ABSTRACT
A series of nonsymmetrical 2,3-diaminoquinoxaline derivatives prepared straight-forwardly by the C-N coupling between chloro-quinoxaline (2,3-DCQ) and various amino substrates. Synthesized products (4a-z) with excellent purity (1HNMR) without performing flash column chromatography purification. Investigated the antibacterial activity of selected nine compounds against Gram-positive, Gram-negative bacterial strains, and additional fungal strains. Overall, the selected screened compounds (4b, 4c, 4 h, and 4 n) presented significant antibacterial activity against bacterial strains. The resulting lead, displaying a novel quinoxaline compound (4c), showed antibacterial activity between 10.5 and 14.89 mm against all strains except A. Niger. Molecular docking studies revealed the binding poses and critical interactions of 2,3-diaminoquinoxaline analogues at the quinolone-binding site of S. aureus DNA gyrase. The docking results are in accordance with the antimicrobial testing data. Binding of these new analogues (as DNA intercalators) at quinolone-binding site involves the contribution from: I) Protein: Ser-1084, II) DNA: DG (G) 9 and DA (H) 13; and III) Mg+2 ion for their binding.
Introduction
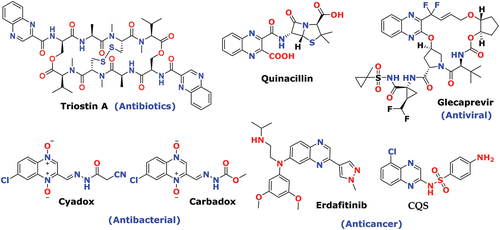
Globally, WHO has documented antimicrobial resistance in many first and second-line medications. [Citation1] Lack of advanced medication, healthcare prescribed most expensive with serve toxicity and even less effective to treat chronic bacterial infection. Because of that, prolonged hospital stays and high cost of treatment with poor outcomes increase the tremendous burden on healthcare and may cause leading cause of death in the future. [Citation2] The most advanced second line, antimicrobial medication (vancomycin and methicillin) has developed resistance against Staphylococcus aureus and a similar trend has been shown in the first line, fluconazole against candida fungal strain. [Citation3] Even though there are many medications existing for antimicrobial treatment, no one causes severe adversative effects [Citation4] such as local inflammation (penicillins), allergic reaction, phototoxicity (tetracyclines), effect on the liver, gray baby disorder, and myelosuppression (chloramphenicol) [Citation5]. The bacterial agent can undergo a mutation that causes resistance to corresponding medication or antibiotics, and finally less effectively binds with target sites like alteration in MOA, chemical alterations of the drug, or by other means. [Citation6] Among that, major threatening is bacteria-produced biofilm to reduced penetration effect or neutralized antibiotics effect. [Citation7,Citation8] Quinoxaline motif is a key structural unit; it exists in various biologically active compounds. In recent research, many small molecules of quinoxaline have a tremendous demand in medicinal chemistry, broadly known as potent antiproliferative and antimicrobial agents. The quinoxaline-bearing antibiotics; including, Triostin A and similar kinds of levomycin and echinomycin to introduce into dsDNA and are advantageously effective toward the bacterial diseases caused by gram-positive bacteria. [Citation9,Citation10]
Quinoxaline has also documented to have promising anticancer activity in various research profiles and the U.S. FDA approved the potential anticancer drug for urothelial cancer and bladder cancer under the brand name Balversa (erdafitinib). [Citation11] Quinoxaline scaffold is pharmacology impressive, displaying an array of interesting biological activities such as antibacterial [Citation12–16], anti-tubercular [Citation17–Citation17], antimalarial [Citation20–Citation20], anti-viral [Citation23,Citation24], anti-HIV [Citation25], anti-inflammatory [Citation26–29], anti-fungal [Citation30–33], anti-amoebic [Citation34], anti-cancer [Citation35–Citation38], anti-proliferative [Citation39,Citation40], antitumor [Citation41,Citation42], antihypertensive [Citation43], anticonvulsant [Citation44–Citation44], kinase inhibitor [Citation47], antiepileptic [Citation48,Citation49], anti-HCV [Citation50,Citation51], analgesic [Citation52,Citation53] and also used in anthelmintic drugs. [Citation54] Quinoxaline-based drugs presenting a broad range of diverse classes of biological activities as an outcome of diverse functionality on central structure . Quinoxaline also used for crop protection in Agro-industries as a major component of insecticides [Citation55], herbicides [Citation56], and fungicides. [Citation57] Besides their medicinal and crop protection applications these compounds widely used as dyes for solar cell applications, [Citation58,Citation59] fluorescent materials [Citation60], organic semiconductors, [Citation61–63], and corrosion inhibitors for metals. [Citation64] Because of the great call of antimicrobial therapy, continuous innovative research are going on quinoxaline scaffolds to hit novel candidates that may be, save the life for future prospects.
Results 2.1 chemistry
The various steps during the synthesis of target compounds (4a-z) illustrated in Scheme 1. The reaction of benzene-1,2-diamine (OPD) with oxalic acid in refluxing 4 N HCl aqueous solution gave quinoxaline-2,3-dione (1). [Citation65] Under reflux conditions, quinoxaline-2,3-dione (1) reacted with SOCl2 in the presence of the catalytic amount of DMF in DCE solvent to afford 2,3-dichloro- quinoxaline (2). [Citation65] Subsequently, intermediate (2) treated with different alkyl amines (a-d) in the presence of K2CO3 to afford key intermediates (3a-d). Finally, treatment of these compounds (3a-d) with arylamine in refluxing ethanol furnished the target compounds of Series (4a-o), (4p-r), (4s-u), and (4 v- z) in .
Table 1. Compounds of the scheme-1 (1–4 series) (N2-alkyl-N3-phenylquinoxaline-2,3-diamine derivatives): reaction time, yield, and M.P. of each compound
K. S. Ahmed et al. (2019) designed and synthesized novel molecules based on N3-alkyl-6-nitro-N2-benzyl quinoxaline-derivatives. The nitro-group of quinoxaline alters the DNA structure of bacterial cells and inhibits the DNA synthesis and as a result, it presented an antibacterial profiles. [Citation66] S. Paliwal et al. (2017) designed and synthesized small drug-like novel compounds of substituted Phenyl-3-Hydrazinyl-Quinoxaline-2-amines derivatives showed potent anti-microbial activities. From docking study of compounds revealed, it binds with the specific amino acid of dihydrofolate reductase protein of Staphylococcus aureus (PDB ID-4XE6). [Citation67] From the above research work [Citation66,Citation67], we have designed a novel hybride scaffold (4) in , which have combination effect and their binding site from 1,3 nitrogen interaction of quinoxaline ring with alkyl amine and similar interaction with aromatic amine. From the reported antimicrobial activites, [Citation66,Citation67] similar trends has been also adopted in selected screened compounds (4b, 4c, 4 h, 4 l, 4 m, 4 n, 4 w, 4x and 4z) in scaffold (4), among them, fluorinated compound (4b, 4c, 4 h and 4 n) presented broad and moderate activity on all bacterial strains.
Scheme 1. Reagents and conditions: (a) (COOH)2.2H2O, 4 N HCl, 100°C, 6 h; (b) SOCl2, DCE, Cat. DMF, 100°C, 6 h; (c) Alkyl amine (derivatives), K2CO3, DMF, rt, 12 h; (d) Aniline (derivatives), ethanol, reflux, 12 h.
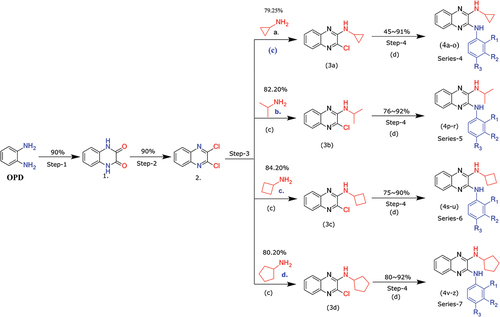
Figure 2. Novel designed from the privileged structure (a) Antibacterial activity from DNA intercalators [Citation66] (b) Hydrazine binding with specific amino acid of Dihydrofolate reductase protein. [Citation67].
