ABSTRACT
Palm oil (PO), the most utilized vegetable oil, continues to be the object of adulteration, especially with Sudan-IV dye (S4D), notwithstanding the health implications of such acts. This study investigated the effects of S4D-adulterated PO on tissue damage/function and inflammatory status. Thirty male albino rats were grouped into five (- n=6); Control, PO, PO+S4D (100 mg/kg), PO+S4D (250 mg/kg), and S4D (250 mg/kg) and treated for 21 days. Exposure to S4D occasioned significant (p < 0.05) elevations in the serum activities of ALT, AST, ALP, and LDH. Contrastingly, the activities of these enzymes were reduced in the liver and kidneys. Serum levels of uric acid, BUN, and creatinine were also elevated in the serum of S4D-exposed rats. Gene expression analyses revealed upregulated expression of CRP and COX-2 in the liver of S4D-exposed rats, while the expression of IL-10 and BAX were downregulated compared to the control group. In the kidneys, exposure to S4D upregulated the expressions of TNFα, IL-1β, and KIM-1 compared to control. Our resultspresent empirical evidence of the adverse effects of adulterating PO with S4D, highlighted by the impaired hepatic and renal function, as well as pro-inflammatory responses elicited in rats exposed to S4D, via PO and alone.
Introduction
Despite human efforts, the world’s population has continued to increase, and there is an ever-present demand for food, among other amenities [Citation1]. Subsequently, the food industry has been forced to evolve, leading to the intensified efforts to improve the organoleptic attributes of food products, which has translated into the increased occurrence of food additives in these food products [Citation2,Citation3]. However, consumers and food control agencies have continued to query the need and safety of these additives, given the numerous reports of the adverse effects associated with exposure to these additives. For example, monosodium glutamate has been implicated in testicular damage [Citation4], neurodegenerative diseases [Citation5], and metabolic syndrome [Citation6]. Increased risks of various cancer have been associated with the intake of nitrites and nitrosamine (found in processed meat and other similar products as a preservative) [Citation7], while [Citation8] reported hepato-renal dysfunction and oxidative stress biomarkers in rats exposed to tartrazine and carmoisine (typical examples of food azo dyes).
The use of some of these food additives is currently prohibited, owing to their evident adverse effects on humans [Citation2]. However, this has not deterred the blatant use of these substances as food additives, especially in Western Africa, some parts of Asia, and even in the United States, with palm oil being a major victim of this adulterating act [Citation9,Citation10]. Palm oil (PO), one of the most consumed edible oil, is a vegetable oil obtained from the mesocarp of oil palm fruits. The intense red color of PO is typically used to appraise its quality [Citation9,Citation11]. Color is one of the most conspicuous organoleptic attributes of food products, easily appraised without necessarily purchasing the product [Citation12]. Therefore, food vendors frequently add dyes to PO to enhance its color [Citation9,Citation10]. Unfortunately, the upsurge in the use of palm oil, particularly in homemade and ready-to-eat meals, means that even more people will be affected by the adverse effects associated with the intake of these additives.
The primary colorant used to adulterate PO, according to the surveys by [Citation9,Citation10] is Sudan IV dye (S4D). S4D (C24H2ON4O, also called Scarlet Red) is a lysochrome diazo dye used as a colorant in shoe polish, petrol, soaps, plastics, oil, printing inks, etc. It is also used to color foods because of its deep scarlet color and low cost [Citation13]. Although illegal, S4D has continued to be found in PO at levels far greater than the EU detection limits of 0.6–1.2 µg/ml [Citation10]. Following ingestion and absorption, S4D, like most other azo dyes, is metabolized by azoreductases and peroxidases, producing aromatic amines and semiquinone radicals, which can further decompose to generate superoxide (O2•-) and hydroxyl (•OH) radicals, leading to oxidative stress and subsequent cell or tissue damage [Citation13,Citation14]. Also, the reactive species may attack cellular and sub-cellular components, thereby impairing organ function [Citation15]. Besides its potential tissue-damaging effects, oxidative stress (potentially induced via S4D metabolism) may provoke inflammatory responses via the activation of the NLRP3 inflammasome and NF-κB signaling pathways, resulting in the downstream induction and activation of the several pro-inflammatory cytokines [Citation15,Citation16].
