ABSTRACT
This study evaluated the anxiolytic and antioxidative potential of DRLC on behavioral deficits, and neuronal perturbations in the hippocampus following paradoxical sleep deprivation in adult Wistar rats. Animals were paradoxically sleep deprived for 7 days ; DRLC (75 mg/kg b.w of RiboceineTM) was pre- and post-administered for 21 days before and after paradoxical sleep deprivation. Thereafter, behavioral study was conducted to assess fear and anxiety , animals were sacrificed and biochemical analysis of oxidative stress markers and histomorphology of the hippocampus was performed. Behavioral assessments revealed that PSD elicited increased anxiety levels as demonstrated by reduced line crossing, reduced habituation time, and increased freezing time in OFT as well as increased time spent in closed arms of EPM. Also, oxidative stress levels were elevated by PSD with significantly decreased activities of catalase (CAT), superoxide dismutase (SOD) and glutathione (GSH) concentration as well as increased Malondialdehyde concentrations in the blood serum. However, pre-and post-treatment with DRLC significantly prevented and reduced anxiety levels and oxidative stress levels as well as prevented and repaired neuronal hippocampal damage associated with PSD respectively. In conclusion, DRLC was able to modulate oxidative stress-driven alterations and perturbed emotionality linked with PSDthrough its anxiolytic and antioxidative properties.
Abbreviations: D-ribose-L-cysteine(DRLC); Catalase(CAT); Glutathione transferase(GSH); Superoxide dismutase(SOD); Non-rapid eye movement(NREM); Rapid eye movement(REM); Paradoxical sleep deprivation(PSD); Elevated plus maze(EPM); Open Field test(OFT)
Introduction
Sleep is a ubiquitous phenomenon and most species, including humans spend a significant time asleep. It is widely acknowledged that sleep is crucial for proper brain function. There are five phases of sleep: the wake phase, non-rapid eye movement (NREM) phase, which has 3 stages (N1 to N3), and the rapid eye movement (REM) phase. Wakefulness is characterized by more than 50% alpha waves and opening of the eyes. The N1 stage of the NREM phase is the most superficial stage of sleep characterized by skeletal muscle tone and regular breathing rate. The N2 stage, where the majority of sleep is spent, typifies a deeper stage of sleep characterized by lowered body temperature and heart rate. As deeper sleep develops, there is a transition to the N3 stage of NREM. This is the deepest stage of sleep when the body repairs worn-out tissues, builds bones and strengthens the immune system. REM sleep usually begins about 90 minutes after an individual falls asleep. Apart from the eye and diaphragmatic breathing muscles which remain active, all other skeletal muscles are inactive. The REM stage of sleep is characterized by dreaming and erratic breathing rate [Citation1].
Each sleep phase is characterized by specific chemical, cellular and anatomic events of vital importance for normal neural functioning [Citation2] Different forms of sleep deprivation may lead to a decline of cognitive functions in individuals [Citation1].
Sleep deprivation is the state of inadequate sleep which may either be acute or chronic. Chronic sleep deprivation is associated with fatigue, clumsiness, weight loss or gain, and daytime sleepiness [Citation1]. Apart from causing physical health problems, sleep deprivation also has adverse effects on the brain [Citation3] especially on hippocampal function [Citation4].
Although several models of hippocampal function focus on its well-known role in cognitive functions, historically it has also been viewed as a neural mediator of emotion. Accumulating evidence that may accommodate some of this variability is that anxiety is functionally segregated within the hippocampus, with ventral subregions more involved in anxiety-related processes, and dorsal subregions more involved with cognitive processes. Hippocampal cellular and molecular processes critical for memory consolidation and emotionality are affected by the amount and quality of sleep attained. Chronic sleep deprivation triggers reduction of hippocampal cell proliferation and neurogenesis, which may eventually lead to hippocampal volume reduction. Hence, the impaired hippocampal plasticity and function contribute to cognitive disorders and psychiatric diseases [Citation4].