![Figure 2. Novel designed from the privileged structure (a) Antibacterial activity from DNA intercalators [Citation66] (b) Hydrazine binding with specific amino acid of Dihydrofolate reductase protein. [Citation67].](/cms/asset/e8576be3-542c-42e7-bac6-47d4c37fea2d/teba_a_2049085_f0002_oc.jpg)
Evaluation of biological activity
Antimicrobial and antifungal activities
They bought the test microorganisms from the National Collection of Industrial Microorganisms, National Chemical Laboratory (NCL), Pune (India). Nutrient agar, MGYP, and Potato Dextrose agar (25°C, 24 h) were used to cultivate bacteria and fungi, respectively. It measured the zone of inhibition in mm. Disc diffusion method [Citation68,Citation69] using sterile paper disk (diameter 6 mm), against bacterial strain in the concentration of 100 μg/disk screened for antibacterial activity and screened these compounds for antifungal activity against fungi Aspergillus Niger and Candida albicans by the disc diffusion method () in the same concentration. The antibacterial activities compared with standard drugs viz. Chloramphenicol (10 μg/disk) and antifungal activity with Amphotericin B (100 μg/disk). The selected nine compounds were dissolved in DMSO, which were used as a control.
Table 2. Antimicrobial and antifungal activity, zone of inhibition (mm)
Table 3. Physicochemical property estimation (QikProp properties) of new designs
Table 4. Docking scores, residue contacts and ∆G of binding scores of proposed designs. Ch; Chloramphenicol
S. aureus; Staphylococcus aureus, B. subtilis; Bacillus subtilis, E. coli; Escherichia coli, K. pneumoniae; Klebsiella pneumoniae, S. typhi; Salmonella typhi, C. albicans; Candida albicans, A. Niger; Aspergillus Niger, Ch; Chloramphenicol, Am; Amphotericin B., (NA; Not applicable, Nt; Not tested).
Structure–activity relationship study
The above biological data ( or ) of the newly synthesized compounds revealed that all the nine novel derivatives adopted a significant antimicrobial and antifungal profile against the tested bacterial and fungal strains.
Figure 3. The comparison between the inhibition zones of compounds (4b-4z) and standard drugs against bacterial and fungal strains.
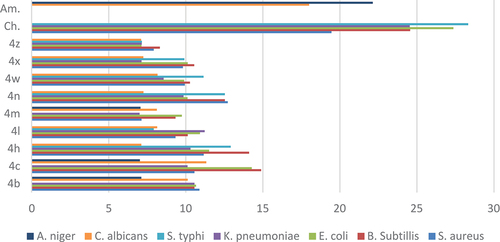
The results of antimicrobial screening showed that most of the studied compounds displayed variable growth inhibitory effects on the tested gram-positive, gram-negative bacterial and pathogenic fungal strains. As expected, we distinguish a vibrant dissimilarity in the activity between derivatives within the entire series, directing to the strengthening and diminishing effects of substitution at C-2 and C‐3 of the quinoxaline scaffold. The diameter of (Zone of inhibitions) IZs of the antifungal activity has shown almost the same tendency as the antibacterial activities. Concerning the outcome of substitution at the C-3 position on aryl ring is clear that the change of such a substituent may have a remarkable effect on the antimicrobial activity, which may improve or decreased, depending on the electronic nature on the ring. Individually, aryl ring at C-3 having diverse group, fluorinated compound (4c, 4 h) [Citation67] enhanced the activity because of an electron-withdrawing group and the same trend shown in the compound (4b, 4 n) because of the CF3 group [Citation66] but directing CH3 and – OCH3 compound (4 m) decreases activity. Similar steric effect, diminished activities at C-2 position, cyclo-pentane series of compound (4x and 4z) presented inferior activities compare to the cyclo-propyl series of compound (4b and 4 l). The zone of inhibition (mm) shown in or , revealed that compound (4c) could inhibit the growth of the gram-positive bacteria; B. subtilis and Gram-negative bacteria; E. coli in vitro having a zone of inhibition between 14.28 and 14.89 mm. Among the synthesized derivatives, compound 4c presented moderate activity on C. albicans is 11.33 mm. We do not exactly explain the SAR from few compounds but further, modification in structure can enhance the activity and may identify lead molecules from the same scaffolds.
Molecular modeling study
The synthesized and screened nine 2,3-diamino quinoxaline derivatives were subjected for physicochemical property estimation to understand their drug-likeness. Next, molecular docking was employed for predicting the binding modes, interactions and binding affinities of these compounds.
Physicochemical property estimation
The estimated physicochemical properties of top nine compounds are shown in Table-3. Almost all the compounds possess nearly favorable drug-like properties with minor violations (acceptable) for Lipinski’s rule of five. These data of screened hit hints for their possibility as potential drug-like candidates.
Binding mode analysis from docking studies
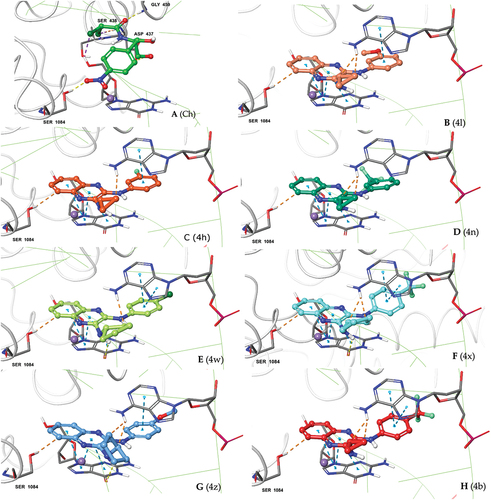
Putative binding modes of both orthosteric or allosteric binders can be accuractly predicted using molecular docking approach [Citation70–72]. The molecular docking can also be effectively used for virtual-screening of compounds [Citation73]. Here, we also used molecular docking approach to predict the binding modes of 2,3-diamino quinoxaline derivatives for their antibacterial activity via DNA intercalation. The results of binding mode analysis are summerized in Table-4 . We used chloramphenicol as a reference compound to mimick our earlier experimental design. The binding modes of 2,3-diamino quinoxaline derivatives and reference compound corroborate quite well and is nearly comparable with antimicrobial testing data. The posing of our designs at quinolone-binding site of S. aureus DNA gyrase with DNA is shown in . The consistency in binding poses of our screened designs at quinolone-binding site is evident from the pose analysis as shown in (4). The interaction with Ser-1084 was seen for all the docked designs. Additionally, the intercalation through pi-pi stacking of quinoxaline moiety between DG (G) 9 and DA (H) 13 DNA base pairs were evident (2D ligand interaction maps in Supplementry . The important role of metal ion (Mg+2 or Mn+2) in mediating coordinated quinolone-metal-bridging is well documented [Citation74]. Similarly in our all docked poses the metal-coordination of pyrazine ring nitrogen with crystal bound Mg+2 ion is observed (indicated in SI 2D ligand interaction maps). The Glide docking scores for nine tested designs were ranged from −5.06 to −7.18 kcal/mol. However the prime MM-GBSA scores of the selected poses ranged from 0.57 to −50.26 kcal/mol showing nearly comparable values to the values from antimicrobial testing data.
Computational modeling
Initially the physicochemical properties of most promising quinoxaline designs were calculated using Schrödinger [Citation75] QikProp module [Citation76]. Then their binding modes were estimated using GLIDE [Citation76,Citation77,] docking module of Schrodinger Suite. The prime MM/GBSA [Citation78,Citation79] G∆ of binding was calculated for evaluating the binding affinities of new designs.
Generation of 3D molecular structures
The quinoxaline derivatives were modeled and minimized using Hyperchem7.5 [Citation80] using MM+ force field. The output mol2 files were subjected for ligand preparation at physiological pH of 7.4 with LigPrep module.
Protein structure selection and preparation
The Quinoxaline derivatives binds as DNA intercalators [Citation66] thereby shows antibacterial [Citation12–16], activity. Accordingly, S. aureus DNA gyrase crystal structure (PDB ID: 2XCT) [Citation74] complex with ciprofloxacin and DNA was selected for performing molecular docking studies. Fully automated Protein preparation wizard protocol was used to prepare the protein structure at ionization pH of 7.4. The H-bonding network was optimized and restrained minimization was performed using OPLS-2005 force field with the RMSD convergence criteria of 0.30 Å for all heavy atoms.
Molecular docking study
The fully prepared protein (containing DNA and ciprofloxacin) and ligand structures were considered for the molecular docking study. The GLIDE receptor grid was generated using the centroid of crystal-bound ciprofloxacin molecule within the search space of 14 Å box size for docking ligands 14 Å length at equally spaced 2 Å grids. The standard precision (SP) mode Glide docking was performed with a soft potential function at van der Waals radii scaling of 0.5 with applied core constraints for generating consistent poses of quinoxaline derivatives. The obtained ligand poses were finally selected based on the docking scores and presence of Ser1084 interaction. The images of the ligand poses were generated using Pymol (Schrödinger LLC, 2010) [Citation81–82]. The 2D ligand interaction maps of poses were obtained using ligand-interaction tool from Schrödinger suite.
