Abstract
In South Africa, novel fungal pathogens have emerged as an important cause of disease in HIV-infected individuals. The clinical presentation is characteristically prolonged, insidious and the diagnosis elusive, often being made post mortem. We report an HIV-infected individual with a systemic mycosis due to Aureobasidium pullulans, a ubiquitous dematiaceous mould which has not been described in HIV-infected individuals.
Introduction
Invasive opportunistic systemic mycoses are common in immunosuppressed individuals, with mortality in HIV-infected individuals reaching 48%.Citation1,2 The high mortality is in part due to the difficulty in making the diagnosis: fungal culture is slow, hence positive cultures may be missed by the treating clinician. Additionally, the clinical manifestations of disseminated fungal infections are protean, with deep fungal infections in some HIV-infected patients misdiagnosed as tuberculosis, varicella, Kaposi’s sarcoma and papular pruritic eruption of HIV.Citation1 Thus, the diagnosis is often delayed, and made after death in up to 24% of cases.Citation1
The recent description of a novel systemic mycosis, caused by Emmonsia spp., in HIV-infected individuals highlights the need for heightened surveillance in this high-risk population.Citation3–5 We describe an HIV-infected patient with a disseminated fungal infection caused by Aureobasidium pullulans, a ubiquitous dematiaceous mould that is not known to cause disease in HIV-infected individuals.Citation6
Case report
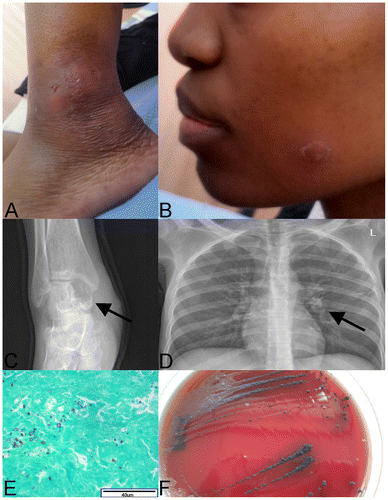
In August 2014, a 28-year-old HIV-infected female presented to Chris Hani Baragwanath Academic Hospital (CHBAH) with a four-month history of an insidious, asymmetrical oligoarthritis involving her ankles, knees and left elbow (Figure A). She had been previously admitted to CHBAH in June 2013 when she had been diagnosed with cryptococcal meningitis, based on cerebrospinal fluid culture. She was successfully treated with amphotericin B and fluconazole and discharged on fluconazole secondary prophylaxis. During the same admission, she had been diagnosed with HIV-infection, with a baseline CD4+ cell count of 15 cells/mm3. She had initiated antiretroviral treatment (tenofovir/lamivudine/ efavirenz) after discharge in July 2013. Five months after initiation of antifungal treatment, her fluconazole prophylaxis was stopped prematurely.Citation7 She had a period of good health, until April 2014, when her joints became warm, swollen and tender, ultimately resulting in her current presentation. She had an associated rash that was well-circumscribed with scaling plaques on her face, trunk and thighs (Figure B). The remainder of her clinical examination was non-contributory. Her chest radiograph showed a small left hilar consolidation, while limb radiographs revealed lytic lesions suggestive of osteomyelitis in her right talus and tibia (Figure C and D, respectively). Her current CD4+ cell count was 168 cells/mm3 and her HIV-1 viral load was less than 20 copies/ml. A clinical diagnosis of septic arthritis was made and she was initiated on ceftriaxone and cloxacillin, treating empirically for Neisseria gonorrhoeae and Staphylococcus aureus whilst awaiting microbial confirmation from blood and joint aspirate cultures. An orthopaedic consult was obtained, and an arthrotomy of her right ankle and knee was performed. Tissue histology revealed necrotising granulomatous inflammation with unspecified fungal elements (Figure E). A fungus was cultured from blood, synovial fluid and a tissue specimen. This fungus was initially identified as C. neoformans (AuxaColor, BioRad, USA), leading to a diagnosis of disseminated cryptococcosis. Her therapy was altered to amphotericin B and fluconazole. A lumbar puncture revealed normal, sterile cerebrospinal fluid (CSF) while her serum and CSF cryptococcal latex antigen test (CrAg) (ImmunoMycologics, USA) was negative. A repeat serum CrAg test two weeks later was positive (titre 2048). The discordant serological results led to questioning of the diagnosis of relapsed disseminated cryptococcosis. Microscopic review of the isolates revealed atypical fungal morphologies not fully in keeping with C. neoformans. Two isolates, cultured from blood and synovial fluid, were submitted to a reference laboratory for phenotypic and molecular identification. The synovial fluid isolate was identified as A. pullulans phenotypically (urease-positive cream-coloured yeast-like colonies that darkened with age) and genotypically by sequencing of the internal transcribed spacer (ITS) region of the fungal ribosomal gene. The second isolate was contaminated and could not be identified further. The patient was continued on amphotericin B and fluconazole for 22 days, resulting in full resolution of the skin lesions and arthritis. She was discharged, in good health and on antiretroviral treatment with fluconazole prophylaxis.