Worryingly, reports on the impact of adulterating PO with S4D in vivo, particularly on organ function and inflammatory response, are sparse in Literature and remain largely unexplored. Thus, this study investigated the effects of S4D-adulterated PO on tissue damage/function and the inflammatory status of male albino rats.
Methodology
Test materials and chemicals
Unadulterated PO was procured personally from an oil mill in Abeokuta, Ogun State, Nigeria, while S4D was purchased from NICE Chemicals Pvt. Ltd. (Kochi, Kerala, India). TRIZOL and DNAse-I enzyme were products of ThermoFisher Scientific (Waltham, Massachusetts, USA). Gene-specific primers were synthesized by ShineGene Bio-Technologies, Inc. (Xuhui District, Shanghai, China). All other chemicals, unless otherwise specified, were products of the British Drug House Chemicals Limited (Poole Dorset, England).
Ethical approval
All conditions of animal handling followed the ARRIVE guidelines [Citation17], while this study received approval from the Animal Ethical Committee of the Department of Biochemistry, Federal University of Agriculture, Abeokuta, Nigeria (FUNAAB), with approval number: FUNAAB/CBS/BCH/20150888.
Experimental protocol
Thirty male albino rats (150–200 g) were procured from the animal house of the College of Veterinary Medicine, FUNAAB, housed in the departmental animal house, and fed diet and water ad libitum. Diet was composed of 65% carbohydrates, 20.3% protein, 5% fat, 5% fiber, 3.7% AIN-93 mineral mixture, and 1% AIN-93 vitamins mixture [Citation8]. Acclimation was for a week, after which rats were randomly distributed into five groups of six: Control, palm oil (PO), PO + S4D (100 mg), PO + S4D (250 mg), and S4D (250 mg). Control group received diet alone; PO group received diet supplemented with 10% PO (i.e. 100 g PO/kg of diet); PO + S4D (100 mg) and PO + S4D (250 mg) groups received diet supplemented with PO (10%) containing 100 mg or 250 mg of S4D per liter of PO, respectively, which is equivalent to 10 and 25 mg of S4D/kg of diet. The S4D (250 mg) group received diet supplemented with an equivalent amount of the 250 mg of S4D per liter of PO, albeit without PO (i.e. 25 mg of S4D/kg of diet). The control and the S4D (250 mg/kg) diets were supplemented with soy oil so that all diets contained similar amounts of fats.
The study lasted for three weeks, after which the rats were sacrificed. Rats fasted overnight, and blood was collected via retro-orbital plexus into plain tubes, allowed to clot, and spun for 10 minutes at 3500 rpm to obtain the serum. The animals were dissected after cervical dislocation; the liver and kidneys were excised. Ten percent homogenates were prepared in phosphate buffer (0.05 mM, pH 7.4) for biochemical analyses. Small portions (~40 mg) of the liver and kidneys were also preserved in TRIZOL for gene expression analyses.
Biochemical assays
The activities of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were determined in the serum, as well as in the liver and kidney homogenates, using commercially available kits and following the manufacturers’ instructions. The kits for ALT, AST, and ALP were products of Randox Laboratories Limited (Crumlin, United Kingdom), while the LDH kit was a product of Biosystems Diagnostics [Costa Brava, Barcelona, Spain). The total protein concentration was determined using the Coomassie blue method of [Citation18] with serum albumin as standard. Serum levels of uric acid, blood urea nitrogen (BUN], and creatinine were also determined using commercially available kits produced by Randox Laboratories Limited, as per the manufacturer’s instructions.
Gene expression profiling
The expression levels of genes were assessed relative to the house-keeping gene (β-actin) using the reverse transcriptase-polymerase chain reaction technique [Citation19]. Briefly, total mRNA was isolated from the liver tissues using TRIZOL and purified by DNAse-I treatment. Reverse transcription of the purified DNA-free mRNA to cDNA was done using ProtoScript® First Strand cDNA Synthesis Kit (New English Biolabs (NEB), Ipswich, Massachusetts, USA) and then amplified via a polymerase chain reaction, using OneTaq® 2X Master Mix (NEB) with gene-specific primers (). Representative snapshots of reverse transcription-polymerase chain reaction-agarose gel electrophoresis were taken, and the band density [Image-J software; Citation20] plotted as a bar graph (mean ± SEM, n = 6).