D-ribose-L-cysteine (DRLC), a precursor to the antioxidant glutathione [Citation5], is a very simple and unique molecule that is produced naturally all the time in the body. It is a combination of three simple building blocks of protein or amino acids-cysteine, glycine, and glutamine. It is believed to enhance wound healing [Citation5], attenuates testicular damage [Citation6], attenuates memory deficit induced by lipopolysaccharide [Citation7]. DRLC primarily protects and detoxifies the cell and studies have shown that glutathione production declines with age from age 20, at a rate of about 1% per year [Citation8]. Depletion of the body’s glutathione level is triggered by air toxicants, food, stress, radiation, and pharmaceutical medications [Citation9–12]. DRLC is a synthetic antioxidant that is effective in combating oxidative stress. Studies have shown that DRLC supplementation has both protective and ameliorative impacts on tissue damage that results from oxidative stressors [Citation13–15]. There is evidence linking oxidative stress to the occurrence of depression and anxiety. Since, brain lipid peroxidation cannot be directly assessed in living subjects, the need to measure oxidative stress markers in other biological fluids such as the serum is paramount. An increased concentration of oxygen reactive species (ROS) in cells and tissues and the inability of a biological system to detoxify these reactive products often result in harmful effects on important cellular structures like proteins, nucleic acids, and lipids. Sleep deprivation tends to cause increased generation of reactive oxygen species which leads to the buildup of oxidative stress in different brain regions that can predispose a person to various neurodegenerative disorders. Obtaining adequate sleep is challenging in a society that values ‘work around the clock’. Hence, the development of interventions to combat the negative cognitive effects of sleep deprivation is key. Studies have shown that sleep deprivation is a potent oxidative stressor [Citation16,Citation17] that leads to various neurodegenerative disorders. Therefore, this study intends to investigate the impact of D-ribose-L-cysteine (DRLC) on behavioral deficits, and neuronal perturbations in the hippocampus triggered by increased oxidative stress parameters in the serum, induced by paradoxical sleep deprivation.
Materials and Methods
Animal Care
Thirty-five (35) adult male Wistar rats weighing between 180 and 200 g were procured, acclimatized, and housed in the animal house of the Faculty of Basic Medical Sciences, Osun State University. The rats had liberal access to rat chow and water. Animal care and experimental procedures were performed according to the ARRIVE guidelines, and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Animal Grouping and Treatment
Thirty-five (35) Wistar rats weighing between 180 and 200 g were randomly divided into 5 groups with n = 7.
Group I:Control group.
Group II:PSD group paradoxically sleep deprived for 7 days
Group III:PSD+DRLC group paradoxically sleep deprived for 7 days and post-treated with DRLC (75 mg/kg b.w) [Citation7,Citation15] for 21 days
Group IV:DRLC+PSD group pretreated with DRLC (75 mg/kg b.w) for 21 days to evaluate the protective impact of DRLC and then paradoxically sleep deprived for 7 days;
Group V:DRLC group treated with DRLC (75 mg/kg b.w) only for 21 days. This serves as positive control for groups III & IV
DRLC was procured from Max International, dissolved in distilled water, and administered orally using oral gavage. Pre and post-treatment with DRLC were to access the protective and ameliorative potentials of D-Ribose-L-Cysteine.
Paradoxical Sleep Deprivation (PSD) Protocol
The multiple platform technique was used to induce PSD according to the method described by Nunes et al., 1994 [Citation18]. The water tank with dimensions 42 cm x 35 cm x 17 cm) contained 12 platforms (3 cm in diameter) surrounded by water up to 1 cm beneath the surface. Each group was placed on the 6 platforms with the animals being able to move from one platform to another. Animals were sleep-deprived both day and night. They were only taken out of the tank and fed in conventional cages for 3 hours daily after which they were returned to the tank. PSD lasted for 7 days.
Neurobehavioural Paradigms
Open field test (OFT)
The locomotor activity and anxiety-like behavior were measured in the open field test [Citation19]. Rats were gently placed into a corner of the box and allowed to explore the apparatus for 3 minutes. Exploratory motor activity (EMA) which includes horizontal locomotion (the number of squares crossed) and vertical activity (rearing) were recorded. At the end of the test, the rat was gently picked from the maze, returned to home cage and fecal boli removed and urination spots cleaned. The maze was cleaned with 95% ethanol before the commencement of the next test period.
Elevated plus maze test (EPM)
The elevated plus maze is used for assessing anxiety responses of rodents [Citation20]. At the commencement of this test, animals were centrally placed between the open and closed arms, and the behavior of each animal was consistently recorded using a video recorder mounted in the room for 5 min. The training session lasted for 5 min before the test session. The number of times the rats entered the open arm or closed arm and the time spent in each arm were recorded.