Experimentals
Instruments and appparatus
General reagents and solvents for the synthesis of compounds were analytical grade (AR) and used without further purification. Air-sensitive reactions were carried out under dry nitrogen or argon atmosphere. Thin-layer chromatography was performed on silica gel plates (Merck Silica Gel 60, F254), and the spots were visualized under UV light (254 and 365 nm). All melting points were recorded on open glass capillary tubes using the Stuart Digital Melting Point SMP10 and are uncorrected. 1H NMR was recorded at 500 MHz (Bruker DPX) frequency and 13C NMR spectra were recorded at 150.85 MHz (Bruker DPX) in DMSO-d6 or CDCl3 solvent using tetramethylsilane (TMS) as the internal standard. Mass spectra were measured with ESI ionization in MSQ LCMS mass spectrometer. Flash column chromatography was carried out using silica gel (Merck, 230–240 mesh) and Key intermediate (3a-d) were eluted in n-pentane/ethyl acetate as a mobile phase and the pure product (4a-z) were purified from recrystallization (diether ether). The coupling constant was recorded in Hz. The following abbreviations are used to explain the multiplicities: s = singlet, d = doublet, dd = doublet of doublets, dt = doublet of triplets, t = triplet, q = quartet, m = multiplet.
Chemistry
Synthesis of 1,4-dihydroquinoxaline-2,3-dione (1)
It was prepared according to previously reported method. [Citation65] The product was recrystallized from ethanol to give compound (1). White solid (13.5g, 90%). m.p. 360–362°C (lit. > 300°C). [Citation65] 1H NMR (500 MHz, DMSO-d6): δ 11.90 (br s, 2H), 7.11 (dd, J = 5.0 Hz, 10 Hz, 2H), 7.06 (dd, J = 5.0 Hz, 10 Hz, 2H). 13C NMR (150.85 MHz, DMSO-d6) δ: 155.Citation36, 125.79, 123.18, 115.30
Synthesis of 2,3-dichloroquinoxaline (2)
According to reported method. [Citation65] The product was recrystallized from ethanol to give compound (2). White needle (11.0 g, 90%). m.p. 110–115°C (lit. 100–102°C). [Citation65] 1H NMR (500 MHz, CDCl3): δ 7.98 (dd, J = 5.0, 5.0 Hz, 2H), 7.77 (dd, J = 10.0, 5.0 Hz, 2H). 13C NMR (150.85 MHz, 500 MHz, CDCl3): δ 145.31, 140.52, 131.18, 128.18
General procedure for the synthesis of 3-chloro-N-alkylquinoxaline-2-amine (3a-d)
To a stirred solution of 2,3-dichloroquinoxaline (4.0 g, 20 mmol) in DMF (40 mL). Alkyl amine derivative (a-d), (20 mmol) and anhydrous K2CO3 (40 mmol) were added. The reaction mixture was stirred at room temperature, under nitrogen for 12 h. The completion of the reaction was monitored by TLC and spots were visualized under UV light. The reaction mixture was poured into ice-cold water (400 ml) and stirred for 15 min, then aqueous layer was extracted with ethyl acetate (50 ml X 2). The organic layers were combined, wash with brine solution (50 ml), dried over anhydrous Na2SO4, filtered and concentrated under-reduced pressure which affords to give crude material that was purified by flash chromatography by using silica gel (230–240 mesh) using 15% ethyl acetate in n-pentane to obtain as white solid product (3a-d)
3-chloro-N-cyclopropylquinoxaline-2-amine (3a)
White solid (3.49 g, 79.25%). m.p. 237–240°C. 1H NMR (500 MHz, DMSO-d6): δ 7.74 (dd, J = 5.0, 10 Hz, 2H), 7.68 (dd, J = 5.0 Hz, 1H), 7.63–7.57 (m, 1H), 7.53 (d, J = 5.0 Hz, 1H), 7.43–7.36 (m, 1H), 2.91 (dt, J = 5.0 Hz, 1H), 0.77 (td, J = 5.0 Hz, 2H), 0.67 (td, J = 5.0 Hz, 2H). 13C NMR (150.85 MHz, DMSO-d6): δ 149.58, 141.17, 137.80, 135.90, 130.32, 127.61, 125.85, 124.87, 24.72, 6.55. MS (ESI) m/z Calcd. for C11H10ClN3 [M + H]+ 219.67 Found 220.06
3-chloro-N-isopropylquinoxaline-2-amine (3b)
White solid (3.48 g, 78.20%). m.p. 237–240°C. 1H NMR (500 MHz, DMSO-d6): δ 7.76 (dd, J = 5.0, 10 Hz, 1H), 7.68 (dd, J = 5.0, 10 Hz, 1H), 7.57–7.50 (m, 1H), 7.Citation37–7.33 (m, 1H), 5.34 (d, J = 5.0 Hz, 1H), 4.38 (dq, J = 5.0 Hz, 1H), 1.32 (d, J = 5.0 Hz, 6 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 147.5, 141.0, 137.7, 135.3, 130.1, 127.4, 125.5, 124.3, 42.4, 21.7. MS (ESI) m/z Calcd. for C11H12ClN3 [M + H]+ 221.69 Found 222.07.
3.2.3.3. 3-chloro-N-cyclobutylquinoxaline-2-amine (3c)
White solid (3.95 g, 84.20%). m.p. 237–240°C. 1H NMR (500 MHz, DMSO-d6): δ 7.76 (dd, J = 5.0, 10 Hz, 1H), 7.68 (dd, J = 5.0, 10 Hz, 1H), 7.56–7.53 (m, 1H), 7.37–7.34 (m, 1H), 5.65 (d, J = 5.0 Hz, 1H), 4.69–4.61 (m, 1H), 2.54–2.49 (m, 2H), 2.02–1.94 (m, 2H), 1.83 (dt, J = 5.0 Hz, 2H). 13C NMR (150.85 MHz, DMSO-d6) δ: 147.4, 140.9, 137.6, 135.5, 130.1, 127.4, 125.5, 124.5, 46.2, 29.9, 14.8. MS (ESI) m/z Calcd. for C12H12ClN3 [M + H]+ 233.70 Found 234.56
3.2.3.4. 3-chloro-N-cyclopentylquinoxaline-2-amine (3d)
White solid (3.4g, 80.20%). m.p. 237–240°C. 1H NMR (500 MHz, DMSO-d6): δ 7.76 (dd, J = 5.0, 10 Hz, 1H), 7.69 (dd, J = 5.0, 10 Hz, 1H), 7.54 (dd, J = 5.0 Hz, 1H), 7.36–7.33 (m, 1H), 5.48 (d, J = 5.0 Hz, 1H), 4.50–4.43 (m, 1H), 2.18 (td, J = 5.0, 10 Hz, 2 H), 1.76 (dd, J = 5.0 Hz, 2 H), 1.68 (dd, J = 5.0 Hz, 2 H), 1.53 (dd, J = 5.0 Hz, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 148.0, 141.0, 137.7, 135.3, 130.1, 127.4, 125.5, 124.4, 52.6, 31.7, 23.7. MS (ESI) m/z Calcd. for C13H14ClN3 [M + H]+ 247.73 Found 248.09
General procedure for the synthesis of compounds (4a-z)
A solution of 3-chloro-N-alkylquinoxaline-2-amine (200 mg, 0.91 mmol) and aniline derivatives (0.91 mmol) in ethanol (4 mL) were heated to reflux for 6 h until the reaction completed (monitored by TLC). Then the reaction mixture was cooled, filtered, and the solid was washed with petroleum ether to obtain a solid product (4a-z).