Table 1. Gene-specific primers
Statistical analysis
The data obtained are as mean ± standard deviation of six rats. Differences between groups were compared with one-way Analysis of Variance (ANOVA), and where heterogeneity occurred, means were separated using Dunnett’s Multiple Comparison. All analyses and graphs were plotted using Graph Pad Prism (Version 8.01).
Results
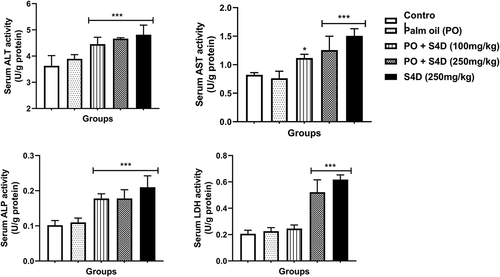
Our results illustrated that exposure to S4D-adulterated PO (100 and 250 mg) and S4D (250 mg) occasioned significant elevations in the serum activities of ALT, AST, ALP, and LDH in comparison to the control group, except for the LDH activity in the PO + S4D (100 mg) group that was not significantly (p > 0.05) different from control ().
Figure 1. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the activities of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) in the serum of control and experimental rats. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used)
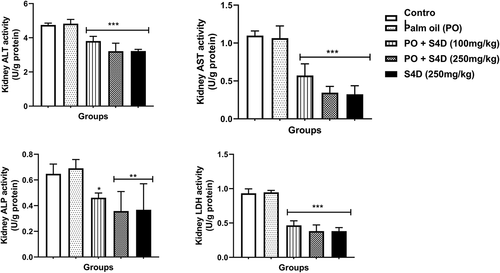
In the liver, the activities of ALT, AST, ALP, and LDH were significantly reduced compared to the control, except for the S4D + PO (100 mg) group, which had statistically similar ALP activity as the control. These S4D exposure-induced depletions in liver enzymes followed a dose-dependent trend and were more pronounced in the S4D group (). The activities of these organ function markers (ALT, AST, ALP, and LDH) in the kidneys followed a parallel trend as in the liver, with exposure to S4D, via PO and alone, provoking significant reductions in the activities of these enzymes (). Furthermore, exposure to PO alone did not cause any significant difference (p > 0.05) in the activities of these enzyme biomarkers in the serum, liver, and kidneys.
Figure 2. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the activities of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) in the liver of control and experimental rats. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used)
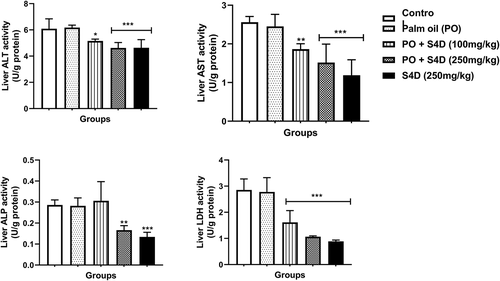
Figure 3. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the activities of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) in the kidneys of control and experimental rats. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used)
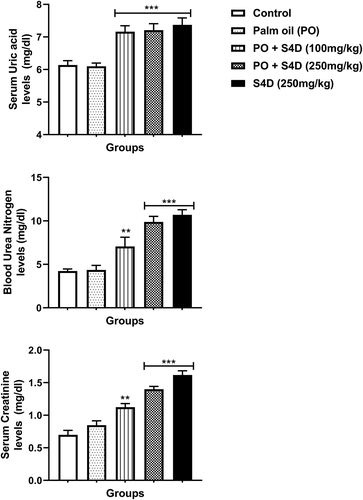
The serum levels of kidney function biomarkers (uric acid, BUN, and creatinine) were significantly (p < 0.05) increased in the S4D-exposed groups compared to the control and PO-groups, which were more pronounced in the groups exposed to 250 mg S4D (alone or in PO) ().
