?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Psoriasis is a chronic inflammatory cutaneous disorder, which is prone to methicillin-resistant Staphylococcus aureus (MRSA) infection. We aimed to to investigate prevalence of MRSA skin colonization among psoriatic patients and if there is a relation between Panton-Valentin leukocidin (PVL) expression and disease severity. A case-control study was conducted on 90 patients with active psoriatic lesions and 90 healthy controls. Scales from psoriasis plaques and skin swabs from healthy control were collected. Identification of MRSA and antibiotic susceptibility patterns were performed using routine microbiological methods. mecA and PVL genes were detected using PCR assay and pulsed field gel electrophoresis (PFGE) was done for MRSA typing. Prevalence of MRSA among psoriatic patients was 23.3%. Significant difference was noticed in MRSA colonization between psoriasis and control groups. Five (23.8%) MRSA isolates from skin lesions were positive for mecA and PVL genes. Antibiotic resistance among PVL negative MRSA was higher, specially towards clindamycin. Patients harboring PVL positive isolates had significantly lower mean age and higher PSAI score than those with negative isolates. Fourteen PVL negative isolated MRSA from psoriasis patients were the same pulsotype. There was significant MRSA colonization in psoriasis group and presence of PVL may contribute to increased severity of this disease.
Introduction
In the middle of the twentieth century, the advances in antibiotics development were a hope for controlling infections, but the overuse of it and the ability of bacteria to mutate resulted in drug-resistant strains. In 1959, methicillin was introduced and within 2 years hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) was reported. In 1982, the first MRSA was isolated outside the hospital. By the late nineties, MRSA was isolated from people in communities with no known risk factors. By 2004, community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) had reached the proportions of a worldwide epidemic [Citation1]. Twenty percent of individuals are persistently colonized with S. aureus, and 30% are intermittently colonized. Colonization clearly increases the risk for subsequent infection as it provides a reservoir from which bacteria can be introduced [Citation2]. MRSA accounted for 70% of the infections by S. aureus strains. In the USA, MRSA causes 94,000 life-threatening infections and 19,000 deaths a year [Citation3]. The epidemiologic changes in MRSA over the years have made the distinction between CA-MRSA and HA-MRSA is no longer clear [Citation4]. In 2005, the prevalence of MRSA was high in Egypt (52%) [Citation5], and even more, Abouelfetouh reported more increase in its prevalence (82%) in 2017 [Citation6]. MRSA strains possess a mobile genetic element called staphylococcal cassette chromosome mec (SCCmec) which includes the ccr gene complex encoding for site-specific recombinases and the mec gene complex which typically divided into five types of I, II, III, IV (IVa, IVb, IVc and IVd) and V. Other regions of the SCCmec have been referred to as the J regions and contain a variety of genes, including those coding for resistance to other antibiotics [Citation7]. SCCmec types can be further sub-typed into variants based on differences in J regions [Citation8]. Furthermore, Staphylococcus protein A gene (spa) can be used for accurate and discriminatory typing of MRSA even greater than that of the multi-locus sequence typing [Citation9]. SCCmec also carries the Panton-Valentin leukocidin (PVL) virulence genes that compose of lukS-PV and lukF-PV which form the PVL toxin [Citation10]. The presence of PVL genes appears to be associated with increased severity in some diseases and screening for it may become a routine laboratory procedure [Citation11]. Psoriasis is a chronic inflammatory cutaneous disorder, affecting approximately 2–4% of the general population, and these patients are more prone to MRSA infection due to injured keratinocyte with impaired barrier function [Citation12]. MRSA is characterized by enhanced ability to bind, persist, invade skin cells than other strains do and produce a unique toxin, PVL protein, with leukocytolytic activity. MRSA invades skin, increases the production of proinflammatory products inside leukocytes, and PVL produces pores in the leukocyte membranes resulting in lysis and release of inflammatory enzymes and cytokines even with low concentrations of PVL and this may induce or worsen psoriasis [Citation13]. Furthermore, MRSA reacts with antigen-presenting cells that stimulate the T helper-cells which produce IL17 & IL22 that are well known to be important immunological mediators in psoriasis pathogenesis [Citation14]. Panton-Valentin leukocidin toxin is uncommonly found in methicillin-sensitive Staphylococcus aureus (MSSA) isolates but there is a strong epidemiological association between it and the emergence of CA-MRSA infections [Citation15]. This study is to investigate the prevalence of MRSA skin colonization in psoriasis and correlate PVL expression and the disease severity.