Sample Collection
After the completion of treatments and neurobehavioral testing, animals were euthanized by intraperitoneal anesthetic injection of ketamine hydrochloride (50 mg/kg) and a mid-abdominal incision was made on the anterior abdominal wall toward the thoracic region to expose the heart. Blood was collected via cardiac puncture using a sterile needle and 5 ml syringe into plain bottles. The blood sample was allowed to clot for 30 mins, centrifuged at 2000 g for 10 mins and serum was pipetted into polypropylene tubes for immediate biochemical analysis. The animal’s brain tissue was excised from the skull, rinsed in 0.25 M sucrose 3 times for 5 minutes each, and thereafter fixed in buffered neutral formalin (BNF). Sacrifice of the animals was done in batches based on the duration and treatments of animals in each group.
Determination of Oxidative stress markers
Superoxide dismutase (SOD), Catalase (CAT), Glutathione (GSH), and Malondialdehyde (MDA) assay kits procured from Bio Legend Inc., San Diego, CA, USA were used to determine the activities of SOD and CAT, and the concentrations of GSH and MDA in the blood serum of the experimental animals for the quantification of oxidative damage in the experimental animals.
Superoxide dismutase activity
Superoxide dismutase scavenges superoxide anion, converting it into hydrogen peroxide and oxygen thus preventing peroxynitrite production. The activities of Superoxide dismutase in the serum was determined according to the method of Misra and Fridovich [Citation21] based on the ability of SOD to inhibit the autoxidation of adrenaline to adrenochrome in a basic medium. Summarily, 75 mM tris-HCl, 30 mM EDTA, 2 mM pyrogallol (pH 8.2) was added to 50 microliter of the serum. Absorbance was read at 420 nm using a microplate reader
Glutathione assay
The glutathione (GSH) concentration was estimated following the procedure developed by Ellman [Citation22] and described by Beutler et al. [Citation23], based on the ability of the Ellman reagent, 5, 5-dithiobis-2-nitrobenzoic acid (DTNB), to react with compounds containing sulfhydryl groups, yielding a mixed disulfide (GS-TNB) and 2-nitro-5-thiobenzoic acid (TNB) with the absorbance of the anion (TNB2-) read at 412 nm.
Catalase activity
Determination of catalase activity in the serum was performed according to the dichromate method described by Sinha [Citation24] based on the utilization of hydrogen peroxide by catalase using the potassium dichromate K2Cr2O7/acetic acid reagent. Briefly, when heated in the presence of hydrogen peroxide, the dichromate in acetic acid reduces to chromic acetate, which is measured spectrophotometrically at 610 nm.
Malondialdehyde assay
MDA concentration was determined by measuring thiobarbituric acid reactive substances present in the blood serum according to the modified method of Buege and Aust [Citation25], 1 ml of the serum was added to 2 ml of the reagent comprised of trichloroacetic acid, thiobarbituric acid, and hydrochloric acid (TCA/TBA/HCl) at ratio 1:1:1. After vigorously shaking, it was boiled for 15 min, cooled on ice, centrifuged at 3000 rpm for 10 min, and the absorbance was read at 532 nm against a blank.
Histology and histochemistry
Fixed brain tissues in BNF were dehydrated with ascending grades of alcohol, clear in xylene, infiltrated and embedded with paraffin wax, and mounted on a paraffin cassette. Thereafter, hippocampal sections of 6 microns were cut using a microtome, mounted on a clean slide, and stained with Hematoxylin and eosin for general histology according to the method of Bancroft & Layton [Citation26] and Cresyl fast violet for histochemical demonstration of Nissl substances according to the method of Pilati et al. [Citation27], Photomicrographs were captured using a Biobase binocular research microscope (China) connected to a 5.0 MP Amscope.
Statistical Analysis
Quantitative outcomes of neurobehavioral and biochemical examinations were analyzed using GraphPad Prism® (version 8) software. The outcomes were plotted in One-way ANOVA followed by Turkey’s multiple comparisons test. Significance was set at p < 0.05*. The staining polarity of the Cresyl fast violet stain was measured using the Image J software.
