ABSTRACT
The present study aimed to study the effect of Silver nanoparticles (AgNPs) on the reproductive health of female Wistar rats. Twenty-one adult female rats in the estrus phase were divided into three groups. Seven rats each, Group (I) was the control group, Group (II) received a low dose of 2 mg/kg of AgNPs, and Group (III) received a high dose of 4 mg/kg of AgNPs. Rats were intraperitoneally (I.P.) injected three days per week for 30 days. After 24 hours from the last treatment, the rats were sacrificed under anesthesia. The results indicated no change in body weight or relative and absolute weight of reproductive organs. The biochemical results showed decreased estrogen hormone levels and no change in progesterone hormone levels. High dose group revealed a significant increase in follicle-stimulating and luteinizing hormone. AgNPs caused a significant decrease in total antioxidant capacity and a significant increase in malondialdehyde levels. AgNPs induced ovarian follicles degeneration. The uterine epithelial cells showed a high level of hyperplasia, losing their columnar shape showing apoptosis and inflammation. Immunohistochemistry studies showed a significant increase in activated caspase-3. This study showed the harmful effect of AgNPs on reproductive tissues, which may increase the chance of infertility.
Introduction
Reproduction is needed to ensure the continuity of species. Problems in reproduction may lead to infertility. Infertility is the incapability of a couple to achieve pregnancy over a usual period of one year (in a woman under 35 years of age) or six months (in a woman above 35 years of age) despite adequate, regular unprotected sexual intercourse [Citation1]. Infertility may be a result of a disturbance in reproductive health which is a state of complete physical, mental and social safety and not just the absence of disease or disability, in all matters relating to the reproductive system and its functions and processes. Reproductive health and the environment focus on exposures to environmental contaminants during serious periods of human development.
Conclusion: The female reproductive system is critical for embryonic development and the survival of the species. Female reproductive systems are much more delicate than male reproductive systems. This is due to female gametes’ limited production in comparison to reproductive male gametes, the reproductive organs’ periodic growth and regression, as well as their sensitivity to foreign bodies and stress, which is influenced and regulated by hormones; and the disruption of female reproduction, which inevitably leads to abnormal fetal development [Citation2] so it can be more sensitive to environmental contaminants
One of these contaminants is Nanoparticles (NPs) which are generally defined as particulate matter with at least one dimension of less than 100 nm. They have unique thermal, mechanical, magnetic, and optical capabilities, allowing them to be used in various biomedical and industrial applications, which would elevate the risk of human exposure to NPS One of the most popular nanoparticles is silver nanoparticles. AgNPs are important because of their unique properties, which can be included in antimicrobial applications, biosensor materials, cosmetic products, and many other fields [Citation3–5]. Silver nanoparticles have also been added to medical products, including wound dressings, contraceptives, surgical instruments, bone prostheses, and cardiac catheters [Citation6].
Studies have shown that N.P.s are likely to have toxic effects on the brain, liver, and lungs, the most studied target organs. According to recent research, nanoparticles can infiltrate cells and disrupt their biological structures and activities by producing reactive oxygen species (ROS) or increasing intracellular oxidative stress [Citation7]. Many N.P.s can also pass across the blood-testis barrier (BTB), placental barrier, and blood-brain barrier (BBB) to collect in a variety of cells [Citation8]. Reproductive toxicity caused by NPS is becoming increasingly significant. Most studies on the toxicity of NPS in the female reproductive system focus on effects on reproductive capacity, teratogenic effects during embryonic development, and perinatal effects on children [Citation8]. According to a recent study, inhaled, swallowed, or dermally absorbed NPS can easily diffuse via the circulatory system and collect in many reproductive organs, including the fetus, according to a recent study [Citation9].
According to toxicity studies in rats and mice, in vivo biodistribution and AgNPs directed via inhalation, ingestion, or intravenously (i.v)/ (I.P.) injection are detected in blood and cause harm to several organs, including the lung, liver, kidney, intestine, and brain. According to the study, short-term (14 days) nose-only delivery of 20 nm AgNPs at different concentrations resulted in alterations in brain gene expression. In rats given 18 nm AgNPs, researchers detected minor, dose-dependent lung inflammation and alterations in pulmonary function over a 90-day inhalation period [Citation10]. AgNPs accumulated in the brain, olfactory bulb, kidney, and spleen [Citation11]. Inhalation studies in which pregnant females were exposed to newly generated aerosols for either 1 or 4 hours per day during the first 15 days of pregnancy indicated that AgNPs (18–20 nm) could pass through the mouse placenta [Citation10–12]. An in vivo study verified a significant change in levels of serum Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estrogen (E2) in the polycystic ovary-induced mice treated with both concentrations of silver N.P.s as compared to the control [Citation13]. Many investigations have also reported on the reproductive toxicity of N.P.s in several germ cell lines in vitro and animal models in vivo [Citation14].
So, according to the known previous studies, no study explains the adverse effect of AgNps on sexual hormones, oxidative stress and histopathological variations in both ovaries and uteri of female albino rats.
Materials and methods
Materials
Silver nitrate and trisodium citrate from Nanotechnology Department in Cairo University Al Sheikh Zayed Branch. Estrogen (E2), Progesterone (P4), Follicle-stimulating hormone (FSH), and Luteinizing hormone (LH) level by ELISA using kits from SunLong Biotech Co., LTD. Lipid peroxidation (MDA) and total antioxidants capacity (TAC) Kits were obtained from Bio diagnostic company (Egypt). Caspase-3 (rabbit polyclonal antibody, ab13847) was purchased from Abcam, USA.
Synthesis of silver nanoparticles
Silver nitrate and trisodium citrate were used as precursor materials to synthesize silver nanoparticles. The silver colloid was synthesized by using the co-precipitation method. This synthesis was done in the Nanotechnology Department in Cairo University Al Sheikh Zayed Branch.