Figure 1: A: Swollen, fluctuant left ankle with sinus formation. B: Well-circumscribed, scaling plaque on the patients face that resolved after treatment with amphotericin B and fluconazole. C: Lytic lesion in the patient’s right talus (arrow), consistent with osteomyelitis. D: Chest X-ray showing a small infiltrate in the left hilar region (arrow). E: Grocott’s stain (40x) showing fibrous connective tissue from the patient’s knee with variable sized fungal elements. F: Aureobasidium pullulans culture on blood agar from blood culture. Colonies were initially small and white, becoming black two weeks later.
Microbiology
The admission aerobic blood culture was cultured at 37 °C, and was positive after 32 h, with the initial Gram stain showing no bacteria or yeasts. On subculture, no growth was noted after 24 h; but, after 48 h, small white, dry (almost chalky) colonies grew on both the blood and chocolate agar plates. After approximately 14 days, the colonies darkened and became black (Figure F). A Gram stain from this culture revealed ellipsoidal yeast-like cells of varying size, with budding and hyphal structures, not in keeping with typical C. neoformans morphology. These cultures were initially identified as C. neoformans (AuxaColor, BioRad, USA). The AuxaColor kit identifies a limited number of commonly encountered yeast species based on biochemical and morphological criteria. Uncommon fungal isolates with similar morphology and biochemistry may generate a profile similar to a species in the database of the AuxaColor identification kit, as we believe occurred between C. neoformans and A. pullulans. Upon sub-culture, the aerobic blood culture was found to be contaminated, and could not be identified further. Culture of the synovial fluid from the patient’s right ankle revealed identical colony morphologies and Gram stain characteristics to those from the blood culture. Again, initial culture identification revealed C. neoformans (AuxaColor, BioRad, USA). However, upon sequencing of the ITS region of the multi-copy ribosomal gene of this isolate, A. pullulans was identified (99% sequence homology to A. pullulans, GenBank accession EF595769.1).
Discussion
Invasive fungal infection is rare, and is associated with immunosuppression and medical instrumentation.Citation2 Worldwide, common human fungal pathogens include Aspergillus and Candida, while other fungi such as Histoplasma, Blastomyces, Coccidioides, Cryptococcus, and Sporothrix are rarer causes. These fungi can be difficult to diagnose.Citation8 In South Africa, misidentification of invasive fungal pathogens is common in HIV-infected patients, while profound immunosuppression from HIV-infection allows novel fungal pathogens to cause disseminated disease.Citation1,3,4 Strong clinical and laboratory interrogation is, therefore, warranted when HIV-associated fungal infections are suspected. In our case, C. neoformans was initially cultured but, upon more robust interrogation, A. pullulans was identified. This is possible as A. pullulans is morphologically similar to C. neoformans on early culture, but A. pullulans colonies tend to darken after one to two weeks. Molecular techniques are often required to make a definitive diagnosis.
A. pullulans fungemia has been previously described in patients with either malignancy, recent major surgery, indwelling catheters or bone marrow transplantation.Citation9,10 Care must be taken in making the diagnosis of A. pullulans infection, as it has been a contaminant in previous studies.Citation6 As the phenotypic characteristics of the fungus cultured from all sites in this case were consistent with A. pullulans and sequencing of the ITS region identified A. pullulans, we favour a diagnosis of disseminated A. pullulans infection. The lack of molecular confirmation of the second A. pullulans isolate from our patient, combined with the discordant serum CrAg results make a definite diagnosis hard to achieve. The serum CrAg test has been shown to be falsely reactive in the presence of streptococcal antigenaemia, and at the time of the second CrAg test, our patient was diagnosed with an infection of viridans group Streptococcus based on three positive blood cultures.Citation11,12 We postulate that this may have caused the high positive serum CrAg test result that was discordant with the first. Unfortunately, no further specimens were available to confirm the diagnosis and the patient has not followed up for a repeat serum CrAg test.