Results
Sleep Deprivation was Associated with Increased Emotionality in the Open Field Test
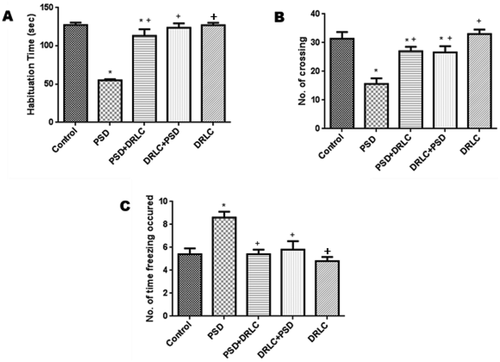
Obvious signs of anxiety and emotionality displayed following PSD were significantly reduced with pre-and post-treatment with DRLC as observed in . PSD group exhibited significantly (p < 0.05) reduced time of habituation with a mean value of 55.13 ± 3.262 when compared to the control group as well as the DRLC treated groups which showed significantly increased habituation time with p-value <0.05 as seen in . In addition, the PSD group displayed significantly reduced number of crossing (15.60 ± 1.217) with a p-value <0.05 in contrast to the control group having a mean value of 31.40 ± 1.230. However, DRLC treated animals (PSD+DRLC, DRLC+PSD groups showed a significantly increased no of crossings with a p-value <0.05 when compared with the PSD group as seen in . Furthermore, PSD animals spent significantly increased time freezing with a mean value of 8.24 ± 2.130 when compared to the control and DRLC treated groups as observed in .
Figure 1. Open field neurobehavioral test. (A) Time of habituation (B) No of crossing (C) No of time freezing occurred. The PSD animals displayed obvious signs of poor memory and emotionality. In the open field test, the PSD group showed significantly reduced habituation time and horizontal locomotion (crossing), as well as increased freezing time when compared to the control and other treatment groups (p < 0.05). the values are expressed as mean ± SEM. P < 0.05 is considered to be statistically significant; * indicate significant level of difference in compared with control; + indicate significant level of difference in comparison with PSD group.
Sleep Deprivation Increases Anxiety
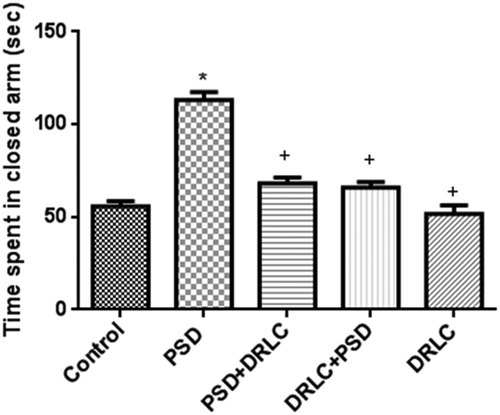
The elevated plus maze test was done to evaluate anxiety and fear. PSD+DRLC, DRLC+PSD, DRLC treated animals, as well as the control group, showed reduced time spent in the closed arm (68.00 ± 1.412, 65.80 ± 0.851, 51.60 ± 1.920, and 53.040 ± 1.213 respectively) whereas PSD animals significantly spent more time (113 ± 1.406) in the close arm of the apparatus as seen in .
Figure 2. Elevated plus maze test. the PSD group exhibited low time of habituation and spent more time in the closed arm when compared with the control group (p < 0.05). animals pre and post-treated with DRLC in this study showed little or no emotional impairment as indicated by the significantly reduced time spent in the closed arm when compared with the PSD group (p < 0.05). the values are expressed as mean ± SEM. P < 0.05 is considered to be statistically significant; * indicate significant level of difference when compared with control; + indicate significant level of difference in comparison with PSD group.
SOD, GSH, and CAT Profiles increases following D-Ribose-L-Cysteine Administration
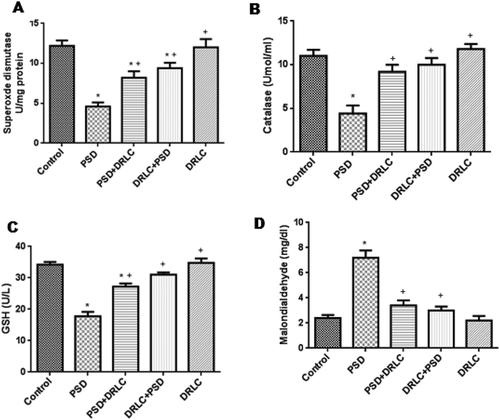
Differential expression of superoxide dismutase and catalase activities, as well as glutathione concentrations, were assessed in the experimental rats as seen in respectively. Results showed significant increase (p < 0.05) in the activities of catalase (CAT) and superoxide dismutase (SOD), as well as the levels of glutathione (GSH) in DRLC, treated animals (PSD+DRLC, DRLC+PSD, DRLC) when compared with PSD animals (group B). PSD rats expressed significantly decreased levels in the activities of these enzymes (CAT & SOD) and concentration of glutathione (GSH).
