 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Roselle and Beetroots are essential plants used individually for hepatoprotective abilities. This study investigated the preventive potentials of the combinations of Roselle and beetroots against carbon tetrachloride (CCl4)-induced and Escherichia coli (E. coli)-induced hepatic stress. Total phenolic content (TPC), total flavonoids (TF), 1,1-diphenyl-picrylhydrazin (DPPH) and ferric-reducing antioxidant power (FRAP) were evaluated. Oxidative stress was induced in rats with CCl4 and E. coli. Glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT) and histopathology were evaluated in the liver. Roselle had the highest TPC and TF of 274.4 and 1140.9 GAE mg/mL, respectively. Roselle and beetroots inhibited DPPH and FRAP by (95.7% and 73.8%) and (887.6 and 662.6 µmol/L), respectively. In the extract-treated animal groups, a significant increase (P < 0.05) in the GSH, SOD and CAT was observed with a decrease in the MDA and histology indicates preserved hepatocellular architecture. The investigated plant extracts combination therefore exhibited antioxidant activities and hepatic cell protection. The results confirmed the combined functionalities of these plants as protective against oxidative stress induced in the liver, which can be incorporated as a dietary strategy to combat hepatic stress after due clinical trials. .
Graphical abstract
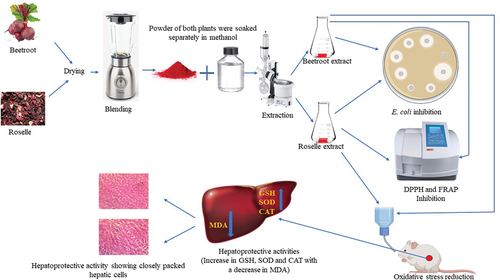
Introduction
Roselle (Hibiscus sabdariffa L.) is an edible plant, which is rich in vitamins, minerals and bioactive compounds such as organic acids, phytosterols and polyphenols [Citation1–3]. In addition, calyces of Roselle rich in polyphenolic acids, flavonoids, anthocyanins and protocatechuic acid have been reported to possess strong antioxidant and antitumor properties [Citation2–4]. Many investigations highlight the additional role of polyphenolic acid, flavonoids and anthocyanins that may act as antioxidants or via other mechanisms contributing to the hepatoprotective actions. The extract of Roselle inhibits low-density lipoprotein (LDL) oxidation in vitro and decreases serum lipids in cholesterol and high fructose-fed rats. Therefore, dietary extracts of Roselle may reduce the incidence of liver stress through their antioxidant activity [Citation4,Citation5]. Red beet (Beta vulgaris L.) is a taproot plant grown for food sources [Citation6,Citation7]. The concentrated red coloration of beetroots is from betalains, which is a group of phenolic compounds of plant origin. The antioxidant mechanism present in beetroots has been reported to be due to the inhibition of lipid peroxidation [Citation6,Citation7], increased resistance to the oxidation of low-density lipoproteins [Citation6] and chemo-preventive effects [Citation8]. In addition to betalains produced by red beets, other bioactive compounds present in minute quantities are flavonoids and hydroxycinnamic acids such as gallic, caffeic and syringic acids [Citation6–9]. Several types of research have described betalains potentials as very high in antioxidants and anti-inflammatory activities in an in vitro system and in vivo in animal models [Citation6,Citation10]. The functional food concept was developed from the consumption of a diet rich in fruits and vegetables with added health benefits leading to a wholesome lifestyle. Studies have reported the significance of plants in the management of human health.