N2-cyclopropyl-N3-phenylquinoxaline-2,3-diamine (4a)
Creamish white solid (0.22 g, 87.69%). m.p. 245–246°C. 1H NMR (500 MHz, DMSO-d6): δ 11.01 (br s, 1H), 10.36 (br s, 1H), 8.06 (d, J = 5.0 Hz, 2 H), 7.96 (dd, J = 10.0, 5.0 Hz, 1H), 7.60 (dd, J = 10.0, 5.0 Hz, 1H), 7.42 (dd, J = 10.0, 5.0 Hz, 2 H), 7.38 (t, J = 5.0 Hz, 2 H), 7.11 (t, J = 5.0 Hz, 1H), 3.07 (s, 1H), 1.05 (d, J = 10.0 Hz, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.2, 141.2, 139.3, 133.9, 128.6, 126.0, 125.6, 123.5, 120.8, 24.8, 6.9. MS (ESI) m/z Calcd. for C17H16N4 [M + H]+ 276.34 Found 277.14
N2-cyclopropyl-N3-(4-(trifluoromethyl)phenyl)quinoxaline-2,3-diamine (4b)
White solid (0.25 g, 79.87%). m.p. 237–238°C. 1H NMR (500 MHz, DMSO-d6): δ 10.61 (br s, 2 H), 8.31 (d, J = 5.0 Hz, 2 H), 7.94 (d, J = 5.0 Hz, 1H), 7.74 (d, J = 5.0 Hz, 2 H), 7.65 (d, J = 5.0 Hz, 1H), 7.48–7.43 (m, 2 H), 3.06 (d, J = 5.0 Hz, 1H), 1.06–10.1 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.2, 141.2, 133.5, 126.8, 126.2, 126.0, 125.9, 125.8, 123.2, 123.1, 122.9, 120.3, 24.9, 6.9. MS (ESI) m/z Calcd. for CCitation18H15F3N4 [M + H]+ 344.34 Found 345.14
N2-cyclopropyl-N3-(4-fluorophenyl)quinoxaline-2,3-diamine (4c)
Yellow solid (0.23 g, 86.14%). m.p. 254–256°C. 1H NMR (500 MHz, DMSO-d6): δ 11.01 (br s, 1H), 10.45 (br s, 1H), 8.08 (s, 2 H), 7.94 (s, 1H), 7.59 (s, 1H), 7.42 (s, 2 H), 7.23 (s, 2 H), 3.06 (d, J = 5.0 Hz, 1H), 1.06–10.2 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.3, 133.8, 126.1, 125.6, 122.6, 122.5, 118.5, 115.4, 115.2, 24.8, 6.9. MS (ESI) m/z Calcd. for C17H15FN4 [M + H]+ 294.33 Found 295.13
N2-(4-chlorophenyl)-N3-cyclopropylquinoxaline-2,3-diamine (4d)
Yellow solid (0.24 g, 85.10%). m.p. 238–240°C. 1H NMR (500 MHz, DMSO-d6): δ 10.92 (br s, 1H), 10.43 (br s, 1H), 8.13 (d, J = 5.0 Hz, 2 H), 7.92 (s, 1H), 7.62 (dd, J = 5.0 Hz, 1H), 7.43 (dd, J = 5.0 Hz, 4 H), 3.05 (d, J = 5.0 Hz, 1H), 1.06–1.02 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.3, 141.1, 138.5, 136.7, 128.5, 126.9, 126.3, 126.1, 125.8, 122.1, 24.8, 6.9. MS (ESI) m/z Calcd. for C17H15ClN4 [M + H]+ 310.79 Found 311.10
N2-cyclopropyl-N3-(p-tolyl)quinoxaline-2,3-diamine (4e)
Yellow solid (0.24 g, 90.90%). m.p. 245–246°C. 1H NMR (500 MHz, DMSO-d6): δ 11.06 (br s, 1H), 10.35 (br s, 1H), 7.93 (d, J = 5.0 Hz, 3 H), 7.57 (d, J = 5.0 Hz, 1H), 7.41 (s, 2 H), 7.18 (d, J = 5.0 Hz, 2 H), 3.07 (s, 1H), 2.29 (s, 3 H), 1.06–1.02 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.2, 141.2, 136.7, 132.6, 129.0, 126.0, 125.8, 125.5, 120.9, 24.7, 20.5, 6.9. MS (ESI) m/z Calcd. for C18H18N4 [M + H]+ 290.37 Found 291.17
N2-cyclopropyl-N3-(4-methoxyphenyl)quinoxaline-2,3-diamine (4 f)
Brown solid (0.24 g, 89.55%). m.p. 135–136°C. 1H NMR (500 MHz, DMSO-d6): δ 8.89 (br s, 1H), 8.79 (br s, 1H), 7.81 (d, J = 10.0 Hz, 2 H), 7.51 (d, J = 10.0 Hz, 1H), 7.47 (d, J = 10.0 Hz, 1H), 7.27 (t, J = 5.0 Hz, 2 H), 6.97 (d, J = 10.0 Hz, 2 H), 3.77 (s, 3 H), 3.02 (s, 1H), 0.88 (d, J = 5.0 Hz, 2 H), 0.68 (d, J = 5.0 Hz, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 162.1, 155.0, 140.9, 125.2, 124.6, 124.1, 122.1, 113.9, 55.2, 24.3, 6.5. MS (ESI) m/z Calcd. for C18H18N4O [M + H]+ 306.37 Found 307.15
N2-(4-bromophenyl)-N3-cyclopropylquinoxaline-2,3-diamine (4g)
Brown solid (0.27 g, 83.83%). m.p. 242–243°C. 1H NMR (500 MHz, DMSO-d6): δ 10.85 (br s, 1H), 10.36 (br s, 1H), 8.08 (d, J = 10.0 Hz, 2 H), 7.92 (s, 1H), 7.63 (s, 1H), 7.58 (d, J = 10.0 Hz, 2 H), 7.45 (s, 2 H), 3.05 (s, 1H), 1.03 (d, J = 10.0 Hz, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 141.1, 140.2, 138.9, 136.4, 133.8, 131.4, 126.3, 126.0, 125.8, 125.7, 122.6, 122.5, 115, 24.8, 6.9. MS (ESI) m/z Calcd. for C17H15BrN4 [M + H]+ 355.24 Found 355.05
N2-cyclopropyl-N3-(3-fluorophenyl)quinoxaline-2,3-diamine (4 h)
Off-white solid (0.24 g, 89.55%). m.p. 258–261°C. 1H NMR (500 MHz, DMSO-d6): δ 10.85 (br s, 1H), 10.Citation45 (br s, 1H), 8.12 (d, J = 10.0 Hz, 1H), 7.91 (d, J = 5.0 Hz, 1H), 7.87 (d, J = 5.0 Hz, 1H), 7.66 (d, J = 5.0 Hz, 1H), 7.48–7.38 (m, 3 H), 6.92 (t, J = 10.0 Hz, 1H), 3.04 (s, 1H), 1.03–1.01 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 163.2, 160.8, 143.2, 141.4, 141.2, 133.6, 130.2, 126.3 (d, C-F), 125.9, 116.3, 109.7 (d), 107.1 (d), 24.8, 6.9. MS (ESI) m/z Calcd. for C17H15FN4 [M + H]+ 294.33 Found 295.15
N2-(3-chlorophenyl)-N3-cyclopropylquinoxaline-2,3-diamine (4i)
Off-white solid (0.25 g, 86.87%). m.p. 250–251°C. 1H NMR (500 MHz, DMSO-d6): δ 11.09 (br s, 1H), 10.60 (br s, 1H), 8.28 (s, 1H), 8.08 (d, J = 10.0 Hz, 1H), 7.97 (d, J = 5.0 Hz, 1H), 7.62 (d, J = 10.0 Hz, 1H), 7.44 (dd, J = 5.0 Hz, 2 H), 7.40 (t, J = 5.0 Hz, 1H), 7.14 (d, J = 5.0 Hz, 1H), 3.07 (d, J = 5.0 Hz, 1H), 1.06–1.02 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.2, 141.2, 141.0, 133.6, 132.9, 130.2, 126.5, 126.2, 125.8, 123.0, 119.8, 118.9, 118.2, 24.9, 6.9. MS (ESI) m/z Calcd. for C17H15ClN4 [M + H]+ 310.79 Found 311.10
N2-(3-bromophenyl)-N3-cyclopropylquinoxaline-2,3-diamine (4 j)