Figure 3. Oxidative stress markers. (A) Activities of superoxide dismutase, (B) Activities of catalase, (C) Concentration of glutathione, and (D) Concentration of malondialdehyde in the blood serum of rats. SOD and Catalase activities as well as glutathione concentration in PSD group was significantly reduced compared to DRLC rats. Malondialdehyde levels were significantly increased in PSD animals when compared with control and DRLC groups. the values are expressed as mean ± SEM. p < 0.05 is considered to be statistically significant; * indicate significant level of difference when compared with control; + indicate significant level of difference in comparison with PSD group.
DRLC Reduced Lipid Peroxidation
MDA profiles as shown in , increased significantly in the PSD animals (7.200 ± 2.131) as against the reduced MDA mean value (2.400 ± 1.208) of the control group, as well as the DRLC treated groups. There was no observable significant difference in the malondialdehyde levels of animals pre and post-treated with DRLC when compared with the control.
Histological and Histochemical Evaluation
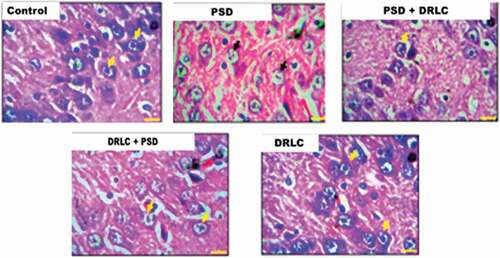
In , the control group showed a well-outlined array of cells within the hippocampus as seen from the cornu ammonus (CA) to the dentate gyrus (DG). Normal cellular densities were observed in the different hippocampal layers characterized by normal dendritic spines (yellow arrows). Group B animals, however, showed fragmentation in the pyramidal and granular cell layer with the presence of pyknotic cells. The pre-and post-treated DRLC animals showed regenerative changes (black arrows) mildly similar to the morphologic appearance of control treatments; their cortical layers appeared better structured and delineated with a distinct layering when compared with PSD animals (group B). The DRLC group showed similar morphological observation to that of the control group.
Figure 4. Representative photomicrographs showing the general morphology of the hippocampus of the Wistar rats across the various groups stained with H & E. (Scale bars: 50 µm). the Dentate Gyrus (DG) composed of granule cells, Cornu amonus (CA1-3) containing pyramidal cells, are well demonstrated across the experimental groups.
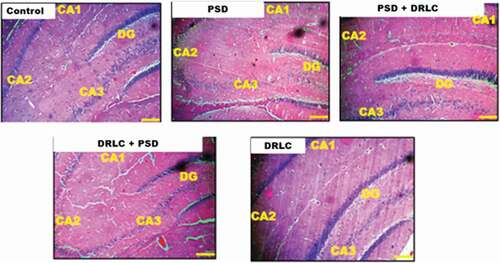
Figure 5. Representative photomicrographs showing the granule and pyramidal cells (neurons) of the hippocampus of the wistar rats across the experimental groups stained with H & E stain (scale bars: 25 µm) .
Also, in , normal cytoplasmic Nissl substance staining was observed in the control (A) and pre-and post-treated DRLC animals characterized with well-stained neurons (yellow arrows) whereas, the PSD group showed chromatolytic changes in the pyramidal and granular cell layers with reduced cytoplasmic Nissl staining intensity (black arrow).
Figure 6. Representative photomicrograph showing the expression of nissl substance in the hippocampus of the wistar rats across the experimental groups stained with cresyl fast violet stain (scale bars: 50 µm) .
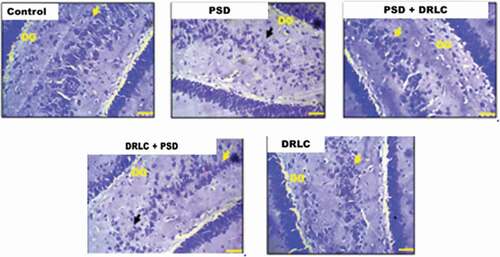
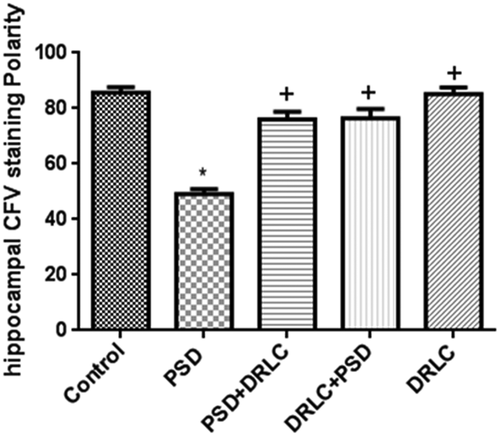
Figure 7. Cresyl fast violet staining intensity in the hippocampus. Reduced CFV staining was observed in the hippocampus of PSD groups when compared to DRLC treated groups. p < 0.05 is considered to be statistically significant; * indicate significant level of difference when compared with control; + indicate significant level of difference in comparison with PSD group.