Reactive oxygen and nitrogen species (RONS) are produced constantly during normal cellular metabolism. At low concentrations, RONS plays a vital role in various cellular and biochemical processes, which include the proliferation of cells, expression of genes, contraction of muscles and apoptosis. However, cells may be attacked by severe oxidative stress, which may weaken the antioxidant capacities; these could be through infectious pathogens or toxigenic substances [Citation7,Citation10,Citation11]. Carbon tetrachloride (CCl4) is a commonly employed chemical compound used to initiate oxidative stress and liver toxicity in experimental models. The liver injury induced by CCl4 lipid peroxidation leads to the impairment of the cellular mechanisms after oxidative damage and aids in the further assessment of the therapeutic potentials of dietary antioxidants in the experimental models [Citation12]. Escherichia coli (E. coli) is a significant infectious foodborne pathogen, which is responsible for various bacterial diseases and disorders such as hemolytic uremic syndrome (HUS), septicemia, gastroenteritis and pyelonephritis [Citation13,Citation14]. In the course of the infection, E. coli secretes different virulent factors such as Shiga toxin (Stx) and hemolysin, which can induce oxidative stress in the blood and cells of humans [Citation13,Citation14]. The symptoms of HUS are pancreatitis (inflammation of the pancreas), liver necrosis (death of liver cells or tissues), brain dysfunction, seizure and coma [Citation14]. The liver is a vital organ in the body, which regulates several physiological processes such as metabolism and storage of carbohydrates, synthesis of bile acids and aid in the detoxification of drugs and xenobiotics in the body [Citation12,Citation15]. This makes it susceptible to injury that can result in different debilitating conditions such as hepatitis, cirrhosis or hepatocellular carcinoma [Citation15]. More so, oxidative stress plays an integral role in the pathology and progression of liver toxicity, with most pharmacotherapeutic agents contributing to hepatotoxicity [Citation7]. The protective influence of free radicals in the liver is balanced by the action of antioxidants enzymes (glutathione peroxidase, superoxide dismutase, catalase), antioxidant compounds (thioredoxin, lipoic acid), dietary antioxidants (Vitamin C, Vitamin E, carotenoids, flavonoids) and plant-derived bioactive compounds [Citation3,Citation16,Citation17]. The separate use of Roselle and beetroot as an antioxidant and in the prevention of carbon-tetrachloride-induced oxidative stress is well documented in literature [Citation2,Citation3,Citation6,Citation7,Citation10]. However, there are no reports on the potentials of these plants in different combinations against E. coli-induced oxidative stress.
Hence, this study was aimed at evaluating the hepatoprotective potentials of methanolic extracts of Roselle and beetroots in different combinations in rats challenged with CCl4 and E. coli.
Materials and methods
Collection and preparation of Roselle and beetroot samples
The dried calyces of Roselle (Hibiscus sabdariffa) and fresh beetroots were obtained from a retail market in Ibadan, southwest Nigeria. The calyces of the Roselle were handpicked to remove dirt and other debris, while the beetroots were thoroughly washed and oven-dried at 40°C for 8 h until well dried. The plant samples were milled and subjected to methanolic extraction separately [Citation18].
Preparation of crude methanolic extract
The dried samples of Hibiscus sabdariffa calyces and beet roots were finely ground into powder (approx. 2.0 mm) by a grinder. The samples (500 g) were extracted by a cold extraction procedure, with continuous stirring using pure methanol (1000 mL) for 72 h. After this procedure, the filtrate obtained was passed through a muslin cloth and Whatman (No. 1) filter paper. The combined filtrate was evaporated in a rotary evaporator (Heidolph, Laborota 4000 efficient, Schwabach, Germany) set at 40°C at 700 mmHg pressure and concentrated in a vacuum oven (Thermo Fisher Scientific, United Kingdom) set at 40°C with a pressure of 700 mm Hg to obtain the crude methanolic extract. The resultant methanolic extracts were dried and stored in the refrigerator (4°C) until use [Citation18].
The bioactive contents of the samples
The crude methanolic extracts were assayed for phytochemical properties [Citation19]. Total phenolic content (TPC) of the extracts was determined as described by Gulcin et al. [Citation20] with slight modifications. Briefly, the mixture of 0.5 mL of the extract and 2.5 mL of 10% (v/v) Folin-Ciocalteu reagent was allowed to stand for 8 min, and then 2 mL of 7.5% (v/v) Na2CO3 was added and incubated in the dark at room temperature for 1 h. The absorbance of the mixture was measured at 765 nm by UV–VIS spectrophotometer (Spectrumlab 752S, Shanghai, China), and the TPC was expressed as milligrams of gallic acid equivalents per gram of dried sample (mg GAE/g) using a standard curve.