Methodology
Subjects
This case–control study was conducted over the period from February 2015 till April 2017 on 180 adults attending Mansoura University Hospital after informed consent. They were allocated in two groups: Gr1 (n = 90) patients with active psoriatic lesions, and Gr2 (n = 90) healthy controls with no history of psoriasis. Exclusion criteria were as follows: oral or local antibiotic, ultraviolet light therapy and systemic therapies at least 2 weeks before enrollment. All subjects were subjected to history taking and clinical examination. Psoriasis patients were diagnosed in a dermatology clinic, psoriasis area and severity index (PASI) of patients were reported [Citation16], which involves the assessment over four body regions (head [h], trunk [t], upper [u] and lower [l] extremities) of erythema (E), infiltration (I), desquamation (D), and body surface area involvement (A), as in . Since the head, upper extremities, trunk, and lower extremities correspond to approximately 10%, 20%, 30%, and 40% of body surface area, respectively, the PASI score is calculated by the formula:
Table 1. Method for calculating the psoriasis area and severity index (PASI).
Samples collection
For psoriasis patients, skin scales were collected from psoriasis plaques after decontamination by alcohol. For normal skin in healthy control subjects, two skin swabs were collected for each person from the volar site of the elbow using cotton applicators immersed in NaCl 0.9%.
Isolation and identification of S. aureus
All samples were inoculated on blood agar and mannitol salt agar plates (Oxoid, UK) and incubated aerobically for 24 h at 37°C. After incubation, S. aureus strains were identified on the basis of their colonies morphology, catalase test, Gram staining, coagulase activity, and biochemical features using the API Staph identification test kit (Bio-Mérieux, France).
Antimicrobial resistance
Methicillin susceptibility was studied by using agar screen plates on Müller-Hinton agar (MHA) (Oxoid, UK) with 30 μg/ml of cefoxitin disc. Plates were incubated at 37°C for 18 h and zone diameters were measured. An inhibition zone diameter of ≤19 mm was reported as oxacillin resistant and ≥20 mm was considered as oxacillin sensitive [Citation17]. Quality control strains, methicillin-sensitive S. aureus ATCC 25923 and methicillin-resistant S. aureus ATCC 43300 were used as negative and positive controls, respectively. Antibiotic sensitivity of MRSA was obtained using the agar disc diffusion method on MHA (Oxoid, UK) according to the recommendations given by the CLSI (2015). The following antimicrobial agents (Oxoid, UK) were tested: tetracycline (30 μg), erythromycin (15 μg), lincomycin (15 μg), gentamicin (10 μg), clindamycin (2μg), rifampicin (5 μg), ciprofloxacin (5 μg), cotrimoxazole (1.25/23.75 μg), and fusidic acid (10 μg). D-test was also performed to diagnose inducible clindamycin resistance in erythromycin-resistant clindamycin-susceptible isolates. This test was performed by placing an erythromycin disk at a distance of 15–20 mm of clindamycin disk. Isolates with blunting of the inhibition zone around the clindamycin disk adjacent to the erythromycin disk (D-zone positive) considered to have inducible clindamycin resistance and are presumed to be resistant [Citation17].
Multiplex PCR assay for detection of mecaand PVL genes among MRSA isolates
Genomic DNA was extracted by using a simple boiling method and the supernatant was used as a DNA template for PCR analysis. The multiplex PCR assay targets the lPV genes with primers Luk-PV-1 (5′-ATCATTAGGTAAAATGTCTGGACATGATCCA-3′) and Luk-PV-2 (5′-GCATCAAGTGTATTGGATAGCAAAAGC-3′) [Citation18], and the mecA gene with primers MecA1 (5′-GTAGAAATGACTGAACGTCCGATAA-3′) and MecA2 (5′-CCAATTCCACATTGTTTCGGTCTAA-3′). The PCR yielded fragments of the expected sizes, i.e. 433, and 310 bp for the lPV, and mecA genes, respectively. The optimized multiplex PCR conditions were, as follows: 2 μl of template DNA in a 25 μl final reaction volume containing 1μl of each primer. The multiplex PCR conditions set at 94°C for 10 min, followed by 10 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 75 s and 25 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 75 s. Amplification in a single multiplex PCR produced distinct bands, corresponding to their respective molecular sizes, that were easily recognizable in agarose gels stained with ethidium bromide [Citation19].
