.5) of the asymptomatic settlement population (p = 0.050).
Treatment interval
The median time from diagnosis to treatment start was 1 day (IQI = 0–1.5) for all subpopulations, except for the asymptomatic town population which had a median time interval of 2 days (IQI = 1–2.5) (p = 0.018) ().
Table 4. The treatment intervals for the 153 TB patients shown for all and divided into patients in towns and settlements
System interval
The median time from first relevant contact to chest imaging was 4 days (IQI = 0–14) for the total study population (). In the settlements, the symptomatic patients waited a median of 49.5 days (IQI = 7.5–88.5) compared to contact tracing cases who waited 0.5 days (IQI = 0–4.5) (p = 0.0018). In the towns, this difference was not seen ().
Table 5. Time intervals from contact to chest imaging for the 153 TB patients shown for all and divided into patients in towns and settlements
The median system interval for expectorate sample in the settlements was 0.5 days (IQI = 0–4) for contact tracing cases compared to 66.5 days (IQI = 8–144.5) for the symptomatic cases (p = 0.002) (). The symptomatic cases of the settlements waited longer for expectorate sample collection than symptomatic cases in the towns (p = 0.008).
Table 6. Time intervals from contact to expectorate sample collection for the 153 TB patients shown for all and divided into patients in towns and settlements
Discussion
Main findings
Patients diagnosed with active TB in Greenland experienced a median diagnostic interval of 19 days, and 25% waited more than two months for the TB diagnosis. The results show a substantially longer diagnostic interval for the symptomatic settlement population. Here, half of the TB cases waited nearly three months or more for a diagnosis, and 25% waited more than five months. If the diagnosis was part of contact tracing, the diagnostic interval was lower and 50% of the settlement population had a diagnosis within 16 days. Thus, living in settlements was strongly associated with a longer diagnostic interval for TB compared with living in towns.
The main reason for a delayed TB diagnosis in settlements was longer waiting times for imaging and expectorates. In the settlements, half of the symptomatic cases waited at least 1½ months for a chest x-ray and more than two months for expectorate. For the latter, 25% waited nearly five months for expectorate.
For most cases, treatment was started within 2 days after diagnosis. For contact tracing cases, it was seen that the diagnostic interval and system interval was less than one month for the majority, which means that it may be possible to shorten the time intervals.
Strengths and weaknesses
We included patients from all of Greenland under the assumption that the registration of patients with active TB in the database would be complete and updated in all five healthcare regions. While some variation in registration may occur, the quality of the database is regarded as high[Citation3].
In our study, we defined settlement and town cases according to residency address at the time of diagnosis. It is conceivable that some cases may have migrated during the medical investigation process. If the migration was to affect our data, the relocating of the case would need to occur between the first symptomatic contact with health care personnel and the time of diagnosis and treatment. We estimate that such cases would be very limited in our data set. However, we cannot be certain, as the place of residence at the time of symptomatic contact is not taken into account.
Also, of the 24 settlement cases included, eight were discovered in one settlement. Some of the differences observed between towns and settlements may be explained by this particular episode with increased contact tracing. However, excluding these cases when calculating the diagnostic intervals for the settlement cases did not change the results.
All patients within the restricted age group of 18–75 years and diagnosed with active TB in the study period were initially included, and restriction added to approximate a homogeneous population and increase generalisability. Children, adolescents, and older people may have different health-care seeking patterns. Also, some people leave the settlements at old age. Finally, TB diagnoses may be missed if the patients die of comorbidities. The current life expectancy in Greenland is approximately 71 years [Citation13].
Our data relied on information extracted from medical records. A certain misclassification may be seen in the registration of signs and symptoms and the date for these. However, this would tend to underestimate the intervals and the time intervals we present are thus merely lower than in reality.
The statistical precision was low, as we were only able to include 24 cases from the settlements. Therefore, we might not have been able to identify all statistically significant differences.
Comparisons with other studies
This study is, to our knowledge, the first study that investigates the difference in time intervals from contact to treatment start of active TB in the towns and settlements of Greenland. However, similar studies have been conducted in other countries.