Discussion
This current study was designed to investigate the anxiolytic and antioxidative potential of D-Ribose-L-Cysteine following paradoxical sleep deprivation in male Wistar rats. Laboratory rats normally sleep for about 12 hrs/day of which 15–20% of the sleep time corresponds to the REM sleep stage. This study confirmed REM sleep deprivation as a stressor, which may have deleterious effects on learning and memory sub-served by the hippocampus via its anxiogenic and oxidative mechanisms.
In an attempt to establish the anxiety-related behavior associated with PSD and DRLC treatment, the EPM and OFT were used to assess fear and anxiety in the experimental groups. Our findings confirmed previous results of behavioral deficits in sleep-deprived animals [Citation28,Citation29]. While sleep deprivation was associated with increased exploratory activities in open field test following tread-mill sleep deprivation [Citation30], in our study, sleep-deprived rats exhibited reduced motor activity characterized by decreased crossings, decreased time of habituation and increased time spent freezing. This difference in exploratory response may be due to the difference in the technique and duration employed to induce sleep deprivation. In addition, although some studies showed that sleep deprivation reduced anxiety-like behavior [Citation31,Citation32], however, in this study, paradoxical sleep deprivation was associated with increased anxiogenic effect as reflected by increased time spent in the closed arm of the maze. This finding was similar to the report of Silva et al. [Citation33], and Omotoso et al. [Citation34]. However, treatment with DRLC before and after PSD attenuated the anxiety-like behaviors characterized by increased crossings, decreased freezing time, increased time of habituation as well as reduced time spent in the closed arm. Hence, DRLC was confirmed as an anxiolytic agent in this study. Though the mechanism by which DRLC exerts its anxiolytic property is yet to be fully elucidated, our study suggests that this is likely to be related to the antioxidative property of DRLC to reduce the oxidative stress attenuated with PSD
Sleep has been confirmed to limit metabolic requirements whereas sleep deprivation, on the other hand, enhances metabolic rate and in turn increases oxidative stress. Previous research has shown that sleep deprivation elicits psychotic episodes by induction of increased oxidative stress levels [Citation35], triggered by increased metabolic activities. Lipid peroxidation is a known degenerative process of oxidation that leads to destruction of cell membranes, lipoproteins, and other lipid-containing structures [Citation36]. Lipid peroxidation has been linked to a variety of neuropsychiatric disorders, including depression, schizophrenia, bipolar mood disorders, attention deficit hyperactivity disorder, and Alzheimer’s disease [Citation37,Citation38]. Previous studies have shown that increased serum lipid by-products, MDA and Lipid hydroperoxide (LOOH), are linked with patients with anxiety disorders [Citation39,Citation40]. This conforms to the findings in our present study, with increased serum MDA level observed in PSD which elicits lipid peroxidation and eventually elevated anxiety levels as seen in . Whilst high anxiety levels have been established to significantly increase oxidative stress [Citation41], it has also been shown that oxidative stress could trigger anxiety-related behavior [Citation42]. However, D-Ribose-L-cysteine supplementation in this study revealed decreased serum lipid peroxidation evidenced by reduced MDA levels. This could be associated with its ability to prevent anxiogenic-like effects of sleep deprivation and its role as a bioactive source of antioxidants, therefore, reducing oxidative stress elicited by PSD.