Yellow solid (0.27 g, 85.32%). m.p. 246–247°C. 1H NMR (500 MHz, DMSO-d6): δ 10.94 (br s, 1H), 10.49 (br s, 1H), 8.40 (d, J = 5.0 Hz, 1H), 8.11 (d, J = 10.0 Hz, 1H), 7.94 (d, J = 5.0 Hz, 1H), 7.61 (dd, J = 5.0 Hz, 1H), 7.45 (t, J = 5.0 Hz, 2 H), 7.34 (t, J = 5.0 Hz, 1H), 7.28 (d, J = 5.0 Hz, 1H), 3.05 (d, J = 5.0 Hz, 1H), 1.03 (t, J = 5.0 Hz, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.3, 141.2, 130.6, 126.5, 126.1, 125.8, 122.8, 122.3, 119.2, 24.8, 6.9. MS (ESI) m/z Calcd. for C17H15BrN4 [M + H]+ 355.24 Found 355.05
N2-cyclopropyl-N3-(m-tolyl)quinoxaline-2,3-diamine (4k)
Yellow solid (0.24 g, 90.15%). m.p. 250–251°C. 1H NMR (500 MHz, DMSO-d6): δ 11.06 (br s, 1H), 10.28 (br s, 1H), 7.95 (d, J = 5.0 Hz, 1H), 7.89–7.86 (m, 2 H), 7.60 (dd, J = 10.0 Hz, 1H), 7.42 (dd, J = 5.0 Hz, 2 H), 7.26 (t, J = 10.0 Hz, 1H), 6.93 (d, J = 10.0 Hz, 1H), 3.06 (d, J = 5.0 Hz, 1H), 2.33 (s, 3 H), 1.05–1.02 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.1, 141.3, 139.2, 137.8, 133.8, 128.5, 126.1, 125.6, 124.3, 121.3, 118.1, 24.8, 21.3, 6.9. MS (ESI) m/z Calcd. for C18H18N4 [M + H]+ 290.37 Found 291.15
N2-cyclopropyl-N3-(3-methoxyphenyl)quinoxaline-2,3-diamine (4 l)
Yellow solid (0.24 g, 87.45%). m.p. 228–229°C. 1H NMR (500 MHz, DMSO-d6): δ 11.11 (br s, 1H), 10.38 (br s, 1H), 7.97 (s, 1H), 7.85 (s, 1H), 7.66 (d, J = 5.0 Hz, 1H), 7.61 (dt, J = 5.0 Hz, 1H), 7.43 (dd, J = 5.0 Hz, 2 H), 7.27 (t, J = 5.0 Hz, 1H), 6.69 (dd, J = 5.0 Hz, 1H), 3.77 (s, 3 H), 3.06 (t, J = 5.0 Hz, 1H), 1.05 (d, J = 5.0 Hz, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 159.3, 143.1, 141.2, 140.5, 133.7, 129.3, 126.2, 125.7, 113.0, 109.0, 106.5, 55.0, 24.9, 7.0. MS (ESI) m/z Calcd. for C18H18N4O [M + H]+ 306.37 Found 307.15
N2-cyclopropyl-N3-(o-tolyl)quinoxaline-2,3-diamine (4 m)
Brown solid (0.12 g, 45.45%). m.p. 148–149°C. 1H NMR (500 MHz, DMSO-d6): δ 8.15 (s, 1H), 7.50 (dd, J = 5.0 Hz, 1H), 7.35 (dd, J = 5.0 Hz, 1H), 7.30–7.26 (m, 3 H), 7.26–7.20 (m, 2 H), 7.14 (td, J = 5.0 Hz, 20.0 Hz, 2 H), 3.02 (dd, J = 5.0 Hz, 1H), 2.15 (s, 3 H), 0.83 (dt, J = 5.0 Hz, 2 H), 0.61–0.58 (m, 2 H). MS (ESI) m/z Calcd. for C18H18N4 [M + H]+ 290.37 Found 291.05
N2-cyclopropyl-N3-(3-(triflouromethyl)phenyl)quinoxaline-2,3-diamine (4 n)
Off white solid (0.26 g, 83.06%). m.p. 232–233°C. 1H NMR (500 MHz, DMSO-d6): δ 11.10 (br s, 1H), 10.79 (br s, 1H), 8.60 (d, J = 5.0 Hz, 1H), 8.38 (d, J = 10.0 Hz, 1H), 7.98 (d, J = 5.0 Hz, 1H), 7.60 (dd, J = 10 Hz, 5.0 Hz, 2 H), 7.50–7.35 (m, 3 H), 3.08 (s, 1H), 1.06–10.1 (m, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.2, 129.8, 129.5, 127.0 (q, CF3), 126.6, 126.3, 125.8, 125.6, 123.9, 119.5, 116.5, 24.9, 6.9. MS (ESI) m/z Calcd. for C18H15F3N4 [M + H]+ 344.34 Found 345.15
3.2.4.15. 4-((3-(cyclopropylamino)quinoxalin-2-yl)amino)benzonitrile (4o)
White solid (0.23 g, 83.94%). m.p. 231–233°C. 1H NMR (500 MHz, DMSO-d6): δ 10.64 (br s, 2 H), 8.57 (br s, 1H), 8.36 (d, J = 10.0 Hz, 1H), 7.94 (d, J = 5.0 Hz, 1H), 7.63–7.59 (m, 2 H), 7.44 (dd, J = 10 Hz, 5.0 Hz, 3 H), 3.07 (d, J = 5.0 Hz, 1H), 1.03 (t, J = 10 Hz, 4 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.4, 141.2, 140.4, 133.6, 129.8, 126.5, 126.1, 125.8, 125.1, 123.9, 123.3, 119.4, 116.5, 24.8, 6.9. MS (ESI) m/z Calcd. for C18H15N5 [M + H]+ 301.35 Found 302.13
N2-isopropyl-N3-(4-(triflouromethyl)phenyl)quinoxaline-2,3-diamine (4p)
White solid (0.24 g, 76.28%). m.p. 276–278°C. 1H NMR (500 MHz, DMSO-d6): δ 10.56 (br s, 2 H), 8.27 (d, J = 5.0 Hz, 2 H), 7.84 (s, 1H), 7.73 (d, J = 5.0 Hz, 2 H), 7.60 (d, J = 10.0 Hz, 1H), 7.43–7.37 (m, 2 H), 4.54 (s, 1H), 1.41 (d, J = 6.0 Hz, 6 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.3, 140.9, 133.1, 126.5, 125.8, 125.7, 125.4, 124.5 (q, CF3), 123.6, 120.5, 44.9, 21.5. MS (ESI) m/z Calcd. for C18H17F3N4 [M + H]+ 346.36 Found 347.14
N2-(4-chlorophenyl)-N3-isopropylquinoxaline-2,3-diamine (4q)
Yellow solid (0.27 g, 86.17%). m.p. 275–277°C. 1H NMR (500 MHz, DMSO-d6): δ 10.43 (br s, 2 H), 8.07 (d, J = 5.0 Hz, 2 H), 7.83 (d, J = 5.0 Hz, 1H), 7.55 (d, J = 5.0 Hz, 1H), 7.44 (d, J = 10.0 Hz, 2 H), 7.40–7.34 (m, 2 H), 4.53 (s, 1H), 1.41 (d, J = 5.0 Hz, 6 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 141.0, 138.5, 128.5, 127.0, 125.6, 125.4, 122.5, 45.1, 21.6. MS (ESI) m/z Calcd. for C17H17ClN4 [M + H]+ 312.80 Found 313.13
N2-isopropyl-N3-(4-methoxyphenyl)quinoxaline-2,3-diamine (4 r)
Brown solid (0.25 g, 92.08%). m.p. 245–246°C. 1H NMR (500 MHz, DMSO-d6): δ 10.37 (br s, 2 H), 7.83 (d, J = 10.0 Hz, 3 H), 7.51 (dd, J = 5.0 Hz, 1H), 7.34 (t, J = 5.0 Hz, 2 H), 6.99 (d, J = 5.0 Hz, 2 H), 4.53 (s, 1H), 3.77 (s, 3 H), 1.40 (d, J = 5.0 Hz, 6 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 156.0, 141.2, 125.6, 124.5, 123.4, 123.2, 114.0, 113.9, 55.3 (OCH3), 45.1, 21.6. MS (ESI) m/z Calcd. for C18H20N4O [M + H]+ 308.39 Found 309.18
N2-cyclobutyl-N3-(4-(triflouromethyl)phenyl)quinoxaline-2,3-diamine (4s)
White solid (0.23 g, 75.01%). m.p. 276–277°C. 1H NMR (500 MHz, DMSO-d6): δ 10.49 (br s, 2 H), 8.28 (d, J = 5.0 Hz, 2 H), 7.81 (d, J = 10.0 Hz, 1H), 7.73 (d, J = 10.0 Hz, 2 H), 7.60 (dd, J = 10.0 Hz, 1H), 7.42–7.37 (m, 2 H), 4.72–4.66 (m, 1H), 2.45–2.30 (m, 4 H), 1.89–1.75 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 143.4, 140.9, 133.4, 126.6, 126.5, 125.9, 125.8 (d), 125.6 (d), 123.2, 120.3, 47.3, 29.0, 15.2. MS (ESI) m/z Calcd. for CCitation19H17F3N4 [M + H]+ 358.37 Found 359.14