Pulse field gel electrophoresis typing (PFGE)
The clonal relationships between the MRSA isolates were studied by evaluating the genomic DNA with PFGE analysis that was performed according to the CDC protocol [Citation20]. DNA was restricted with the SmaI enzyme and was separated on an agarose gel using a CHEF DR III apparatus (Bio-Rad laboratories). The running conditions were 6 V per cm, with pulses ranging from 5 to 40 s for 18 h at 14°C. The DNA banding patterns were visualized under UV light after staining with ethidium bromide (0.5 mg/mL). The similarities between the isolates were determined by visual comparison of the isolates band patterns. The interpretation of the PFGE results was carried out by eye according to the criteria described by Tenover et al. [Citation21].
Statistical analysis
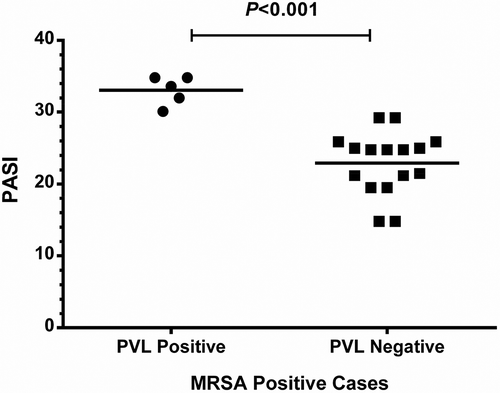
Data were analyzed using SPSS statistical software, version 16.0 (IBM, Chicago, USA). Chi-Square test was used to assess the differences between proportions of S. aureus strains isolated in psoriasis and control skin. P-value <0.05 was considered as statistically significant. Quantitative data were presented as means and standard deviation and also described as numbers and percentages. Mann–Whitney U test was used for comparing PVL positive and PVL negative groups relation to PASI.
Results
Two groups were included in this study: the first was patients with psoriasis (36 ± 12.4 years, 54 females and 36 males) and the second was a control group (38 ± 11.7 years, 49 females and 41 males).
Prevalence of S. aureus in psoriasis and control groups
S. aureus was isolated from 31 cases (34.4%) in psoriatic skin lesions, and from 16 cases (17.8%) in the control group, which makes a statistically significant difference (P= 0.01).
Prevalence of MRSA in psoriasis and control groups
The prevalence of MRSA colonization in our psoriatic patients from skin lesions was 23.3% (21 out of 90 samples). A high statistically significance difference (P< 0.001) was noticed in MRSA colonization between psoriasis and control groups (23.3% versus 3.3% in psoriasis and control groups, respectively).
Expression of mecaand PVL genes in MRSA isolates from psoriasis and control groups
In psoriasis patients, five out of 21 (23.8%) MRSA isolates from skin lesions were positive for mecA and PVL genes using PCR assay. None of the isolated MRSA from the control group was positive for PVL genes.
PFGE
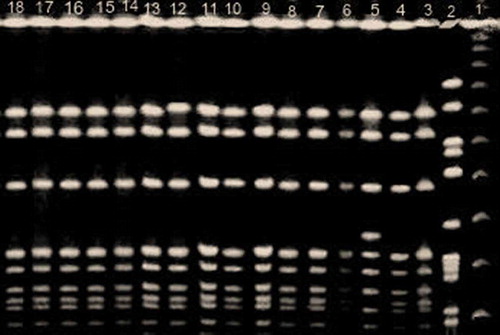
The restriction endonuclease patterns obtained with the PFGE following the SmaI treatment for the 24 MRSA isolates showed 10 different patterns. Fourteen isolates belonged to the same pulsotype, as in , another two carried similar pulsotypes (control group), and other eight showed un-similar pulsotype. Of interest, the 14 MRSA isolates with similar pulsotypes were isolated from psoriasis patients out of 16 with PVL negative.
Antibiotic resistance in PVL-positive and PVL-negative MRSA isolates
The results of antimicrobial susceptibility testing of 21 MRSA isolates from psoriatic skin lesions are shown in . Generally, the antibiotic resistance among PVL negative MRSA isolates was found higher as compared to PVL positive MRSA. There was a statistically significant difference in the resistance pattern only toward clindamycin. Ten out of 16 isolates were D-zone positive and considered to have inducible clindamycin resistance and are presumed to be resistant.
Table 2. Antibiotic resistance pattern of PVL positive and PVL negative MRSA isolates.
Clinical comparisons of psoriatic patients with PVL-positive and -negative MRSA
Psoriatic patients with PVL-positive and PVL-negative MRSA did not show significant difference with respect to sex, family history, nail involvement, pruritis and arthritis. In contrast, we had noticed a significantly higher PSAI score among patients harboring PVL-positive isolates compared to PVL negative isolates (33.06 ± 0.9 vs. 22.94 ± 1.08; p< 0.001) beside a significant difference in the age in which PVL positive cases were younger than those with negative isolates as shown in and .