In addition, reductions in glutathione level, and catalase and superoxide dismutase activities are commonly used as markers of oxidative stress [Citation43–45]. Sleep deprivation has been established to induce reduced antioxidant levels in different brain regions [Citation16,Citation46] and this conforms to our findings which revealed reduced serum glutathione level and catalase and SOD activities following paradoxical sleep deprivation which may be due to increased free radical production triggered by PSD. However, DRLC treatment in this study was associated with an increased antioxidant level characterized by elevated GSH levels as well as increased catalase and SOD activities. It is suggested that DRLC supplementation augments the synthesis of glutathione [Citation10] which in turn scavenges the free radicals produced thereby reducing oxidative stress. Hence oral supplementation with DRLC increased glutathione level by making available the raw nutritional materials such as cysteine which can be used in glutathione synthesis. Furthermore, positive correlation in glutathione and other antioxidant markers assayed for in this study were observed in animals pre- and post-treated with DRLC. Superoxide dismutase (SOD) and catalase (CAT) are two very important enzymes capable of protecting the brain from oxidative damage by ROS [Citation47]. This study has shown that DRLC supplementation can enhance the activities of SOD and CAT which may subsequently lead to a reduction in oxidized lipid content buildup, thus providing a defense against cell damage caused by ROS
The morphological demonstration of the hippocampus of PSD animals in this study showed pathological changes characterized by necrotic and chromatolytic neurons (–9). Consistent with findings from this study, decreased nissl staining intensity is often seen in pathological conditions [Citation48] (fig 10). Uncontrolled generation of ROS is a known cause of necrotic neuronal death which is the hallmark of neurodegenerative diseases. Rats treated with DRLC presented with normal hippocampal morphology. The findings of this study confirm the neuroprotective and ameliorative potentials of DRLC against PSD-induced neuronal damage.
Conclusion
Though numerous studies emphasize the possible chronic impact of long-term sleep deprivation or chronic sleep restriction on the occurrence of neurodegenerative diseases such as Alzheimer’s disease and dementia [Citation49–51]; however, our present study suggests that D-Ribose-L-Cysteine (DRLC) supplementation possibly attenuates and ameliorates behavioral deficits and neuronal damage of the hippocampus caused by paradoxical sleep deprivation via its anxiolytic and antioxidative properties.
Acknowledgments
We acknowledge the laboratory personnel of the Department of Medical Biochemistry, Osun State University, for assistance with the biochemical analyses of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ranasinghe AN, Gayathri R, Vishnu Priya V. Awareness of effects of sleep deprivation among college students. Drug Invention Today. 2018;10(9):1806–1809. Corpus ID: 188837567.
- Alkadhi K, Zagaar M, Alhaider I, et al. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11(3):231–249.
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–3.
- Kreutzmann JC, Havekes R, Abel T, et al. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–190.
- Saltman AE. D-Ribose-L-cysteine supplementation enhances wound healing in a rodent. Am J Surg. 2015;210)153–158. DOI:10.1016/j.amjsurg.2014.11.014
- Falana B, Adeleke O, Orenolu M, et al. Effect of D-ribose-L-cysteine on aluminum induced testicular damage in male Sprague-Dawley rats. JBRA Assist Reprod. 2017;2:94–100.
- Emokpae O, Ben-Azu B, Ajayi AM, et al. D-Ribose-L-cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;7:1–7.
- Erden‐İnal M, Sunal E, Kanbak G. Age‐related changes in the glutathione redox system. Cell Biochem Funct. 2002;20(1):61–66.
- Bhatia AL, Jain M. Spinacia oleracea L. protects against gamma radiations: a study on glutathione and lipid peroxidation in mouse liver. Phytomedicine. 2004;11(7–8):607–615.
- Kader T, Porteous CM, Williams MJ, et al. Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein (a) mice. Atherosclerosis. 2014;237(2):725–733.
- Ismail AF, Salem AA, Eassawy MM. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J Photochem Photobiol B Biol. 2016;160:1.
- Roychoudhury S, Chakraborty S, Choudhury AP, et al. Environmental factors-induced oxidative stress: hormonal and molecular pathway disruptions in hypogonadism and erectile dysfunction. Antioxidants. 2021;10(6):837.
- Cukrov D, Newman TA, Leask M, et al. Antioxidant treatment ameliorates phenotypic features of SMC1A-mutated Cornelia de Lange syndrome in vitro and in vivo. Hum Mol Genet. 2018;27(17):3002–3011.
- Osinubi AA, Medubi LJ, Akang EN, et al. A comparison of the anti-diabetic potential of D-ribose-L-cysteine with insulin, and oral hypoglycaemic agents on pregnant rats. Toxicol Rep. 2018;5:832–838.
- Abayomi TA, Tokunbo OS, Ajayi M, et al. Riboceine regimen attenuates ethanol-induced neuronal damage in the cerebellum of adult male wistar rats. Iran J Toxicol. 2021;15(4):249–256.
- Mathangi DC, Shyamala R, Subhashini AS. Effect of REM sleep deprivation on the antioxidant status in the brain of Wistar rats. Ann Neurosci. 2012;19(4):161.
- Rizk NI, Rizk MS, Mohamed AS, et al. Attenuation of sleep deprivation dependent deterioration in male fertility parameters by vitamin C. Reprod Biol Endocrinol. 2020;18(1):1–3.