Characterization
The characterization of silver nanocomposite aimed to determine physicochemical properties(X-RD). The microscopic class was carried out using a transmission electron microscope (TEM). TEM was done to ensure that (Ag NPs) are homogeneous in shape and size with\ without agglomeration.
Animals
The international law set up the study protocol for protecting animals in the laboratory, and Ethical approval for animal use was obtained from Institutional Animal Care and Use Committee (CU-IACUC) Cairo-University. (CUFS/Comp&Emb/CU/I/F/75/19).
The Faculty of Veterinary Medicine at Cairo University provided 21 healthy adult female Wistar rats weighing between 150 and 160 g. Animals were housed in hygienic cages with sawdust-covered floors. Rats were kept under controlled circumstances of heat (23 ± 1°C) and humidity (50–60%).
A week of acclimatization was given to the animals before therapy began. Commercial rat chow was fed to the animals, who also had free access to tap water.
Animal grouping
Animals were randomly assigned into three experimental groups. They were divided into seven rats per Group. Group I (Control group) received distilled water, Group II (Treated 1) received a low dose of AgNPs 2 mg/kg, and Group III (Treated 2) received a high dose of AgNPs 4 mg/kg”.
Dosage
Then the powder of AgNPs was diluted in distilled water (200 mg/50 ml) to get a suspension and sonicated to obtain a pure solution. The doses were determined based on a previous study that found the LD50 of AgNPs to be larger than 5000 mg/kg [Citation15]. So, the low dose was 2 mg/kg while the high dose was 4 mg/kg based on [Citation16].
Experimental procedure
Rats were intraperitoneally injected three days per week for 30 days. After 24 hours from the last treatment, the rats were sacrificed under anesthesia with intraperitoneal sodium pentobarbital injection (100 mg/kg body weight). Blood was collected by cardiac puncture, then centrifuged at 4000 rpm for 15 minutes. Serum was stored at −20°C and then used in biochemical and hormonal analyses. Ovary and uterus were dissected and washed with 0.9% saline, dried on filter paper, and weighed.
Serum hormone analysis
The sera obtained from all groups were analyzed for Estrogen (E2), Progesterone (P4), Follicle-stimulating hormone (FSH), and Luteinizing hormone (LH) level by ELISA using kits from SunLong Biotech Co., LTD. Plates were washed two times before adding Standard, Sample and Control (blank) wells! Then 50ul Sample was added to each well. Immediately 50ul Biotin-labeled Antibody was added into each well, gently tapped the plate to ensure thorough mixing, then incubated for 45 minutes at 37°C. Then we washed the plates three times. Then Add 100 µl Working Solution into each well and incubate for 30 minutes at 37°C. Then washed the plates five times. Then Add a 90 µl Substrate Solution. Incubated for 10–20 minutes at 37°C. Then Add a 50 µl Stop Solution. Then Read at 450 nm immediately and calculated [Citation17–19]
Oxidative stress investigation
The left ovary and uterus were separately homogenized (poly, P.T. 2500E, Switzerland) with phosphate buffer saline PBS buffer as 10% (W/V) at pH 7.4, the homogenate was then centrifuged at 10,000 rpm for 20 min, and the clear supernatant was collected and stored in −20c for biochemical studies.
Lipid peroxidation (MDA) based on [Citation20] and total antioxidants capacity (TAC) based on [Citation21] were done for ovarian and uterine tissue. Kits were obtained from the biodiagnostic company (Egypt).
Histological investigation
Three ovaries and three uteri from each Group were fixed in 10% formal saline for twenty-four hours for histological analysis by light microscopy. After washing with tap water, dehydration was accomplished using serial dilutions of alcohol (methyl, ethyl, and absolute ethyl). Specimens were cleared in xylene and embedded in paraffin for twenty-four hours at 56 degrees in a hot air oven. Using a sliding microtome, paraffin beeswax tissue blocks were created for sectioning at a thickness of 4 microns [Citation22].
Immunohistochemistry studies for activated caspase 3
Caspase-3 (rabbit polyclonal antibody, ab13847) was purchased from Abcam, MA, USA and applied at a dilution of 1:100 on the cells [Citation23].
Three ovaries and three uteri from each Group were used. The positive cells were determined with the streptavidin-biotin- peroxidase staining method [Citation23]. Caspase-3 positive reactions were visualized and evaluated by high-power light microscopy. Twenty sections were selected from the groups, and the quantitative analysis of caspase-3 was performed using Image J software (Version 1.53i). The DAB signal was quantified using Image J software to estimate the differences in immunoreactivity. Fifteen fields were selected from each group (5 fields x three rats/ group). The optical density was calculated according to the following equation: O.D. = log (Max. gray intensity/mean gray intensity) to obtain the degree of immunoreactivity (darkness) of the stained cells by the DAB signal [Citation24]
Statistical analysis
All statistical analysis was performed using a package (SPSS Software). Data are presented as mean ± standard error mean (SEM). To determine differences between groups, analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc analysis was used for multiple comparisons between different groups. The level of statistical significance was set at a probability of P < 0.05.
Results
Characterization of silver nanoparticles
X-ray diffraction (XRD)
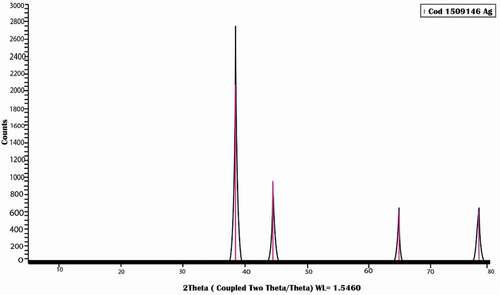
The pattern of AgNPs as shown in () illustrates the presence of five characteristic peaks for silver nanoparticles at 2θ = 38.3°,44.5°,64.8°,77. 8°and82°.The mean crystal size was 4 nm.XRD data illustrate the high crystallinity of silver nanoparticles with a cubic lattice structure.