Table 3. Clinical characteristics of patients with PVL positive MRSA.
Discussion
n the current study, the prevalence of S. aureus was 34.4% among psoriasis cases, this is in agreement with Ng CY et al. who reported 35.3% and Flytström et al. who reported 37.5% [Citation12,Citation22]. A higher prevalence of 64% and 46.2% were reported in previous studies [Citation23,Citation24]. However, other studies reported lower prevalence [Citation25,Citation26]. In the present study, 17.8% of the control group was S. aureus positive; similarly was reported by Fahlén et al. [Citation26]. On the other hand, the lower prevalence was reported in different literatures [Citation22–Citation26]. S. aureus showed a significant skin colonization in psoriasis group when compared with the control group and this is parallel to that reported by Ng CY et al. who mentioned that patients with psoriasis were 4–5 times more likely to be colonized by S. aureus than healthy controls [Citation12]. There is evidence that S. aureus peptidoglycan inhibits apoptosis, and affects keratinocyte proliferation [Citation27]. Also, staphylococcal antigens regulate inflammatory mediators such as interleukin (IL)-22, which inhibit the terminal differentiation of keratinocytes [Citation28]. In the current study, the prevalence of MRSA positive cases was 23.3% in psoriatic patients. A lower prevalence of 11% and 9% were reported by others [Citation12,Citation29]. In the present study, 3.3% of the control group was positive for MRSA, a finding close to that reported by Ng CY et al. (2.6%) [Citation12]. However, a higher prevalence (12%) was reported by others [Citation25]. MRSA colonization was significant in psoriasis group when compared with the control group in our study and this is in agreement with Hsu et al. who reported that psoriasis patients were more susceptible to MRSA by twofolds than those without [Citation30]. In this study, we detected five PVL positive MRSA isolates from psoriatic skin lesions and this was in parallel with Pouessel et al. study who reported an association between PVL-positive MRSA and psoriasis [Citation31]. Our results of antimicrobial sensitivity testing revealed higher resistance trend in PVL negative MRSA isolates compared to PVL positive MRSA isolates; however, there were no statistically significant differences except in case of clindamycin. The same result was obtained by Elizuret al. and Shrestha et al. who reported that PVL genes were positively correlated with erythromycin resistance and clindamycin-susceptible phenotype which was confirmed by the presence of a macrolide efflux protein [Citation32,Citation33]. The current study reported an increase in the psoriasis severity score among patients harboring PVL positive isolates when compared to PVL negative isolates, this is in parallel to Zhang et al. who reported that PVL genes are more common among isolates causing a clinical problem and larger skin lesions than among isolates causing asymptomatic colonization [Citation34]. The clinical squeal of PVL positive MRSA is more serious than those with PVL negative MRSA because this toxin stimulates the secretion of pro-inflammatory molecules as LTB4 which is a potent chemokine for macrophages, and neutrophils, decrease the percentage of Treg cells and the expression of Foxp3, and increase the expression of RORγt mRNA, that act as DNA-binding transcription factor and plays an important regulatory role by reducing apoptosis and promoting the differentiation of T-lymphocyte into pro-inflammatory T helper 17 cells [Citation35]. In this study, 14 PVL negative MRSA isolates showed the same pulsotype pattern; however, the rest of MRSA isolates from psoriasis patients, seven isolates showed different pulsotypes. Up to our knowledge, there is no study that makes PFGE for MRSA in psoriasis patients, so this point needs further studying to clear if there is a certain type of MRSA induce or worsen the prognosis of this disease.
Conclusion
MRSA infections remain a significant threat to human health and our study reported a significant MRSA colonization in psoriasis group and showed that the presence of PVL contributes to increase the severity of this disease; however, the number of PVL + MRSA isolates were very limited and further work is needed to investigate these possible differences and to clear if there is a common type of MRSA infection in psoriasis patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8:259–270.
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762.
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–1846.
- Peterson AE, Davis MF, Julian KG, et al. Molecular and phenotypic characteristics of healthcare- and community-associated methicillin-resistant Staphylococcus aureus at a rural hospital. PLoS One. 2012;7:e38354.
- Falagas ME, Karageorgopoulos DE, Leptidis J, et al. MRSA in Africa: filling the global map of antimicrobial resistance. PLoS ONE. 2013;8:e68024.