Bojovic et al [Citation14]. conducted a cross-sectional study in which they estimated a median delay of 27 days from the first consultation with a general practitioner to TB treatment initiation in Montenegro. Likewise, Auer et al [Citation15]. estimated the median time from first health care visit to TB treatment initiation in Switzerland to be two weeks. Getnet et al [Citation16]. performed a systematic review of 27 studies published between 2007 and 2015 that, among other factors, investigated health system delays in diagnosing pulmonary tuberculosis in low- and middle-income countries for participants 15 years or older. The study reported a median of the median health system delay, or diagnostic interval, for each study to be 28 days for the Sub-Saharan countries and 18 days for the rest of the examined low- and middle-income countries.
When compared to these studies, a 19-day median diagnostic interval for the total Greenlandic population seems reasonable. However, the median diagnostic interval of 88.5 days for the symptomatic population of the settlements in Greenland illustrates that these active TB cases most likely expose others to TB months before treatment is started. This may lead to the higher incidence of TB and outbreaks seen in settlements.
Several factors may explain the observed differences in time intervals, such as limited access to diagnostic tools and less educated health care personnel in the settlements [Citation8]. Accordingly, our data shows significant longer time intervals for the symptomatic population in the settlements compared to the towns.
Another important consideration is the provision of health care consultations. While the hospitals and larger healthcare centres offer consultations with doctors, the settlements rely on telemedicine, occasional visits by doctors, and less educated healthcare employees [Citation8,Citation17]. According to our study, this structure may lead to delayed TB diagnosis for symptomatic patients. As geographical challenges in Greenland complicate travelling, better telemedicine visitation is needed as well as the local use of expectorates.
Further, it is conceivable that there may be a disparity in attendance and absence of health care consultations, chest imaging, and expectorate collection between the citizens of settlements and towns. Also, transportation from settlements to towns that offer chest imaging and expectorate sample collection may be complicated due to weather circumstances, or limits in means of transportation. However, we have no data to quantify this possible difference.
Implications
The occurrence of active TB in Greenland has been characterised by local outbreaks that might be attributed to longer diagnostic intervals and an increased risk of disease transmission [Citation3]. Our study documents a significant difference in the diagnostic intervals of the towns and settlements in Greenland. This might lead to increased transmission. Accordingly, reducing the diagnostic interval may in effect reduce the incidence rate of active TB. It may also have an impact on the health care outcome and prognosis of TB.
The disparity in health care delay was smaller for the asymptomatic populations that were contacted by the health care system. This signifies that rapid diagnosis in the settlements is possible when the healthcare staff initially suspects or contacts people with possible TB. An argument can be made for the earlier inclusion of key TB-personnel in symptomatic cases in the settlements. Thus, the healthcare staff in the settlements could contact key TB-personnel as soon as relevant symptoms are presented. The key TB-personnel could then remotely supervise the diagnostic process and initiate expectorate and chest imaging procedures to reduce the system delay. Also, providing settlements with X-ray technology may reduce chest imaging delay, although the cost of such a measure may prove problematic. Alternatively, reinstating a tuberculosis ship with the capability of performing chest X-rays in settlements along the cost may be another option. Further, while telemedicine offers several advantages in regions where geographical challenges might otherwise limit healthcare, it comes with some downsides, as it is conceivable that the same quality of virtual consultations as physical consultations may not be feasible. Thus, it is paramount that the healthcare personnel receive proper training in managing the equipment and adapting their competences to the digital reality of virtual consultations to ensure proper care, and in turn, early discovery of possible TB-cases and optimal treatment.
Conclusion
Our study showed that the median diagnostic interval was significantly longer for patients in settlements than for patients in the towns. Likewise, diagnostic intervals and system intervals were longer for the symptomatic cases in the settlements than for cases discovered by contact tracing.
We have provided insight into one of the areas where an effort can be made to benefit the achievement of reducing TB in Greenland.