Transmission electronic microscope (TEM)
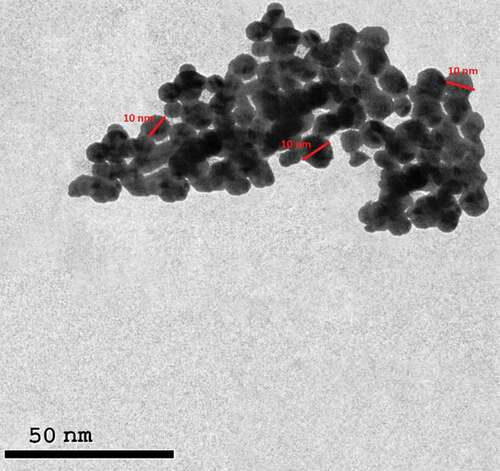
TEM was carried out to identify the morphology of silver nanoparticles and ensure they are homogeneous in shape and size with\without agglomeration ().
Effect of silver nanoparticles on weight change
The administration of AgNPs caused no difference in body weight change of female rats compared with the control group ().
Table 1. Effect of silver nano-particles on weight.
There was no change in the absolute and relative weight of the right ovary after AgNPs injection. In contrast, in the left ovary, the low dosage increased significantly compared to the control ().
There was no change in the absolute uterus weight after AgNPs injection while in relative weight. The high dosage shows a significant increase in weight compared to the control and low dose disturbance ().
Changes in serum hormones
The Administration of AgNps induced a significant reduction in estrogen levels and no change in progesterone levels compared with control ().
Table 2. Effect of silver nano-particle on hormones.
Data shows that AgNPs injection of the low dose caused a significant decrease in both levels of LH&FSH as compared with the control group. However, injection with a high dose of AgNPs causes a significant increase in FSH levels compared with control and low dose groups. It causes a significant increase in LH levels compared with the low-dose group ().
Changes in antioxidants and MDA
After injection with AgNPs, there was a substantial decrease in total antioxidant levels and a significant increase in MDA levels in ovarian tissue when compared to control groups. ().
Table 3. Effect of silver nano-particles on oxidative stress markers in ovarian tissue.
After injection with AgNPs, there was a substantial decrease in total antioxidant levels and a significant increase in MDA levels in uterine tissue when compared to control groups. ().
Table 4. Effect of silver nano-particles on oxidative stress markers in uterine tissue.
Histopathological studies
Ovary
The control group
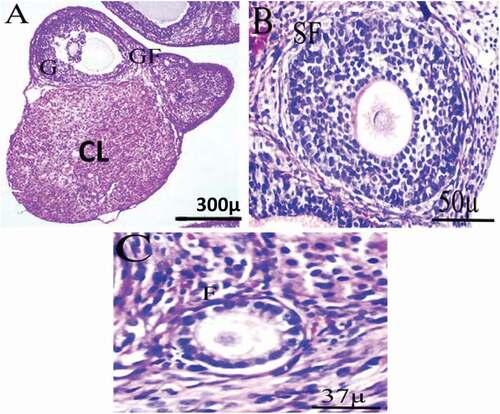
The histology of the control group shows well-developed follicles, normal stromal cells, and well-developed corpus luteal. The preovulatory follicle showed typical oocyte and euchromatin nucleus morphology, surrounded by typical zona pellucida and several layers of granulosa cells resting on the basement membrane. Theca interna and theca externa are developed ().
Low dose group
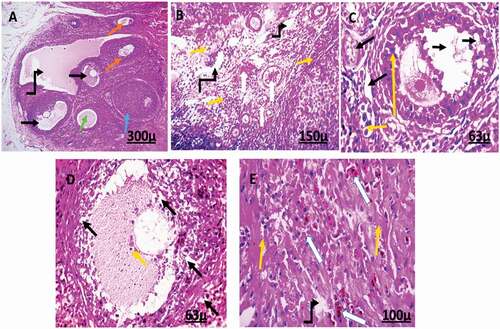
Microscopic examination of the ovarian tissue sections of female rats treated with 2 mg/kg of AgNps shows degenerated follicles. In addition to disorganized zona granulosa of the secondary follicle, degeneration in theca interna and cumulus oophorus of the tertiary follicle and slightly degeneration in the stroma, some atretic follicles and apoptotic cells with pyknotic nuclei, normal and degenerated corpus luteum and some follicular cysts, moreover, hemorrhage of blood vessels, dilated blood vessels with many RBCs, congested blood vessels ().
Figure 4. Photomicrograph of ovarian sections of adult female rats treated with (2 mg/kg of AgNps) low dose H&E stain. Showing: from (a to e) Degenerated tertiary follicle (black arrow), the atretic follicle (Orange arrow), the primary follicle (green arrow), high level of vacuolation with degenerated cells (bent arrow), corpus luteum (blue arrow). High percentage of apoptotic cells with pyknotic nuclei (yellow arrow), hemorrhage of blood vessels, dilated blood vessels with many RBCs (white arrow). High vacuolar degeneration in zona granulosa of the secondary follicle and stroma (black arrow), Degeneration in theca interna and cumulus oophorus of the tertiary follicle and slight degeneration in the stroma (black arrow).
High dose group
Microscopic examination of the ovarian tissue sections of female rats treated with 4 mg/kg of AgNps shows degenerated primary, secondary and tertiary follicles which contain antrum. Also, secondary follicles were disorganized containing red blood cells. Moreover, ovarian tissue shows many corpora lutea, some atretic follicles, hemorrhage, dilated and congested blood vessels, degenerated oocytes containing vacuoles, and high apoptotic cells with pyknotic nuclei to vacuolation and degeneration of stromal cells ().