The optimum treatment of A. pullulans has not been standardised, and previous reports have suggested amphotericin B, flucytosine and fluconazole to be efficacious.Citation6,9,13 Additionally, amphotericin B has been shown to achieve adequate sterilising concentrations in the synovial fluid.Citation14 For these reasons, we chose to treat our patient with amphotericin B and fluconazole in a manner that mirrors the guidelines established for C. neoformans.Citation7 This regimen achieved a complete clinical response in our patient, and should be considered when confronted with HIV-infected patients with disseminated fungal infections, especially A. pullulans.
Conclusion
A. pullulans is a potential opportunistic systemic mycosis in profoundly immunosuppressed HIV-infected individuals. The clinical manifestations of this and other systemic mycoses are protean and often misdiagnosed, leading to high morbidity and often mortality.Citation1 An immune reconstitution inflammatory syndrome is also likely to ensue if highly active antiretroviral treatment is initiated, due to the high pathogen load, and profound immune suppression. Therefore, high clinical suspicion and aggressive pursuit of a diagnosis of disseminated mycosis in all World Health Organisation stage four HIV-infected individuals is warranted.
Declaration
The authors declare that this is their own original work. No conflicts of interest are reported. Informed consent was obtained, and ethical clearance was obtained from the University of the Witwatersrand (Certificate M141203).
References
- Schwartz IS, Govender NP, Corcoran C, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61(6):1001–12.
- Hay RJ. Overview of the treatment of disseminated fungal infections. J Antimicrob Chemother. 1991;28(Suppl B):17–25.10.1093/jac/28.suppl_B.17
- Kenyon C, Bonorchis K, Corcoran C, et al. A dimorphic fungus causing disseminated infection in South Africa. N Engl J Med. 2013;369(15):1416–24.10.1056/NEJMoa1215460
- van Hougenhouck-Tulleken WG, Papavarnavas NS, Nel JS, et al. HIV-associated disseminated emmonsiosis, Johannesburg, South Africa. Emerg Infect Dis. 2014;20(12):2164–6.10.3201/eid2012.140902
- Heys I, Taljaard J, Orth H. An emmonsia species causing disseminated infection in South Africa. N Engl J Med. 2014;370(3):283–4.
- Hofman V, Butori C, Long E, et al. Aureobasidium pullulans contamination in bronchial aspirates mimicking cryptococcosis. Pathology. 2008;40(7):729–32.10.1080/00313020802436782
- Govender N, Meintjes G, Bicanic T, et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. S Afr J HIV Med. 2013;14(2):76–86.
- Bariteau JT, Waryasz GR, McDonnell M, et al. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390–401.10.5435/JAAOS-22-06-390
- Huang YT, Liaw SJ, Liao CH, et al. Catheter-related septicemia due to Aureobasidium pullulans. Int J Infect Dis. 2008;12(6):e137–9.10.1016/j.ijid.2008.02.004
- Joshi A, Singh R, Shah MS, et al. Subcutaneous mycosis and fungemia by Aureobasidium pullulans: a rare pathogenic fungus in a post allogeneic BM transplant patient. Bone Marrow Transplant. 2010;45(1):203–4.10.1038/bmt.2009.111
- Feldmesser M, Harris C, Reichberg S, et al. Serum cryptococcal antigen in patients with AIDS. Clin Infect Dis. 1996;23(4):827–30.10.1093/clinids/23.4.827
- Gade W, Hinnefeld SW, Babcock LS, et al. Comparison of the PREMIER cryptococcal antigen enzyme immunoassay and the latex agglutination assay for detection of cryptococcal antigens. J Clin Microbiol. 1991;29(8):1616–9.
- Mise N, Ono Y, Kurita N, et al. Aureobasidium pullulans peritonitis: case report and review of the literature. Perit Dial Int. 2008;28(6):679–81.
- Evdoridou J, Kremenopoulos G., Roilides E, et al. Multifocal osteoarthritis due to Candida albicans in a neonate: Serum level monitoring of liposomal amphotericin B and literature review. Infection. 1997;25(2):112–6.10.1007/BF02113589