Acknowledgments
We would like to thank Diane Lundholm Nielsen, regional TB-nurse, for providing insight in the TB-database and management of TB-patients, and Hans Christian Florian, regional doctor, for his effort in providing data from Tasiilaq. Also, thanks to Hannah Lind Gleerup for her assistance in revising the language of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Tuberculosis fact sheet. [cited 2020 Oct 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Naalakkersuisut. Tuberculosis in Greenland - the current situation. [cited 2020 Oct 15]. Available from: https://naalakkersuisut.gl/da/Naalakkersuisut/Nyheder/2018/10/0210_tuberkulose#:~:text=Der%20er%20i%20Gr%C3%B8nland%20en,niveau%20i%20en%20l%C3%A6ngere%20%C3%A5rr%C3%A6kke.&text=Tuberkulose%20forekommer%20i%20hele%20Gr%C3%B8nland,udbredt%20i%20Syd%2D%20og%20%C3%98stgr%C3%B8nland
- Department of Health. National TB strategy 2017-2021. 2017; [cited 2022 Feb 11]. Available from: https://nun.gl/-/media/landslaegeembedet/udgivelser/tuberkulose/den-nationale-tb-strategi-2017-21/dknationaltbstrategi20172021.pdf?la=da>
- The Office of the Medical Director of Health. Tuberculosis in Greenland - The current situation. [cited 2020 Oct 15]. Available from: https://nun.gl/emner/borgere/tuberkulose_i_gl/hvordan_er_situationen_i_gl_nu?sc_lang=da>
- World Health Organization. Tuberculosis prevention, control and care in Greenland. 2017; [cited 2022 Feb 11]. Available from: https://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/publications/2017/tuberculosis-prevention,-control-and-care-in-greenland.-report-of-a-review-mission-2016>
- Statistics Greenland. Population of Greenland 2020, [cited 2020 Oct 15]. Available from: http://www.stat.gl/dialog/main.asp?lang=da&sc=BE&version=202001
- Statistics Greenland. Greenland in figures. 2018; [cited 2022 Feb 11]. Available from: https://naalakkersuisut.gl/~/media/Nanoq/Files/Publications/Udenrigs/Greenland%20in%20Figures%202018.pdf
- Peqqik. Catalogue of healthcare services in the regions. 2015; [cited 2022 Feb 11]. Available from: https://www.peqqik.gl/-/media/Files/Patientinfo/Ydelser/Ydelseskatalog-dk-godkendt-Naalakkersuisut.pdf?la=da-DK
- Peqqik. Healthcare offers in your region. [cited 2022 Feb 06]. Available from: https://www.peqqik.gl/da-DK/Emner/Patientinformation/Ydelser-i-SHV>
- The Office of the Medical Director of Health. Tuberculosis - diagnosis, treatment, control, contact tracing, reporting, vaccination, and prevention, 2018; [cited 2022 Feb 11]. Available from: https://nun.gl/-/media/landslaegeembedet/sundhedsprofessionelle/landslaegens-vejledninger-for-sundhedsprofessionelle/tuberkulose/tb-vejledning-2018/tb-vejledning-oktober-2018final-nov.pdf?la=da>
- World Health Organization. Definitions and reporting framework for Tuberculosis - 2013 revision. 2013; [cited 2022 Feb 11]. Available from: https://www.who.int/publications/i/item/9789241505345.
- Statistics Greenland. Population numbers January 1st, 2020, [cited 2020 Oct 15]. Available from: http://bank.stat.gl/pxweb/da/?rxid=876ca726-54bf-4ef9-9ac7-43872b508e70
- The World Bank. Life expectancy at birth, total (years) - Greenland. [cited 2022 Feb 22]. Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=GL>
- Bojovic O, Medenica M, Zivkovic D, et al. Factors associated with patient and health system delays in diagnosis and treatment of tuberculosis in Montenegro, 2015-2016. PLoS One. 2018;13(3):e0193997.
- Auer C, Kiefer S, Zuske M, et al. Health-seeking behaviour and treatment delay in patients with pulmonary tuberculosis in Switzerland: some slip through the net. Swiss Med Wkly. 2018;148:w14659.
- Getnet F, Demissie M, Assefa N, et al. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med. 2017;17(1):202.
- Niclasen B, Mulvad G. Health care and health care delivery in Greenland. Int J Circumpolar Health. 2010;69(5):437–9.