Figure 5. Photomicrograph of ovarian sections of adult female rats treated with (4 mg/kg of AgNps) high dose H&E stain. Showing: from (a to e) corpus luteal (blue), the atretic follicle (Orange arrow), hemorrhage, and dilated blood vessels (green arrow), degenerated stromal cells (double head arrow), Excessive percentage of vacuolation and degeneration (bent arrow), and a high percentage of apoptotic cells with pyknotic nuclei (red arrow). Degenerated tertiary follicle containing antrum (star), degenerated follicle (black arrow).
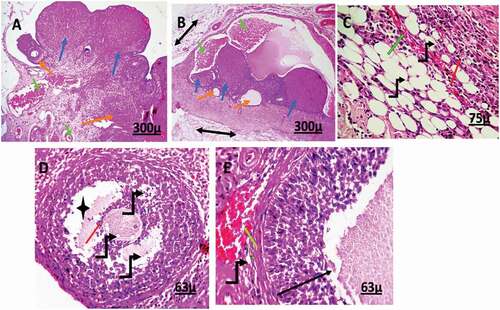
Uterus
The control group
Common uterine histology with normal uterine layers inner endometrium, middle myometrium, outer perimetrium, regular columnar epithelial cells lining the uterine lumen, and glands with normal histology of blood vascularity. The smooth muscles of the myometrium were organized into inner circular and outer longitudinal layers with a layer of blood vessels in between. There was no necrosis, apoptosis, or any other adaptive cellular present in the control uterine tissue sections ().
Figure 6. Photomicrograph of uterine tissue of control adult female rats showing normal histology of different uterine sections, normal blood vessels (black arrow), and normal perimetrium layer (head arrow). MI = Myometrium I (longitudinal muscle layer), M II = Myometrium II (circular muscle layer), G = Gland, E = endometrium.
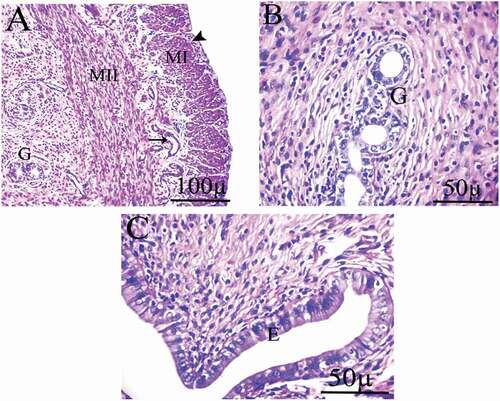
Low dose group
Photomicrograph of the uterus of female rats treated with 2 mg/kg of AgNps shows some degree of degeneration. There is an increase in the number of uterine glands and a severe degree of degenerated signs. It also reveals apoptosis of epithelial cells with cytoplasmic vacuolation. Moreover, inflammation and congested blood vessels were seen. In addition to epithelial hyperplasia, the epithelial cells lose their columnar shape and become flattened, vacuolar degeneration occurs and contains deeply pigmented nuclei ().
Figure 7. Photomicrographs of uterus of female rats treated with (2 mg/kg AgNps).H&E stain. P = Perimetrium, M = Myometrium, E = Endometrium.
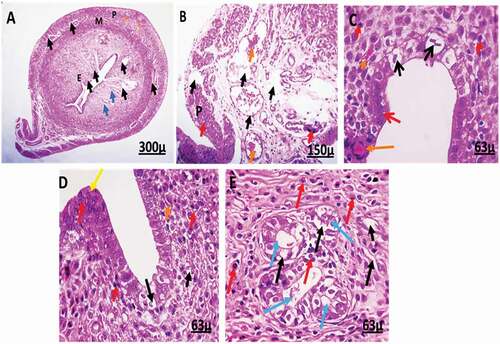
High dose group
Photomicrograph of the uterus of female rats treated with 4 mg/kg of AgNps shows degeneration and apoptosis. It also shows disorganization and vacuolar degeneration in stromal cells of the endometrium and complete degeneration in uterine glands. The width of the lumen becomes very wide. The epithelial cells show a high level of disorganization, folding, hyperplasia, losing their columnar shape, and becoming oval-shaped, showing apoptosis and pyknosis. Moreover, it reveals inflammation in treated uterine tissues ().
Figure 8. Photomicrographs of uterine tissue of female rats treated with (4 mg/kg AgNps).H&E stain. L = Lumen.
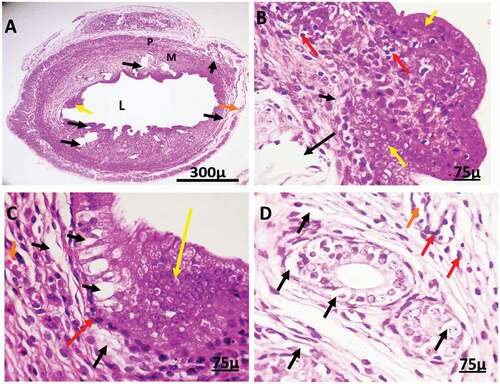
Effect of silver nanoparticles on activated caspase-3
After IP AgNPs injection at low & high doses, the results revealed a significant increase in the level of immunohistochemical expression of Caspase-3 in both ovarian and uterine tissues compared with the control group () and ()
Figure 9. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in ovarian tissue of control adult female rat showing normal expression of immune stain caspase-3.