- Nunes JGP, Tufik S. Validation of the modified multiple platform method (MPM) of paradoxical sleep deprivation in rats. Sleep Res. 1994. 23(1)
- Seibenhener ML, Wooten MC. Use of the open field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;96:e52434.
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525–529.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 25;247(10):3170–3175.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972 1;47(2):389–394.
- Buege JA, Aust SD. Lactoperoxidase-catalyzed lipid peroxidation of microsomal and artificial membranes. Biochim Biophys Acta. 1976 24;444(1):192–201.
- Bancroft JD, Layton C. The hematoxylins and eosin. In: Bancroft’s theory and practice of histological techniques. 7th ed; (Netherlands, Amsterdam:) ed. 2012. p. 173–186.
- Pilati N, Barker M, Panteleimonitis S, et al. A rapid method combining Golgi and Nissl staining to study neuronal morphology and cytoarchitecture. J Histochem Cytochem. 2008;56(6):539–550.
- Ruskin DN, Liu C, Dunn KE, et al. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19(11):3121–3124.
- Fukushiro DF, Calzavara MB, Trombin TF, et al. Effects of environmental enrichment and paradoxical sleep deprivation on open-field behavior of amphetamine-treated mice. Physiol Behav. 2007 23;92(4):773–779.
- Tartar JL, Ward CP, Cordeira JW, et al. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197(2):450–453.
- Martinez-Gonzalez D, Obermeyer W, Fahy JL, et al. REM sleep deprivation induces changes in coping responses that are not reversed by amphetamine. Sleep. 2004;27(4):609–617.
- Hajali V, Sheibani V, Esmaeili-Mahani S, et al. Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behav Brain Res. 2012;17;228(2):311–318.
- Silva RH, Kameda SR, Carvalho RC, et al. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology (Berl). 2004;176(2):115–122.
- Omotoso GO, Olajide OJ, Gbadamosi IT, et al. Kolaviron protects the prefrontal cortex and hippocampus against histomorphological and neurobehavioural changes in cuprizone model of multiple sclerosis. Malays J Med Sci. 2018;25(2):50–63.
- Alzoubi KH, Khabour OF, Salah HA, et al. The combined effect of sleep deprivation and western diet on spatial learning and memory: role of BDNF and oxidative stress. J Mol Neurosci. 2013;50(1):124–133.
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–1542.
- Bilici M, Efe H, Köroğlu MA, et al. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64(1):43–51.
- Ceylan M, Sener S, Bayraktar AC, et al. Oxidative imbalance in child and adolescent patients with attention-deficit/ hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1491–1494.
- Ceylan MF, Guney E, Alisik M, et al. Lipid peroxidation markers in children with anxiety disorders and their diagnostic implications. Redox Rep. 2014 Mar;19(2):92–96.
- Kuloglu M, Ustundag B, Atmaca M, et al. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20(2):171–175.
- Rammal H, Bouayed J, Younos C, et al. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22(8):1156–1159.
- Rammal H, Bouayed J, Younos C, et al. The impact of high anxiety level on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol. 2008;589(1–3):173–175.
- Younes-Mhenni S, Frih-Ayed M, Kerkeni A, et al. Peripheral blood markers of oxidative stress in Parkinson’s disease. Eur Neurol. 2007;58(2):78–83.
- Puertas MC, Martinez-Martos JM, Cobo MP, et al. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol. 2012;47(8):625–630.
- Trivedi MS, Shah JS, Al-Mughairy S, et al. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J Nutr Biochem. 2014;25(10):1011–1018.
- Singh R, Kiloung J, Singh S, et al. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology. 2008;9(3):53–62.
- Alzoubi KH, Khabour OF, Tashtoush NH, et al. Evaluation of the effect of pentoxifylline on sleep‐deprivation induced memory impairment. Hippocampus. 2013;23(9):812–819.
- Sunday FO, Dauda SP, Oladele OJ, et al. Moringa oleifera leaf extract potential in ameliorating MK-801-induced schizophrenia. J Exp Clin Anat. 2019;18(1):69–84.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from adult brain. Science. 2013;342(6156):373–377.
- Li J, Ogrodnik M, Kolachalama VB, et al. Assessment of the mid-life demographic and lifestyle risk factors of dementia using data from the Framingham heart study offspring cohort. J Alzheimers Dis. 2018;63(3):1119–1127.
- Lutsey PL, Misialek JR, Mosley TH, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the atherosclerosis risk in communities study. Alzheimer’s & Dementia. 2018;14(2):157–166.