Figure 4. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the levels of uric acid, blood urea nitrogen (BUN), and creatinine in the serum of control and experimental rats. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used)
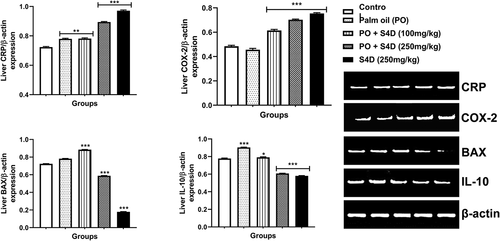
The liver expression of genes coding for C-reactive protein (CRP) and cyclooxygenase-2 (COX-2) were significantly (p < 0.05) upregulated, relative to β-actin, following exposure to PO + S4D (100 and 250 mg) compared to the control. Interestingly, CRP gene expression was significantly (p < 0.01) upregulated in the PO alone group in comparison to the control. On the other hand, the expression of Bcl-2 Associated X-protein (BAX) and interleukin 10 (IL-10) genes were significantly repressed in the PO + S4D (250 mg) and S4D (250 mg) groups while the expressions of BAX and IL-10 were upregulated in the PO + S4D (100 mg) group, compared to the control ().
Figure 5. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the expression of genes coding for C-reactive protein (CRP), cyclooxygenase-2 (COX-2), Bcl-2 Associated X-protein (BAX), and interleukin 10 (IL-10) in the liver of control and experimental rats. Expression levels were normalized against house-keeping gene β-actin. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used). A = control, B = PO alone, C = PO + S4D (100 mg), D = PO + S4D (250 mg), and E = S4D (250 mg)
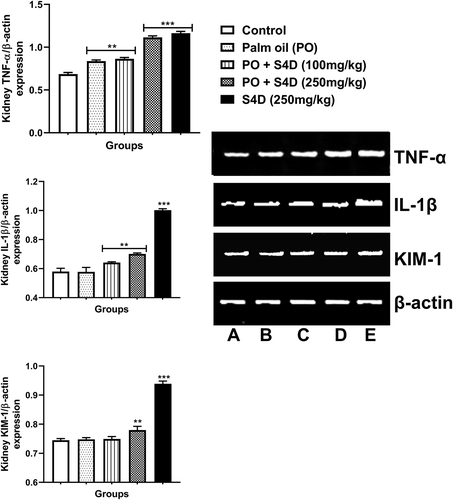
In the kidney, the expressions of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and kidney injury molecule-1 (KIM-1), relative to β-actin, were markedly upregulated in the S4D-exposed groups, particularly the 250 mg groups. Also, TNF-α gene expression was significantly (p < 0.01) upregulated in the PO alone group compared to the control while expression levels of IL-1β and KIM-1 were not statistically (p > 0.05) different to control ().
Figure 6. Effects of sub-acute exposure to Sudan IV dye (S4D)-adulterated palm oil (PO) on the expression of genes coding for tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and kidney injury molecule-1 (KIM-1) in the kidneys of control and experimental rats. Expression levels were normalized against house-keeping gene β-actin. Bars are mean ± standard deviation (n = 6), while *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively, compared to control group (ANOVA followed by Dunnett’s test was used). A = control, B = PO alone, C = PO + S4D (100 mg), D = PO + S4D (250 mg), and E = S4D (250 mg)
Discussion
We report for the first time the deleterious effects of sub-acute exposure to S4D, via PO and alone, on the hepato-renal functions and inflammatory status. Our results indicate that S4D induced hepato-renal damage, evidenced by elevation of ALT, AST, ALP, and LDH in the serum of S4D-exposed rats. These biomarker enzymes are localized in the cellular plasma membrane (ALP), cytosol (ALT and LDH), and mitochondria (AST) of the liver and kidneys, among other tissues, and their elevations in the blood are associated with tissue damage [Citation21,Citation22]. Thus, we attribute the elevation of serum activities of ALT, AST, ALP, and LDH to S4D-induced tissue damage and destruction of cell membrane integrity, resulting in the leakage of these enzymes into the blood. These tissue damaging effects of S4D may be mediated through the propagation of oxidative stress. A previous study reported a dose-dependent increase in reactive species production,following the exposure of HepG2 cells to Sudan I dye[Citation23]. Another in vitro study reported identical results following exposure to S4D, even after just sixty minutes [Citation13]. Indeed, studies have suggested that reactive species are invariably produced by azoreductases, cytochrome P450 monooxygenases, and peroxidases during the metabolism of S4D and other azo dyes [Citation13,Citation14,[Citation23]]. And because the liver and kidneys are major organs involved in the biotransformation of xenobiotics [Citation24], it is plausible to posit that these organs might have undergone oxidative stress instigated by S4D, possibly leading to tissue damage and the observed elevation in serum enzyme activities. Similar results on hepato-renal function enzymes have been reported for other food azo dyes, such as tartrazine, carmoisine [Citation8], and New Coccin [Citation14].