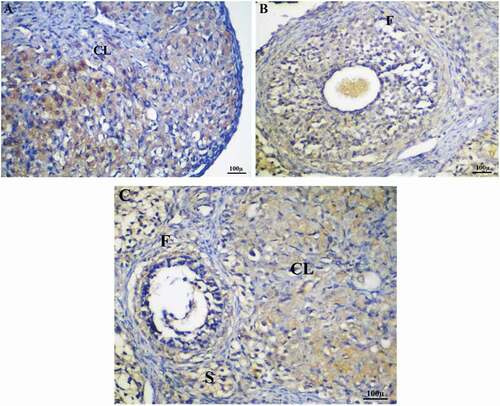
Figure 10. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in ovarian tissue of adult female rats treated with (2 mg/kg of AgNps) low dose. Showing positive and moderate expression of immune stain caspase-3.
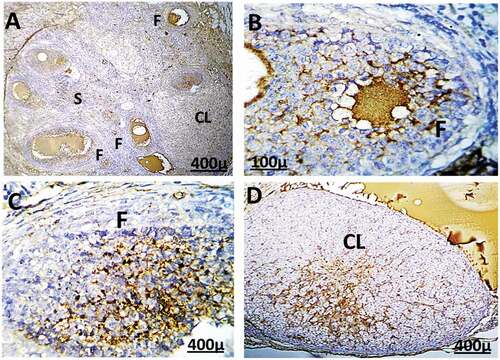
Figure 11. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in ovarian tissue of adult female rat treated with (4 mg/kg of AgNps) high dose, Showing positive and extreme expression of immune stain caspase-3.
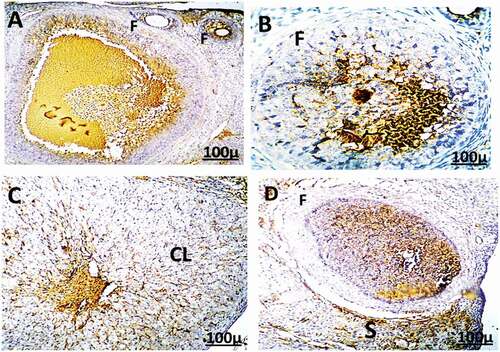
Figure 12. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in the uterus of control adult female rats showing normal expression of immune stain caspase-3. LM = Longitudinal Muscle, CM = Circular Muscle, E = endometrium, G = Gland.
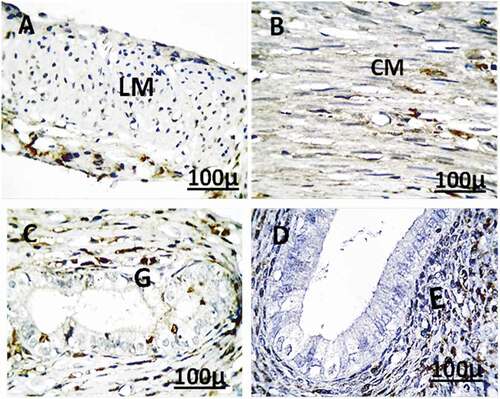
Figure 13. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in the uterus of adult female rat treated with (2 mg/kg of AgNps) low dose showing positive and moderate expression of immune stain caspase-3.
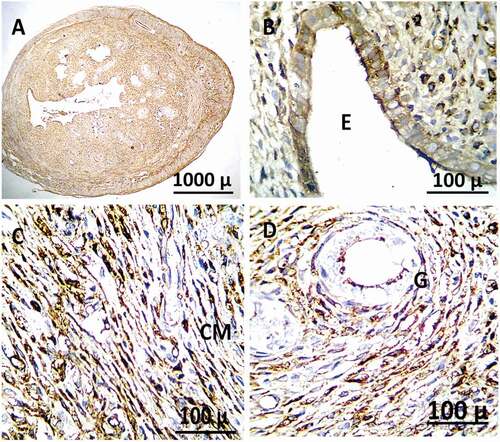
Figure 14. Immunohistochemistry showing caspase-3 streptavidin-biotin- peroxidase staining method in the uterus of adult female rat treated with (4 mg/kg of AgNps) high dose Showing positive and extreme expression of immune stain caspase-3.
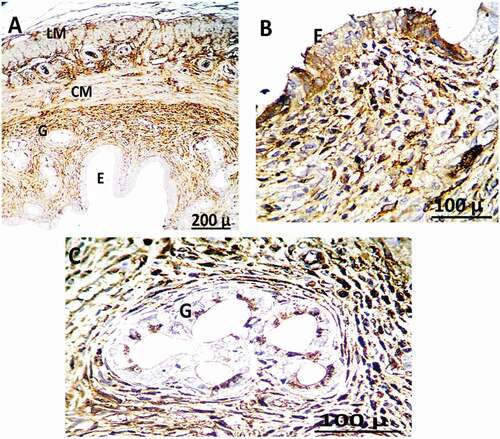
Table 5. Effect of silver nanoparticles on Immunohistochemistry expression for activated caspase-3.
Discussion
The extensive use of AgNPs in medical devices, contraceptives, antibacterial sprays, and many other fields is expected to rise to AgNPs released into our ecosystems [Citation25]. The effects of AgNPs on the reproductive system in female rats were explored in this work. The results showed that intraperitoneal injection of AgNPs caused reproductive system malfunction and disturbed hormone levels in female rats.
The animals given AgNPs in this investigation showed no signs of discomfort (lethargy, nausea, vomiting, or diarrhea) during the duration of the experiment. Moreover, this agrees with wen et al., who stated that animals did not show any discomfort with all tested concentrations of silver nanoparticles [Citation26]. This means that silver nanoparticles did not show any apparent symptoms.
When the two AgNPs treated groups with different doses were compared to the control group, there was no significant difference in total body weight change. These findings corroborated those of Kim et al., who discovered no significant dose-related changes in the body weight of female rats fed orally with (30,125 and 300) mg/Kg AgNPs (56 nm) for 90 days [Citation27]. Furthermore, Sarhan and Hussein demonstrated that short-term intraperitoneal exposure to a dose (2000 mg/Kg) of AgNPs has no significant effect on body weight [Citation28], supporting the findings of this study. On the other hand, a previous study found a significant increase in body weight in rats treated with various doses of AgNPs for 14 and 21 days [Citation29]. In our study, silver nanoparticles did not affect the weight of female rats as we suggested that AgNPs did not alter the food and water intake.