N2-(4-chlorophenyl)-N3-cyclo butylquinoxaline-2,3-diamine (4 t)
ellow solid (0.23 g, 86.33%). m.p. 280–281°C. 1H NMR (500 MHz, DMSO-d6): δ 10.44 (br s, 2 H), 8.10 (d, J = 5.0 Hz, 2 H), 7.82 (d, J = 5.0 Hz, 1H), 7.57–7.55 (m, 1H), 7.45–7.41 (m, 2 H), 7.40–7.35 (m, 2 H), 4.75–4.63 (m, 1H), 2.47–2.32 (m, 4 H), 1.90–1.75 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 140.6, 138.6, 136.6, 133.5, 128.5, 126.9, 126.0, 125.6, 125.5, 125.4, 122.3, 119.5, 47.3, 29.0, 15.2. MS (ESI) m/z Calcd. for C18H17ClN4 [M + H]+ 324.81 Found 325.12
N2-cyclobutyl-N3-(4-methoxyphenyl) quinoxaline-2,3-diamine (4 u)
Yellow solid (0.24 g, 89.41%). m.p. 248–250°C. 1H NMR (500 MHz, DMSO-d6): δ 10.44 (br s, 2 H), 7.87 (d, J = 10.0 Hz, 2 H), 7.81 (s, 1H), 7.51 (dd, J = 5.0 Hz, 1H), 7.34 (dd, J = 5.0 Hz, 2 H), 6.99 (d, J = 10.0 Hz, 2 H), 4.74–4.65 (m, 1H), 3.77 (s, 3 H), 2.48–2.45 (m, 4 H), 1.90–1.75 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 156.0, 141.2, 141.1, 140.2, 140.1, 134.9, 125.8, 125.6, 123.2, 114.0, 55.3 (OCH3), 47.4, 28.9, 15.2. MS (ESI) m/z Calcd. for C19H20N4O [M + H]+ 320.40 Found 321.14
N2-cyclopentyl-N3-phenylquinoxaline-2,3-diamine (4 v)
Yellow solid (0.22 g, 89.79%). m.p. 265–267°C. 1H NMR (500 MHz, DMSO-d6): δ 10.Citation46 (br s, 2 H), 8.00 (d, J = 10.0 Hz, 2 H), 7.88 (s, 1H), 7.57–7.48 (m, 1H), 7.45–7.30 (m, 4 H), 7.12 (t, J = 5.0 Hz, 1H), 4.58 (dt, J = 5.0 Hz, 1H), 2.13 (td, J = 5.0 Hz, 2 H), 1.93 (d, J = 5.0 Hz, 2 H), 1.87–1.75 (m, 2 H), 1.70–1.55 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 141.2, 140.9, 139.2, 133.1, 128.6, 125.9, 125.8, 125.2, 123.6, 121.3, 54.4, 31.4, 23.9. MS (ESI) m/z Calcd. for C19H20FN4 [M + H]+ 304.40 Found 305.19
N2-(3-chlorophenyl)-N3-cyclopentylquinoxaline-2,3-diamine (4 w)
Yellow solid (0.24 g, 87.59%). m.p. 275–276°C. 1H NMR (500 MHz, DMSO-d6): δ 10.52 (br s, 2 H), 8.08 (d, J = 10.0 Hz, 2 H), 7.85 (s, 1H), 7.58–7.52 (m, 1H), 7.45–7.33 (m, 4 H), 4.61–4.52 (m, 1H), 2.11 (dt, J = 5.0 Hz, 2 H), 1.92 (s, 2 H), 1.83 (dd, J = 5.0 Hz, 2 H), 1.63 (dd, J = 5.0 Hz, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 141.1, 138.5, 138.4, 134.3, 133.2, 128.5, 127.0, 126.1, 125.7, 122.6, 54.3, 31.4, 23.9. MS (ESI) m/z Calcd. for C19H19ClN4 [M + H]+ 338.84 Found 339.13
N2-cyclopentyl-N3-(4-(triflouromethyl)phenyl)quinoxaline-2,3-diamine (4x)
White solid (0.24 g, 80%). m.p. 276–277°C. 1H NMR (500 MHz, DMSO-d6): δ 10.79 (br s, 1H), 10.32 (br s, 1H), 8.29 (d, J = 10.0 Hz, 2 H), 7.91 (d, J = 10.0 Hz, 1H), 7.73 (d, J = 10.0 Hz, 2 H), 7.61–7.59 (m, 1H), 7.44–7.38 (m, 2 H), 4.59 (s, 1H), 2.13 (td, J = 5.0 Hz, 10.0 Hz, 2 H), 1.94 (d, J = 5.0 Hz, 2 H), 1.88–1.80 (m, 2 H), 1.67–1.58 (m, 2 H). 13C-NMR (500 MHz, DMSO-d6) δ: 143.2, 141.1, 133.0, 128.5, 125.8, 125.7 (q, CF3), 123.2, 122.9, 120.6, 54.4, 31.4, 23.9. MS (ESI) m/z Calcd. for C20H19F3N4 [M + H]+ 372.40 Found 373.16
N2-cyclopentyl-N3-(p-tolyl)quinoxaline-2,3-diamine (4y)
Yellow solid (0.22 g, 86.38%). m.p. 280–281°C. 1H NMR (500 MHz, DMSO-d6): δ 10.43 (br s, 2 H), 7.85 (d, J = 5.0 Hz, 3 H), 7.55–7.48 (m, 1H), 7.40–7.32 (m, 2 H), 7.20 (d, J = 10.0 Hz, 2 H), 4.58 (s, 1H), 2.30 (s, 3 H), 2.12 (td, J = 5.0 Hz, 2 H), 1.91 (d, J = 5.0 Hz, 2 H), 1.86–1.82 (m, 2 H), 1.66–1.59 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 141.2, 136.5, 132.9, 129.0, 125.7, 125.0, 121.5, 54.3, 31.4, 23.9 (CH3), 20.5. MS (ESI) m/z Calcd. for C20HCitation22FN4 [M + H]+ 318.42 Found 319.18
N2-cyclopentyl-N3-(4-methoxyphenyl)quinoxaline-2,3-diamine (4z)
Yellow solid (0.25 g, 92.59%). m.p. 245–246°C. 1H NMR (500 MHz, DMSO-d6): δ 10.54 (br s, 2 H), 8.25–7.70 (m, 3 H), 7.53 (dd, J = 5.0 Hz, 1 H), 7.40–7.30 (m, 2 H), 7.00 (d, J = 10.0 Hz, 2 H), 4.61(s, 1 H), 3.79 (s, 3 H), 2.14 (td, J = 10.0 Hz, 2 H), 1.94 (d, J = 5.0 Hz, 2 H), 1.88–1.81 (m, 2 H), 1.69–1.60 (m, 2 H). 13C NMR (150.85 MHz, DMSO-d6) δ: 158.4, 156.0, 141.2, 140.8, 125.7, 125.6, 123.4, 123.3, 116.1, 113.9, 55.3 (OCH3), 54.4, 31.5, 23.9. MS (ESI) m/z Calcd. for C20H22N4O [M + H]+ 334.42 Found 335.18
Conclusions
The aim of the work to design and synthesize novel diamino-quinoxaline scaffolds and products were screened against bacterial and fungal strains. Quinoxaline scaffold presented the preliminary significant antimicrobial activity against a panel of pathogenic microbes including two Gram-positive bacteria (S. aureus and B. subtilis), three Gram-negative bacteria (E. coli, K. pneumoniae and Salmonella typhi), and two fungal strains (C. albicans and A. niger). From the quantitative analysis, the compound (4c) presented a broad-spectrum (Zone of inhibitions) on all the strains between 10.11 and 14.89 mm. Our docking-based binding mode analysis results are in accordance with the antibacterial testing data. In summary, docking predicts our diamino-quinoxaline analogues as DNA intercalators where they bind at microbial DNA gyrase quinolone-binding site with critical Mg+2 ion metal-coordination, thereby showing their antibacterial activity.
Author contributions
S.K.S. designed the research and performed the experimental works; Y.K.J. wrote the manuscript; J.M.P. wrote the biological part, P.V.P. done molecular modeling study; N.S.C. and G.P.S. revised the manuscript.