The S4D-exposed rats had significantly reduced ALT, AST, ALP, and LDH activities in the organs (the liver and kidneys), as against the control. This observation may be a sequela of the leakage of these enzymes from the cellular milieu into the extracellular milieu (blood), arising from S4D-induced tissue damage, as discussed earlier. Alternatively, a look into the biochemical functions of these enzymes may provide another perspective. ALT catalyzes the reversible amination of α-ketoglutarate (with alanine as the amino (-NH2) donor) to produce glutamate (while alanine is deaminated to pyruvate), and as a result, is involved in gluconeogenesis (via production of pyruvate) and amino acid metabolism (via breakdown of alanine and liberation of glutamate) [Citation25]. In a like manner, ALT transfers -NH2 from aspartate to α-ketoglutarate, thereby producing oxaloacetate (an intermediate of the citric acid cycle) and glutamate [Citation25]. ALP has the physiological role of dephosphorylating various nucleotides and proteins and is therefore vital to the maintenance of homeostasis [Citation25]. LDH catalyzes the reversible conversion of lactate to pyruvate, using NAD+ and NADH as cofactors, and plays a role in energy metabolism, especially in hypoxic and anaerobic conditions when ATP production by oxidative phosphorylation is disrupted [Citation26]. These enzymes are therefore crucial for optimal organ function and the maintenance of homeostasis. Thus, the repression of their activities in the organs of rats that ingested S4D [via PO and alone) suggests that S4D impairs hepatic and renal function, in consonance with the reports of [Citation8,Citation27]. These authors demonstrated that the ingestion of synthetic food colorants impaired hepato-renal function in male rats.
Uric acid, BUN, and creatinine are clinically important biomarkers of kidney function. Uric acid is the byproduct of purine metabolism, while creatinine is produced by muscle (from creatine phosphate] and during protein catabolism. BUN is a measure of the amount of urea nitrogen present in the blood. Urea is a waste product of protein and amino acid, filtered by the kidneys into the urine. These markers are efficiently eliminated unchanged by the kidney, making them an important serum biomarker for kidney function [Citation28]. Increased levels of these markers (as is the case in S4D-exposed rats) may result from decreased blood volume (hypovolemia) or decreased filtration rate by the kidneys [Citation29]. Thus, the accumulation of these markers further affirms the impairment of renal function by S4D.
Inflammation, a component of the body’s intricate response to deleterious stimuli, such as xenobiotics and pathogens, is a defensive response involving several molecular mediators and the immune cells that secrete these mediators [Citation30]. Mediators involved in the inflammatory process include the interleukins, TNF-α, CRP, and cyclooxygenases [Citation30]. This study appraised the inflammatory response in the liver and kidneys following exposure to S4D-adulterated PO. In the liver, the expression of CRP and COX-2 were significantly elevated in the S4D-exposed groups. CRP is an acute-phase protein secreted predominantly by hepatocytes. CRP then binds to lysophosphatidylcholine present on the surface of dead or dying cells, thereby stimulating the immune system. Thus, the hepatic expression and therefore circulating levels of CRP are elevated during the inflammatory response [Citation31]. Cyclooxygenases are enzymes concerned with the metabolism of arachidonic acid and biosynthesis of prostanoids, which are potent pro-inflammatory compounds [Citation30]. While COX-1 is constitutively expressed in several tissues, the expression of COX-2 is inducible and becomes elevated during inflammation [Citation32]. The elevated expression levels of CRP and COX-2, observed in this study, suggest that S4D dye invoked inflammation, in line with the study of [Citation33]. This study reported that azo food dyes (sunset yellow, tartrazine, Allura red, and carmoisine) elicited cellular inflammatory responses and present potential health risks to the local population. On the other hand, liver expression of IL-10 was significantly repressed. IL-10 is an anti-inflammatory cytokine that predominantly inhibits the production of pro-inflammatory cytokines (e.g. TNFα and IL-1β) [Citation34]. Thus, its repression further substantiates the postulated pro-inflammatory events instigated by S4D. The expression of BAX was also inhibited in the liver tissues of S4D-exposed rats, possibly alluding to its carcinogenic properties [Citation13,Citation35] since BAX is involved in p53-mediated apoptosis of aberrant cells and is inhibited in most carcinomas [Citation36]. Moreover, the expression of acute-phase protein – CRP – was significantly elevated in the PO alone group. We attribute this inflammatory response to the rich saturated fatty acid (SFA) contents of PO, which are known initiators of inflammation via the TLR4/NFκB pathways [Citation37]. However, the expressions of other mediators were comparable to the control, while the anti-inflammatory cytokine IL-10 was elevated in the PO alone group. The latter may be a compensatory response to check the possible inflammatory response elicited by the SFA in PO. However, its expression was progressively repressed as the concentration of S4D increased, suggesting a repressed anti-inflammatory response in the hepatic cells, in alignment with the elevated expression of CRP and COX-2 (both inflammatory mediators).