In the present study, we have measured both the relative and absolute weight of the right and left ovaries and uterus. Results indicate no change in the weight of reproductive organs except for the relative weight of female rats treated with a high dosage of AgNPs, which shows a significant increase compared to the control and low dosage group in uterine tissue. A study by Parveen et al. that evaluated the application of AuNPs at different doses upon the intravenous Administration in mice regularly for 28 days showed an insignificant variation in the weight of all the organs tested in both male and female rats. The analysis has not affected their biological functions [Citation30]
According to one study, treatment with this high Cu-NP dose resulted in a considerable reduction in the relative uterine weight coefficient [Citation31]. Also, according to Fardin et al., the weight of the right ovary and uterine body in the MoO3 Nanoparticles group was considerably lower than in the control group. In addition, there was no change in left ovary weight [Citation32]. Our results indicated that AgNPs disturbed sexual hormone levels. NPs can pass through the blood-brain barrier (BBB) and congregate in the CNS [Citation14]. Neuro-hormones secreted from the hypothalamus and pituitary, including gonadotropin-releasing hormones (GnRH), Follicle Stimulating Hormone (FSH), and Luteinizing Hormone (LH), play an important role in positive and negative feedback control during oogenesis. By disrupting the balance of these sex hormones, NPs may indirectly affect oogenesis and ovarian health [Citation14]. In vivo research has proved that long-term (90 consecutive days) exposure to Titanium dioxide NPs (TiO2 NPs) in female mice can result in an imbalance of sex hormones and mineral element releasing, leading to a decrease in pregnancy rate and oxidative stress and disturbance of ovarian gene expression [Citation33]. These findings contradicted those of Fardin et al. They reported that injection of MoO3 NPs resulted in a significant decrease in E2 concentration and an increase in FSH and LH concentrations in female rats [Citation32]. In addition to stimulating receptor systems for FSH and LH levels, the E2 hormone plays an important role in granulosa cell development. The AgNPs-treated groups showed a considerable reduction in E2. In general, AgNPs may strongly influence the hypothalamus, causing GnRH secretion to rise, increasing FSH levels and promoting E2 synthesis. This discrepancy in E2 levels found in our study could be attributed to the direct effect of nanoparticles on the ovaries, which reduced follicular growth and E2, or interference with follicle function. As a result, the number of estrogen-secreting follicles dropped, and E2 release decreased. Lu kong et al. found that Ni NPs raise serum FSH and LH levels while lowering E2 levels in females and that these effects are dose dependent. These data confirmed the effects of Ni NPs on the ovary of female rats. It could be a symptom of decreased serum E2 and ovarian hormone release resulting from ovarian injury caused by Ni N.P.s, which raise serum FSH and LH levels via negative feedback [Citation34]. According to a prior study, AlNPs exposure decreased E2 and P4, indicating disrupted the reproductive regulating system [Citation35]. Reduced E2 levels may lower P4 biosynthesis and impede the growth and development of ovarian follicles, resulting in decreased reproduction. FSH and LH levels are linked to the number and activity of ovarian follicles. LH treatment of rats with large preovulatory follicles resulted in luteinization of the granulosa cells [Citation36].
Oxidative stress is the inequality between oxidative and anti-oxidative systems of cells and tissues. Oxidative stress is from the major mechanisms by which nanoparticles exert their toxicity. Excessive generation of oxidative-free radicals and related reactive oxygen species (ROS) causes oxidative stress, which can cause immune activation and inflammation, which can activate cell death processes such as apoptosis. Apoptosis is an extremely controlled process that is fundamental for the development and survival of multicellular organisms. ROS plays a key role in cell signaling and the control of apoptosis pathways controlled by mitochondria, death receptors, and the endoplasmic reticulum. Numerous studies have shown that oxidative stress plays a role in the pathophysiology of infertility and assisted fertility. There is some evidence of its role in endometriosis, tubal and peritoneal factor infertility, and unexplained infertility. ROS influences a wide range of physiological reproductive functions, including oocyte maturation, ovarian steroidogenesis, corpus luteal activity, and luteolytic [Citation37]. Toxicity is measured by MDA content in vivo, MDA is formed by lipid peroxidation and can react with protein amino groups. MDA is a by-product of lipid peroxidation and is directly related to the damage caused. MDA levels are a valuable biomarker for determining oxidative stress.
Our study revealed a significant increase in MDA levels and a significant decrease in total antioxidant capacity (TAC) in both ovarian and uterine tissues. So, our findings were inconsistent with a study that found that exposure to AgNPs significantly increased serum level of MDA in male mice compared to the control group, which may denote increased oxidative stress [Citation38]. This result supports some other researchers who reported increased serum and tissue levels of MDA after AgNPs administration in rats and mice compared to the control group [Citation39]. Moreover, results showed that TAC significantly reduced after repeated oral administration of AgNPs for 28 days which is consistent with the results of the previous work [Citation40]. Increased serum levels of MDA and reduced serum levels of TAC indicate accumulation of ROS and oxidative damage. These results are consistent with other studies that reported ROS induction, and oxidative stress has been implicated as reasons for toxicity following AgNPs administration [Citation41]. An increase in lipid peroxidation and disruption of the antioxidant system in the ovaries after exposure to AgNPs was confirmed by an increase in MDA and a decrease in TAC, respectively.