- Abouelfetouh A. The status of methicillin resistance among Egyptian Staphylococcus aureus isolates: an overview. Infect Disord Drug Targets. 2017;17(1):67–69.
- Ito T, Ma XX, Takeuchi F, et al. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651.
- Ma XX, Ito T, Tiensasitorn C, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–1152.
- Robinson DA, Enright MC. Evolutionary models of the emergenceof methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:3926–3934.
- Said-Salim B, Mathema B, Braughton K, et al. Differential distribution and expression of Panton-Valentine leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J ClinMicrobiol. 2005;43:3373–3379.
- Etienne J. Panton-Valentine leukocidin: a marker of severity for Staphylococcus aureus infection? Clin Infect Dis. 2005;41:591–593.
- Ng CY, Huang YH, Chu CF, et al. Risks for Staphylococcus aureus colonization in patients with psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2017;177:967–977.
- Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008 Jun 1;46(5):S350–S359.
- Brembilla NC, Senra L, Boehncke W-H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front Immunol. 2018;9:1682.
- Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819–824.
- Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica. 1978;157:238–244.
- Clinical and Laboratory Standard Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 29th ed. CLSI supplement M100; 2019 ; Wayne, PA: Clinical and Laboratory Standard Institute (CLSI).
- Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132.
- Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947–4955.
- Centers of Disease Control and Prevention (CDC). Pulsed-field Gel Electrophoresis (PFGE); 2016. [cited 2016 Oct 23]; Available from: https://www.cdc.gov/pulsenet/pathogens/pfge.html
- Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced bypulsed-field gel electrophoresis: criteria for bacterial straintyping. J Clin Microbiol. 1995;33:2233–2239.
- Flytström I, Bergbrant I-M, Bråred J, et al. Microorganisms in intertriginous psoriasis: no evidence of Candida. Acta Derm Venereol. 2003;83:121–123.
- Balci DD, Duran N, Ozer B, et al. High prevalence of Staphylococcus aureus cultivation and super antigen production in patients with psoriasis. Eur J Dermatol. 2009;19:238–242.
- Casas C, Ribet V, Alvarez-Georges S, et al. Modulation of interleukin-8 and staphylococcal flora by Avène hydrotherapy in patients suffering from chronic inflammatory dermatoses. J Eur Acad Dermatol Venereol. 2011;25:19–23.
- Elfatoiki FZ, El Azhari M, El Kettani A, et al. Psoriasis and staphylococcus aureus skin colonization in Moroccan patients. Pan Afr Med J. 2016;23:33.
- Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15–22.
- Vázquez-Sánchez EA, Rodríguez-Romero M, Sánchez-Torres LE, et al. Peptidoglycan from Staphylococcus aureus has an anti-apoptotic effect in HaCaT keratinocytes mediated by the production of the cellular inhibitor of apoptosis protein-2. Microbiol Immunol. 2014;58:87–95.
- Niebuhr M, Scharonow H, Gathmann M, et al. Staphylococcal exotoxins are strong inducers of IL-22: a potential role in atopic dermatitis. J Allergy ClinImmunol. 2010;126:1176–1183.
- Ng CY, Huang YH, Chu CF, Wu TC, Liu SH. Risks for Staphylococcus aureus colonization in patients with psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2017 Oct;177(4):967977. doi: 10.1111/bjd.15366. Epub 2017 Sep 8.
- Hsu DY, Gordon K, Silverberg JI. Serious infection prevalence higher for inpatients with psoriasis vs. those without. J Am Acad Dermatol. 2016;75:287–296.
- Pouessel G, Ythier H, Carpentier O. Childhood pustular psoriasis associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Pediatr Dermatol. 2007;24:401–404.
- Elizur A, Orscheln RC, Ferkol TW, et al. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest. 2007;131:1718–1725.
- Shrestha B, Singh W, Raj VS, et al. High prevalence of Panton-Valentine leukocidin (PVL) genes in nosocomial-acquired Staphylococcus aureus isolated from tertiary care hospitals in Nepal. Biomed Res Int. 2014; 10:790350. doi: 10.1155/2014/790350. Epub 2014 Jun 18.
- Zhang K, McClure J-A, Elsayed S, et al. Coexistence of Panton-Valentine leukocidin–positive and –negative community-associated methicillin-resistant Staphylococcus aureus USA400 sibling strains in a large Canadian health-care region. J Infect Dis. 2008;197:195–204.
- Keijsers RRMC, Hendriks AGM, van Erp PEJ, et al. In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J Invest Dermatol. 2014;134:1276–1284.