The total flavonoid content (TFC) of the extracts was determined as described by Sun et al. [Citation21]. Briefly, a mixture of 0.5 mL of extract, 2 mL of deionized water and 0.15 mL of 5% (w/v) NaNO2 was left at room temperature for 6 min. A volume of 0.15 mL of 10% (w/v) of AlCl3 was added and allowed to stand for 6 min, followed by the addition of 2 mL of 4% (w/v) NaOH and 0.7 mL of deionized water, which was mixed thoroughly and left at room temperature for 15 min. The absorbance was measured at 510 nm using a UV–VIS spectrophotometer (Spectrumlab 752S, China) and expressed as milligrams of gallic acid equivalents per gram of dried sample (mg GAE/g) using a standard curve.
Confirmatory test of the bacterial strain and antibacterial screening using the extracts of Roselle and beetroots
The Medical Microbiology Unit of University College Hospital (UCH), University of Ibadan, Oyo State, Nigeria, provided clinically isolated and identified E. coli from a diarrhoeagenic patient. The stock culture was preserved in 1% glycerol in a cryovial. Confirmatory tests such as morphological and biochemical tests and antibiotic susceptibility pattern of the bacterial strain were evaluated. The E. coli strain was grown on tryptic soy broth and subculture on Nutrient agar (CM0003B – Oxoid, Basingstoke, United Kingdom) at 37°C for the tests.
Methanolic extracts of Roselle and beetroots were screened for antibacterial activities against E. coli using the agar well diffusion assay. Briefly, the diluted inoculum of E. coli (0.1 mL) was adjusted to 0.5 McFarland standard (approx. 1.5 × 108 CFU/mL) and seeded on Petri dishes containing Mueller Hinton agar (CM0337 – Oxoid, Basingstoke, United Kingdom). Wells of 6-mm diameter were punched onto the agar medium with a sterile cork borer under aseptic conditions, and the wells were filled with 100 µL of varying concentration of the plant extracts. Gentamicin was used as a positive control, while 10% Dimethyl sulfoxide (DMSO) was used as a negative control. The plates were kept at 4°C for 15 min for diffusion and then incubated for 24–48 h at 37°C. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test microbe [Citation17].
Determination of in vitro antioxidant activity
Determination of 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity
The method described by Kwon et al. [Citation22] with slight modification was used for the DPPH scavenging activities. A stock solution of 100 µM DPPH in methanol was made. A test sample of the extract was made at 100, 200, 400, 600, 800 and 1000 µg/mL in methanol. Similarly, reference samples of ascorbic acid were made at a similar concentration. A volume of 2.0 mL of 100 µM DPPH was mixed with 1.0 mL of the extracts and 1.0 mL of ascorbic acid, respectively. After 5 min in the dark, the samples were vortexed, and absorbance was measured at 517 nm using a spectrophotometer (Spectrumlab 752S, China), and percentage-scavenging activities were calculated by the equation:
Scavenging Activities
Where, AO = Absorbance of the DPPH solution and AT = Absorbance of test or reference sample.
Ferric ion reducing antioxidant power assay (FRAP)
Ferric ion reducing antioxidant power was measured according to the method of Benzie & Strain [Citation23]. To the freshly prepared FRAP reagent, 10 mM of 2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ) (dissolved in 40 mM of HCl), 20 mM of FeCl3 in water and 300 mM of acetate buffer (pH 3.6) in the ratio of 1:1:10 were added. An aliquot of the extracts was added to the measured volume of the working FRAP assay reagent. The absorbance was measured at 593 nm after 4 min of incubation. The FRAP was expressed as µmol/L using a FeSO4 standard curve.