Microscopic examination of the ovarian tissue sections of female rats treated with AgNps shows degenerated follicles, slightly degeneration in the stroma, also shows slightly vacuolar degeneration in the stroma, some atretic follicles and apoptotic cells with pyknotic nuclei, normal and degenerated corpus luteum and some follicular cysts moreover hemorrhage of blood vessels, dilated blood vessels with many RBCs, congested blood vessels. As a result, these findings corroborated those of a prior study [Citation42] which found that continuous exposure to various dosages of AgNPs caused histological alterations in the ovary cells of experimental mice. Another study reported a higher incidence of bile-duct fibrosis, pigmentation, hyperplasia, and necrosis were observed by histopathological examination in treated animals after oral exposure to silver nanoparticles [Citation27]. Metal NPs, particularly AgNPs, have been shown to cause reproductive toxicity in reproductive and somatic cells in many studies [Citation43]. Gao et al. previously showed that long-term exposure to (TiO2NPs) caused ovarian dysfunction and altered cell histology and the expression of genes encoding cell proliferation and inflammatory response in the ovaries [Citation44].
Furthermore, Yang et colleagues; reported severe ovarian histological abnormalities in rats given high-dose Cu NPs, including ovarian atrophy, follicular development disruption, follicular atresia, and a reduction in mature follicles [Citation45]. Also, with varied doses of ZnO NPs injection, Hosseini et al. discovered dose-dependent increases in lesions; the histological examination of hyperemia, enlarged corpus luteum, inflammatory cell infiltration, fibrosis, and follicular cysts [Citation46]. The destructive results of AgNps on uterine tissue histology were consistent with a study that stated that histopathological changes in uterine tissue were observed after intraperitoneal administration of 12.5 mg/kg/d Cu-NPs for 14 days, including inflammatory cell infiltration, disordered epithelial cell arrangement, mitochondrial swelling, and vacuolization, and shortening and decrease of microvilli on endometrial epithelial cells [Citation31].
Caspases play an important role in the apoptosis process. Caspase-3 is a commonly activated death protease that catalyses the precise cleavage of various important cellular proteins. Caspases-3 activation pathways have been identified that are dependent on or independent of mitochondrial cytochrome c release and caspase-9 activity. Caspase-3 is required for appropriate brain development and other apoptotic pathways in tissue-cell-type-and death stimulus-specific ways, including chromatin condensation and DNA fragmentation, in all cell types studied. So, whereas caspase-3 is important for certain processes linked to cell disassembly and the formation of apoptotic bodies, it may also be active prior to or during the decision to lose cell viability [Citation47]. Our results showed a significant increase in caspase-3 expression after AgNPs injection in ovarian and uterine tissues. These results were inconsistent with Cu NP exposure which induced the expression of caspase-9 and caspase-3 levels [Citation45]. Another study on the uterus showed that the expression of caspases 3, 8, and 9 increased after exposure to Cu-NP in the treated rat uterus [Citation31]. Also, El-Nouri et al. stated that there were positive reactions for Caspase 3, indicating apoptosis [Citation42]. AgNPs cause apoptosis in the ovaries and uterus, according to our findings, by inducing oxidative stress and activation of the caspase-3 pathway.
Conclusion
We concluded that intraperitoneal Administration of AgNPs was found to significantly affect sex hormone levels (FSH, LH, E2, P4), histopathology of reproductive organs, and induce oxidative stress, which can lead to oxidative stress apoptosis. So, these results can provide the basis for further investigation of the mechanism of action of silver nanoparticles on the female reproductive system. By targeting and investigating signal pathways of some specific genes related to ovarian failure, follicular atresia, and follicular abnormal development, we also could measure more antioxidants such as SOD and Catalase.
Some limitations faced us as the measurement of total antioxidant capacity and MDA only as an indicator of oxidative stress investigation; it would have been more accurate if we had measured some antioxidants enzymes such as (catalase, glutathione reductase, glutathione peroxidase and superoxide dismutase)
Availability of data and material
data will be available when requested from the corresponding author.
Authors contribution
Yara Mohamed: Data collection, Experimental design, Manuscript writing
Heba Ali: Histopathological studies, Statistical analysis, Manuscript reviewing
Abdel-Wahab El Ghareeb: Manuscript reviewing
Fawzy Ali: Manuscript reviewing
Ethics approval
The international law set up the study protocol for protecting animals in the laboratory, and Ethical approval for animal use was obtained from Institutional Animal Care and Use Committee (CU-IACUC) Cairo-University. (CUFS/Comp&Emb/CU/I/F/75/19).
Acknowledgments
The authors are grateful to all staff members of the zoology department in the Faculty of Science at Cairo University for their help in accomplishing the laboratory work in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245.
- Sun J, Zhang Q, Wang Z, et al. Effects of nanotoxicity on female reproductivity and fetal development in animal models. Int J Mol Sci. 2013;14(5):9319–9337.
- Tan P, Li H, Wang J, et al. Silver nanoparticle in biosensor and bioimaging: clinical perspectives. Biotechnol Appl Biochem. 2021;68(6):1236–1242. DOI:10.1002/bab.2045.
- Said MM, Rehan M, El-Sheikh SM, et al. Multifunctional hydroxyapatite/silver nanoparticles/cotton gauze for antimicrobial and biomedical applications. Nanomaterials (Basel). 2021;11(2):429. DOI:10.3390/nano11020429.
- Harish V, Tewari D, Gaur M, et al. Review on nanoparticles and nanostructured materials: bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and Agro-food applications. Nanomaterials (Basel). 2022;12(3):457. DOI:10.3390/nano12030457.
- Lee JH, Kim YS, Song KS, et al. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part Fibre Toxicol. 2013;10(1):36. DOI:10.1186/1743-8977-10-36.
- Wang R, Song B, Wu J, et al. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018;13:8487–8506. DOI:10.2147/IJN.S170723.
- Muoth C, Aengenheister L, Kucki M, et al. Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine (Lond). 2016;11(8):941–957.