Acknowledgments
The authors extend their appreciation to the Department of Chemistry, Bhupal Nobles’ University, Udaipur, Rajasthan, India, for providing technical support in this project (materials used for experiments).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vithal PM, Ritu S, Khalid UK, et al. First and second line drug resistance among treatment naïve pulmonary tuberculosis patients in a district under Revised National Tuberculosis Control Programme (RNTCP) in New Delhi. J Epidemiol Glob Health. 2015;4:365–373.
- Lynn P, Linus O, Dung TKK, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. Plos one. 2019. DOI:10.1371/journal.pone.0215666
- Ponnam SL, Juhi T, Bibhabati MM, et al. aureus in hospitalized patients. J Global Infect Dis. 2010;2:275.
- Brooks LA, Moushy M. Synthesis of Alkylated Pyrrole Compounds. US2417046 A (11 March 1947).
- Baney LJ Isolation of Pyrrole from Pyridine. US2425220 A, 1947 Aug 5.
- Piddock LK, Webber MA, Baylay AJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51.
- Mah TFC, O’toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39.
- Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113.
- Kim YB, Kim YH, Park JY, et al. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues. Bioorg Med Chem Lett. 2004;14:541–544. [Cross Ref] [Pub Med].
- Bailly C, Waring JM. DNA recognition by quinoxaline antibiotics: use of base-modified DNA molecules to investigate determinants of sequence-specific binding of triostin A and TANDEM. Biochem J. 1998;330:81–87.
- Anthony M. Erdafitinib: first global approval. Drugs. 2019;79: 1017–1021. DOI:10.1007/s40265-019-01142-9
- Badran MM, Abonzid KA, Hussein MHM. Synthesis of certain substituted quinoxalines as antimicrobial agents (part II). Arch Pharm Res. 2003;26:107–113.
- El-Gendy A, El-Meligie S, El-Ansary AAK. Synthesis of some Quinoxaline derivatives containing Indoline-2,3-dione or Thiazolidinone residue as potential antimicrobial agents. Arch Pharm Res. 1995;18:44–47.
- Parhi AK, Zhang Y, Saionz KW, et al. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg Med Chem Lett. 2013;23:4968–4974.
- Ajani OO, Obafemi CA, Ikpo CO, et al. Microwave-assisted synthesis and antibacterial activity of some pyrazol-1-ylquinoxalin- 2(1H)-one derivatives. Chem Heterocycl Comp. 2009;45:1370–1378.
- Caleb AA, Ballo DB, Rachid DH, et al. Synthesis and antibacterial activity of new spiro[thiadiazolinequinoxaline] derivatives. El Mokhtar Arkivoc ii. 2011;217–226.
- Peraman R, Kuppusamy R, Killi SK, et al. New conjugates of Quinoxaline as potent Antitubercular and Antibacterial agents. Int J Med Chem. 2016;1–8. DOI:10.1155/2016/6471352
- Ramalingam P, Ganapaty S, Rao CB. Bioorg. Med Chem Lett. 2010;20:406–408.
- Moreno E, Ancizu S, Pérez-Silanes S, et al. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Eur. J. Med. Chem. 2010;45(10):4418–4426.
- Rangisetty JB, Prasad AL, Srinivas P, et al. Synthesis of new arylaminoquinoxalines and their antimalarial activity in mice. J Pharm Pharmacol. 2001;53(10):1409–1413.
- Chandra Shekhar A, Rao PS, Narsaiah B, et al. Emergence of pyrido quinoxalines as new family of antimalarial agents. Eur J Med Chem. 2014;22:280–287.
- Guillon J, Mouray E, Moreau S, et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: synthesis, and in vitro antimalarial activity – part II. J Med Chem. 2011;46:2310–2326.
- Wilhelmsson LM, Kingi N, Bergman J. Interactions of Antiviral Indolo[2,3-b]quinoxaline Derivatives with DNA. J Med Chem. 2008;51:7744–7750.
- Harmenberg J, Johansson AA, Graslund A, et al. The mechanism of action of the anti-herpes virus compound 2,3-dimethyl-6(2-dimethylaminoethyl)-6H-indolo-(2,3-b). Quinoxaline Antiviral Res. 1991;15:193–204.
- Clercq ED. Toward Improved Anti-HIV Chemotherapy: therapeutic Strategies for Intervention with HIV Infections. J Med Chem. 1995;38:2491–2517.
- Wagle S, Adhikari AV, Suchetha NK. Synthesis of some new 2-(3-methyl-7- substituted-2-oxoquinoxalinyl) −5-(aryl)-1,3,4-oxadiazoles as potential non-steroidal anti-inflammatory and analgesic agents. Indian J Chem. 2008;47B:439–448.
- Rogier AS, Herman DL, Coruzzi G, et al. Fragment based design of New H4 Receptor-Ligands with Anti-inflammatory properties in Vivo. J Med Chem. 2008;51:2457–2467.
- Burguete A, Pontiki E, Hadjipavlou-Litina D, et al. Synthesis and Biological Evaluation of New Quinoxaline derivatives as antioxidant and anti-inflammatory agents. Chem Biol Drug Des. 2011;77(4):255–267.
- Abu-Hashem AA, Gouda MA, Badria FA. Eur. J Med Chem. 2010;45:1976–1981.
- Singh DP, Deivedi SK, Hashim SR, et al. Synthesis and Antimicrobial Activity of Some new Quinoxaline derivatives. Pharmaceuticals. 2010;3:2416–2425.
- Zhang M, Dai ZC, Qian SS, et al. Design, Synthesis, Antifungal, and Antioxidant Activities of (E)-6-((2-Phenylhydrazono)methyl)quinoxaline Derivatives. J Agric Food Chem. 2014;62:9637–9643.
- Ajani OO, Obafemi CA, Nwinyi OC, et al. Bioorg. Med Chem. 2010;18:214–221.
- Carta A, Piras S, Loriga G, et al. Chemistry, Biological Properties and SAR Analysis of Quinoxalinones. Med Chem. 2006;6(22):1179–1200.
- Abid M, Azam AB. Synthesis, characterization and antiamoebic activity of 1-(thiazolo[4,5-b]quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives. Med Chem Lett. 2006;6(10):2812–2816.
- Samir Undevia D, Innocenti F, Ramirez J, et al. A phase I and pharmacokinetic study of the quinoxaline antitumour Agent R(+)XK469 in patients with advanced solid tumours. Eur J Cancer. 2008;44:1684–1692.
- Marcu L, Olver I. Tirapazamine: from Bench to Clinical Trials. Curr Clin Pharmacol. 2006;1:71–79.
- Lee SH, Kim N, Kim SJ, et al. Res. Clin Oncol. 2013;139:1279–1294.
- Waring MJ, Ben-Hadda T, Kotchevar AT, et al. 2,3-Bifunctionalized Quinoxalines: Synthesis, DNA Interactions and Evaluation of Anticancer, Anti-tuberculosis and Antifungal Activity. Molecules. 2002;7:641–656.
- Desplat V, Moreau S, Belisle-Fabre S Synthesis and evaluation of the antiproliferative activity of novel isoindolo[2,1-a]quinoxaline and indolo[1,2-a]quinoxaline derivatives. , et al. Enzyme Inhibition Med Chem. 2011;26:657–667.
- Chung HJ, Jung OJ, Chae MJ, et al. Bioorg. Med Chem Lett. 2005;15:3380–3384.
- Ingle R, Marathe R, Magar D, et al. Sulphonamido-quinoxalines: search for anticancer agent. S J Eur J Med Chem. 2013;65:168–186.
- Noolvi MN, Patel HM, Bhardwaj V, et al. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: search for anticancer agent. J Med Chem. 2011;46:2327–2346.
- Abou-Gharbia M, Freed ME, McCaully RJ, et al. Tetrahydropyrrolo[1,2-a Iquinoxalines and Tetrahydropyrrolo[1,2-a]pyrido[3,2-a Ipyrazines: vascular Smooth Muscle Relaxants and Antihypertensive Agents. J Med Chem. 1984;27:1743–1746.
- Bigge CF, Malone TC, Boxer PA, et al. Synthesis of 1,4,7,8,9,10-Hexahydro-9-methy −1-6-nitropyrido[3,4-f]- quinoxaline-2,3-dione and Related Quinoxalinediones: characterization of a-Amino-3-hydroxy −5-methyl-4-isoxazolepropionic Acid (and N-Methyl-D-aspartate) Receptor and Anticonvulsant Activity. J Med Chem. 1995;38:3720–3740. DOI:10.1021/jm00019a003
- Elhelby AA, Ayyad RR, Zayed MFA. Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents. Drug Res. 2011;61:379–381.