In the kidneys, the expression of TNFα and IL-1β, two potent pro-inflammatory mediators [Citation34], were significantly upregulated in the rats exposed to S4D, via PO and alone, further corroborating the hypothesis that S4D can elicit pro-inflammatory responses in vivo. Unresolved inflammation eventually culminates in tissue damage. Indeed, KIM-1 (a sensitive protein biomarker that is copiously expressed in injured kidneys) was observed to be highly expressed in the kidney tissues of S4D-exposed rats. This may suggest the S4D damages the kidneys probably via inflammatory and oxidative mechanisms.
In conclusion, the results of this study present empirical evidence of the adverse effects of adulterating PO with S4D, highlighted by the impaired hepatic and renal function, as well as pro-inflammatory responses elicited in rats exposed to S4D, via PO and alone. Moreover, these adverse effects followed a dose-dependent manner; it would be interesting to see if these effects were also time-dependent. Nevertheless, we recommend that manufacturers, vendors, and consumers of food products, as well as food agencies, be sensitized about the toxic effects associated with the use of these food dyes.
Data availability
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Crist E, Mora C, Engelman R. The interaction of human population, food production, and biodiversity protection. Science. 2017;356(6335):260–264.
- Martins N, Roriz CL, Morales P, et al. Food colorants: challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci Technol. 2016;52:1–15.
- [WHO] World Health Organization. Food additives. WHO fact sheets; 2020 [cited 2021 Mar 8]. Available from: https://www.who.int/news-room/fact-sheets/detail/food-additives.
- Hamza RZ, Diab AEAA. Testicular protective and antioxidant effects of selenium nanoparticles on monosodium glutamate-induced testicular structure alterations in male mice. Toxicol Rep. 2020;7:254–260.
- Hussein UK, Hassan NE, Elhalwagy ME, et al. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules. 2017;22(11):1928.
- Bautista RJ, Mahmoud AM, Königsberg M, et al. Obesity: pathophysiology, monosodium glutamate-induced model and anti-obesity medicinal plants. Biomed Pharmacother. 2019;111:503–516.
- Hosseini F, Majdi M, Naghshi S, et al. Nitrate-nitrite exposure through drinking water and diet and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Clin Nutr. 2020;40(5):3073–3081.
- Amin KA, Hameid IIHA, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol. 2010;48(10):2994–2999.
- Andoh SS, Nuutinen T, Mingle C, et al. Qualitative analysis of Sudan IV in edible palm oil. J Eur Opt Soc Rapid Publ. 2019;15(1):1–5.
- Genualdi S, MacMahon S, Robbins K, et al. Method development and survey of Sudan I–IV in palm oil and chilli spices in the Washington, DC, area. Food Addit Contam. 2016;33(4):583–591.
- Alabi TD, Olabiyi FA, Oguntibeju OO. Palm oil: its antioxidant potential in diabetes mellitus. In: Preedy VR, editor. Diabetes. Cambridge (MA): Academic Press; 2020. p. 285–291.
- Nisa A, Zahra N, Butt YN. Sudan dyes and their potential health effects. Pak J Biochem Mol Biol. 2016;49(1):29–35.