- Li C, Li X, Suzuki AK, et al. Effects of exposure to nanoparticle-rich diesel exhaust on pregnancy in rats. J Reprod Dev. 2013;59(2):145–150.
- Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21(7):2375. DOI:10.3390/ijms21072375.
- Wiemann M, Vennemann A, Blaske F, et al. Silver nanoparticles in the lung: toxic effects and focal accumulation of silver in remote organs. Nanomaterials (Basel) [Internet]. 2017;7(12):441. DOI:10.3390/nano7120441.
- Campagnolo L, Massimiani M, Vecchione L, et al. silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology. 2017;11(5):687–698.
- Bajilan LASI. Effect of silver nanoparticles on levels of serum fsh, lh and estradiol in pco-induced female mice. World J Pharm Res. 2017;113–125. DOI:10.20959/wjpr201711-9483
- Hou -C-C, Zhu J-Q. Nanoparticles and female reproductive system: how do nanoparticles affect oogenesis and embryonic development. Oncotarget. 2017;8(65):109799–109817.
- Maneewattanapinyo P, Banlunara W, Thammacharoen C, et al. An evaluation of acute toxicity of colloidal silver nanoparticles. J Vet Med Sci. 2011;73(11):1417–1423.
- El Mahdy MM, Eldin TAS, Aly HS, et al. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp Toxicol Pathol. 2015;67(1):21–29.
- Goodman MF. Canine ovulation timing. Probl Vet Med. 1992;4(3):433–444. Review.
- Valdes-Socin H, Salvi R, Daly AF, et al. Hypogonadism in a patient with a mutation in the luteinizing hormone beta-subunit gene. N Engl J Med. 2004;351(25):2619–2625.
- West CD, Mahajan DK, Chavré VJ. Simultaneous measurement of multiple plasma steroids by radioimmunoassay demonstrating episodic secretion. J Clin Endocrinol Metab. 1973;1973(36 No. 6):1230–1236.
- Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43.
- Koracevic D, Koracevic G, Djordjevic V, et al. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54(5):356–361.
- Kiernan JA, Horobin RW. A special issue devoted to hematoxylin, hematein, and hemalum. Biotech Histochem. 2010;85(1):5–6.
- Sternberger LA. Immunocytochemistry. 3rd ed. London England: Churchill Livingstone; 1986.
- Abd-Elhafeez HH, Soliman SA, Attaai AH, et al. Endocrine, stemness, proliferative, and proteolytic properties of alarm cells in ruby-red-fin shark (Rainbow Shark), Epalzeorhynchos frenatum (Teleostei: Cyprinidae). Microsc Microanal. 2021;27(5):1251–1264.
- Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 2010;12(5):1531–1551.
- Wen H, Dan M, Yang Y, et al. Acute toxicity and genotoxicity of silver nanoparticle in rats. PloS One. 2017;12(9):e0185554.
- Kim YS, Song MY, Park JD, et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010;7(1):20.
- Sarhan OMM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine. 2014;9:1505–1517.
- Adeyemi OS, Adewumi I. Biochemical evaluation of silver nanoparticles in Wistar rats. Int Sch Res Notices. 2014;2014:196091.
- Parveen A, Malashetty VB, Mantripragada B, et al. Bio-functionalized gold nanoparticles: benign effect in Sprague-Dawley rats by intravenous administration. Saudi J Biol Sci. 2017;24(8):1925–1932.
- Hu S, Yang J, Rao M, et al. Copper nanoparticle-induced uterine injury in female rats. Environ Toxicol. 2019;34(3):252–261.
- Asadi F, Sadeghzadeh M, Jalilvand A, et al. Effect of molybdenum trioxide nanoparticles on ovary function in female rats. Majallahi Ilmi Pizhuhishii Danishgahi Ulumi Pizishki Khadamati Bihdashtii Darmanii Zanjan. 2019;27(121):48–53.
- Gao G, Ze Y, Li B, et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27.
- Kong L, Tang M, Zhang T, et al. Nickel nanoparticles exposure and reproductive toxicity in healthy adult rats. Int J Mol Sci. 2014;15(11):21253–21269.
- Wang N, She Y, Zhu Y, et al. Effects of subchronic aluminum exposure on the reproductive function in female rats. Biol Trace Elem Res. 2012;145(3):382–387.
- Richards JS, Ireland JJ, Rao MC, et al. Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99(6):1562–1570.
- Newsholme P, Cruzat VF, Keane KN, et al. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473(24):4527–4550.
- Hassan O, Saad A, Hamouda A. Silver nanoparticles induced multiple organ toxicity in mice. Egyptian J Forensic Sci Appl Toxicol. 2019;19(4):31–47.
- Moradi-Sardareh H, Basir HRG, Hassan ZM, et al. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life Sci. 2018;211:81–90.
- Ansar S, Alshehri S, Abudawood M, et al. Antioxidant and hepatoprotective role of selenium against silver nanoparticles. Int J Nanomedicine. 2017;12:7789–7797.
- Patlolla AK, Hackett D, Tchounwou PB. Silver nanoparticles-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem. 2015;399(1–2):257–268.
- El-Nouri MA, Azmy OM, Elshal AO, et al. Study of the effects of silver nanoparticles exposure on the ovary of rats. Life Sci Jour. 2013;10:17–25.
- Taylor U, Barchanski A, Garrels W, et al. Toxicity of gold nanoparticles on somatic and reproductive cells. Adv Exp Med Biol. 2012;733:125–133.
- Gao G, Ze Y, Li B, et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27.
- Yang J, Hu S, Rao M, et al. Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int J Nanomedicine. 2017;12:5959–5971.
- Mohammad Hosseini S, Hossein Moshrefi A, Amani R, et al. Subchronic effects of different doses of Zinc oxide nanoparticle on reproductive organs of female rats: an experimental study. Int J Reprod Biomed (Yazd). 2019;17(2):107.
- Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104.