- Olayiwola G, Obafemi CA, Taiwo FO. Afr. J Biotechnol. 2007;6:777–786.
- Levitzki A. Protein kinase inhibitors as a therapeutic modality. Acc Chem Res. 2003;36:462–469.
- Zarnowski T, Kleinrok Z, Turski WA, et al. 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(F)quinoxaline enhances the protective activity of common antiepileptic drugs against maximal electroshock-induced seizures in mice. Neuropharmacology. 1993;32:895–900.
- Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69:273–294.
- Yan W, Qing J, Mei H, et al. Discovery of novel small molecule anti-HCV agents via the CypA inhibitory mechanism using O-Acylation-DirectedLead optimization. Molecules. 2015;20:10342–10359.
- Rong F, Chow S, Yan S, et al. Structure–activity relationship (SAR) studies of quinoxalines as novel HCV NS5B RNA-dependent RNA polymerase inhibitors. Bioorg Med Chem Lett. 2007;17:1663–1666.
- Ismai MMF, Ammar YA, Ibrahim MK, et al. Synthesis and pharmacological evaluation of novel quinoxalines as potential nonulcerogenic anti-inflammatory and analgesic agents. Arzneimittelforschung. 2005;55:738–743.
- Campiani G, Morelli EG, Nacci S, et al. Pyrroloquinoxaline Derivatives as High-Affinity and Selective 5-HT3 Receptor Agonists: Synthesis, Further Structure−Activity Relationships, and Biological Studies. T J Med Chem. 1999;42:4362–4379.
- Young VV, Haute T, Bright DR Quinoxaline adducts useful as anthelmintics. US4348389A (07 Sep. 1982).
- Meyes P. Basel, Switzerland. Insection compositions containing quinalphos and thiometon. US4510137A, (1985 Apr 09).
- Mann RK, Franklin, Synergistic herbicidal composition containing fluroxypyr and cyhalofop, metamifop or profoxydim. US 2011/00981-81(Apr 2011).
- Zhang M, Dai ZC, Qian SS, et al. Synthesis, Antifungal, and Antioxidant Activities of (E)-6-((2- Phenylhydrazono)methyl)quinoxaline derivatives. J Agric Food Chem. 2014;62:9637–9643.
- Xu M, Hu X, Zhang Y, et al. Novel organic dyes featuring Spiro[dibenzo [3,4:6,7] cyclohepta[1,2-b]quinoxaline-10,9′-fluorene] (SDBQX) as a rigid moiety for dye-sensitized solar cells. ACS Appl Energy Mater. 2018;5:2200–2207.
- Zhang LP, Jiang KJ, Li G, et al. Pyrazino[2,3-g] quinoxaline dyes for solar cell applications. J Mater Chem A. 2014;2:14852–14857. (b) Lu, X.; Feng, Q.; Lan, T.; Zhou, G.; Wang, Z.S. Molecular engineering of quinoxaline-based organic sensitizers for highly efficient and stable dye-sensitized solar cells. Chem. Mater. 2012, 24, 3179-3187. (c) Jung, C.Y.; Song, C.J.; Yao, W.; Park, J.M.; Hyun, I.H.; Seong, D.H.; Jaung, J.Y. Synthesis and performance of new quinoxaline-based dyes for dye sensitized solar cell. Dyes Pigments 2015, 121, 204-210.
- Chen CT, Wei Y, Lin JS, et al. Doubly ortho-linked Quinoxaline/Diphenylfluorene hybrids as bipolar, fluorescent chameleons for optoelectronic applications. J Am Chem Soc. 2006;128:10992–10993.
- Chen HC, Chen YH, Liu CC, et al. Prominent short-circuit currents of fluorinated Quinoxaline-Based Copolymer solar cells with a power conversion efficiency of 8.0%. Chem Mater. 2012;24:4766–4772.
- Yang J, Uddin MA, Tang Y, et al. Quinoxaline-based wide band gap polymers for efficient nonfullerene organic solar cells with large open-circuit voltages. ACS Appl Mater Interfaces. 2018;27:23235–23246.
- Kim H, Reddy MR, Hong SS, et al. Synthesis and characterization of quinoxaline derivative as organic semiconductors for organic thin-film transistors. J Nano sci Nanotechnol. 2017;17:5530–5538. (b) Iyer, A.; Bjorgaard, J.; Anderson, T.; Kose, M.E. Quinoxaline-based semiconducting polymers: effect of fluorination on the photophysical, thermal, and charge transport properties. Macromolecules, 2012, 45, 6380-6389.
- Pereira JDS, Neri JM, Emerenciano DP, et al. Experimental and theoretical analysis of an oxazinoquinoxaline derivative for corrosion inhibition of AISI 1018steel. Quim Nova. 2018. (b) El Aoufir, Y.; Lgaz, H.; Bourazmi, H.; Kerroum, Y.; Ramli, Y.; Guenbour, A.; Salghi, R.; El-Hajjaji, F.; Hammouti, B.; Oudda, H. Quinoxaline derivatives as corrosion inhibitors of carbon steel in hydrochloridric acid media: electrochemical, DFT and Monte Carlo simulations studies. J. Mater. Environ. Sci. 2016, 7, 4330-4347. (c) Chitra, S.; Parameswari, K.; Vidhya, M.; Kalishwari, M.; Selvaraj, A. Evaluation of quinoxalines as corrosion inhibitors for mild steel in acid environment. Int. J. Electrochem. Sci. 2011, 6, 45934613. 2018;(41):243–250.
- Duane RR. Synthesis of 2,3-dichloroquinoxalines via Vilsmeier reagent chlorination. J Heterocyclic Chem. 2009;46:317–319.
- Ahammed KS, Pal R, Chakraborty J, et al. DNA structural alteration leading to antibacterial properties of 6-Nitroquinoxaline derivatives. J Med Chem. 2019;62:7840–7856.
- Paliwal S, Sharma S, Dwivedi J, et al. Synthesis of novel substituted phenyl-3-Hydrazinyl-Quinoxaline-2-Amine derivatives: evaluation of antimicrobial activity and its molecular docking studies. J Heterocyclic Chem. 2017;54:3689–3695.
- Jorgensen JH, Murray E, R P, et al. Susceptibility Test methods: dilution and Disk diffusion methods. Manual Clin Microbiol. 2007;2:1152–1173.
- Ingroff E, Pfaller MA, et al. Manual of clinical microbiology. Murray PR, Baron EJ, Jorgensen Jorgensen, Editors. Susceptibility test methods: yeasts and filamentous fungi, in manual of clinical microbiology, 2007 (Washington: ASM Press), 2 Institute Name - Medical Mycology Research Laboratory, Division of Infectious Diseases, VCU Medical Center, 1101 East Marshall Street, Sanger Hall Room 7-049, Richmond, VA 23298-0049, USA. 1972-1986.
- Pavan VP, Indrani B, Dhananjay B, et al. Capturing state-dependent dynamic events of GABAA-receptors: a microscopic look into the structural and functional insights. J Biomol Struct Dyn. 2016;34:1818–1837.
- Chandrima J, Pritha B, Pavan VP, et al. Chelerythrine–lysozyme interaction: spectroscopic studies, thermodynamics and molecular modeling exploration. Phys Chem Chem Phys. 2015;17:16630–16645.
- Pavan VP, Sudipendra NR, Dhananjay B, et al. Cross-talk between allosteric and orthosteric binding sites of γ-amino butyric acid type A receptors (GABAA-Rs): a computational study revealing the structural basis of selectivity. J Biomol Struct Dyn. 2019;37(12):3065–3080.
- Indrani B, Pavan VP. Use of molecular dynamics simulations in structure-based drug discovery. Curr Pharm Des. 2019;25(31):3339–3349. PMID: 31480998.
- Bax BD, Chan PF, Eggleston DS, et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–994.
- Schrodinger Release 2009. Schrödinger, LLC, New York, NY, 2009.
- Schrödinger Release 2009. QikProp, Schrödinger, LLC, New York, NY, 2009.
- Schrödinger Release 2009: Glide, Schrödinger, LLC, New York, NY, 2009.
- Friesner RA, Banks JL, Murphy RB, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749.
- Jacobson MP, Pincus DL, Rapp CS, et al. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55:351–367.
- Schrödinger Release 2009: Prime MM/GBSA, Schrödinger, LLC, New York, NY, 2010.
- HyperChem (Version 7.5). Gainesville,FL: Hypercube Inc., 2003.
- Schrödinger Release 2009: Pymol, Schrödinger, LLC, New York, NY, 2010.