- Zhang Y, An Y, Jiang L, et al. The role of oxidative stress in Sudan IV‐induced DNA damage in human liver‐derived HepG2 cells. Environ Toxicol. 2011;26(3):292–299.
- Demirkol O, Zhang X, Ercal N. Oxidative effects of Tartrazine (CAS No. 1934-21-0) and New Coccin (CAS No. 2611-82-7) azo dyes on CHO cells. J Verbrauch Lebensm. 2012;7(3):229–236.
- Hoffmann MH, Griffiths HR. The dual role of reactive oxygen species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic Biol Med. 2018;125:62–71.
- Liang X, Zhang D, Liu W, et al. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation induced by zinc oxide nanoparticles in A549 cells. Toxicol Ind Health. 2017;33(10):737–745.
- Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40(9):1769–1777.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254.
- Omotuyi OI, Nash O, Inyang OK, et al. Flavonoid-rich extract of chromolaena odorata modulate circulating GLP-1 in Wistar rats: computational evaluation of TGR5 involvement. 3 Biotech. 2018;8(2):1–8.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675.
- Chida K, Taguchi M. Localization of alkaline phosphatase and proteins related to intercellular junctions in primary cultures of fetal rat hepatocytes. Anat Embryol. 2005;210(2):75–80.
- Shibabaw T, Dessie G, Molla MD, et al. Assessment of liver marker enzymes and its association with type 2 diabetes mellitus in Northwest Ethiopia. BMC Res Notes. 2019;12(1):1–5.
- An Y, Jiang L, Cao J, and et al. (2007). Sudan I induces genotoxic effects and oxidative DNA damage in HepG2 cells. Mutat Res Genet Toxicol Environ Mutagen, 627(2), 164–170.
- Lindamood C. Xenobiotic biotransformation. In: Meeks RG, Harrison SD, Bull RJ, editors. Hepatotoxicology. Boca Raton (FL): CRC Press; 2020. p. 139–180.
- Aulbach AD, Amuzie CJ. Biomarkers in nonclinical drug development. In: Faqi AS, editor. A comprehensive guide to toxicology in nonclinical drug development. Cambridge (MA): Academic Press; 2017. p. 447–471.
- Alegre E, Sammamed M, Fernández-Landázuri S, et al. Circulating biomarkers in malignant melanoma. Adv Clin Chem. 2015;69:47–89.
- El-Wahab HM, Moram GS. Toxic effects of some synthetic food colorants and/or flavor additives on male rats. Toxicol Ind Health. 2013;29(2):224–232.
- Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165–177.
- Longo DL, Fauci AS, Kasper DL, et al., editors. Harrison’s principles of internal medicine. 19th ed. New York (NY): McGraw-Hill Medical; 2014. p. 611–614.
- Abdulkhaleq LA, Assi MA, Abdullah R, et al. The crucial roles of inflammatory mediators in inflammation: a review. Vet World. 2018;11(5):627–635.
- Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
- Tomić M, Micov A, Pecikoza U, et al. Clinical uses of nonsteroidal anti-inflammatory drugs (NSAIDs) and potential benefits of NSAIDs modified-release preparations. In: Č B, editors. Microsized and nanosized carriers for nonsteroidal anti-inflammatory drugs. Cambridge (MA): Academic Press; 2017. p. 1–29.
- Leo L, Loong C, Ho XL, et al. Occurrence of azo food dyes and their effects on cellular inflammatory responses. Nutrition. 2018;46:36–40.
- Steen EH, Wang X, Balaji S, et al. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care. 2020;9(4):184–198.
- Andoh SS, Nyave K, Asamoah B, et al. Optical screening for presence of banned Sudan III and Sudan IV dyes in edible palm oils. Food Addit Contam. 2020;37(7):1049–1060.
- Zhou L, Gao R, Wang Y, et al. Loss of BAX by miR-365 promotes cutaneous squamous cell carcinoma progression by suppressing apoptosis. Int J Mol Sci. 2017;18(6):1157.
- Li X, Ji R, Cui K, et al. High percentage of dietary palm oil suppressed growth and antioxidant capacity and induced the inflammation by activation of TLR-NF-κB signaling pathway in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019;87:600–608.