In vivo study of the extracts in the experimental rats
Wistar Albino male rats (120–170 g) were used for the acute stress and hepatoprotective studies. The animals were acquired from the Central Animal House, University of Ibadan. The animals were assigned randomly to treatment groups in plastic cages at the Central Animal House, Department of Physiology, University of Ibadan. The animals were exposed to 12 h of light, 12 h of darkness in a well-ventilated room and adapted to the environment for 7 days before the commencement of the experiment. The animals were fed the standard commercial pelleted rat chow and granted free access to water ad libitum. The tap water given to the animals was boiled and cooled to avoid contamination. Ethical clearance was granted for this study by the University of Ibadan, Animal Care, Use and Research Ethics Committee with approval no. UI-ACUREC/App/01/2017/0038.
Acute toxicity (LD50) study
An acute stress study was carried out as described by Lorke [Citation19] with slight modification. Forty-eight rats were randomized into 12 groups of 4 rats per group and given varying concentrations of Roselle and beetroot extracts ranging from 100 to 5000 mg/kg body weight of the rat orally. Animals were observed for 24–72 h after administration for signs of stress and mortality.
Preparation of oxidative stress inducers
The E. coli strain was grown in tryptic soy broth at 37°C, centrifuged at 8,000 g for 15 min at 4°C, washed in phosphate-buffered solution (PBS) pH 7.2 twice before use. Carbon tetrachloride (CCl4) (Sigma-Aldrich, Saint Louis, MO, USA) was provided by the Department of Pharmaceutical Chemistry, University of Ibadan.
Hepatoprotective study
Forty-four rats were divided into 11 groups of 4 rats each. Group 1 was administered with water, while ascorbic acid was given to Group 2 at 50 mg/kg body weight of the rat. An equal proportion of Roselle and beetroot extracts (50:50) was administered to Group 3 at 1000 mg/kg body weight of the rat. The treatment groups were pretreated with varying concentrations of Roselle to beetroots extracts for 14 days at 1000 mg/kg body weight of the rat daily. Groups 4 and 4a received 60% Roselle to 40% beetroot, Groups 5 and 5a received 50% Roselle to 50% beetroot, while Groups 6 and 6a received 40% Roselle to 60% beetroot. Group 7 and 7a received only water.
On the fifteenth day, oxidative stress was orally induced in Groups 4, 5, 6 and 7 with CCl4 in olive oil at a dosage of 2 mL/kg body weight of the rat [Citation24] while the E. coli was administered to Groups 4a, 5a, 6a and 7a according to the method of Sharma et al. [Citation15]. The E. coli strain was diluted in sterile distilled water to achieve a final suspension of 2 × 103 CFU/mL before administration by oral gavage. The CCl4-induced groups were sacrificed after 48 h while the E. coli-induced groups after 72 h.
Processing of the liver for histopathology and antioxidant marker indices
The animals were sacrificed by cervical dislocation after administering light ether anesthesia. Livers were excised and washed in normal saline. For the histological studies, the livers were stored in 10% formalin saline for 48 h. The livers embedded in paraffin were dissected in serial sections of 5 µm thickness using a rotary microtome. The sections were stained with hematoxylin–eosin (H and E) for light microscopy examination. The livers assayed for superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and malondialdehyde (MDA) were preserved in iced phosphate buffer saline (pH 7.2) for the analyses of the antioxidant markers [Citation17,Citation25].
Processing for GSH, SOD, CAT and MDA assay
Preparation of samples for the antioxidant indices
The liver homogenates were prepared by mixing 0.1 g of individual samples of the liver in 1 mL of 0.01 M phosphate buffer (pH 7.2).
Assay for the antioxidant indices
Reduced glutathione (GSH) assay
The GSH activity in the liver homogenates was done according to the method of Sedlak & Lindsay [Citation26]. This is established according to the change in stable yellow coloration resulting from the reaction of 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) and GSH. The color formed was measured spectrophotometrically at 412 nm and expressed as µmoles/g tissue using the molar extinction coefficient of DTNB-GSH conjugate (13.6 × 103/M/cm)
Superoxide dismutase (SOD) assay
This was determined according to the method of Marklund & Marklund [Citation27] in the liver homogenates by the superoxide-driven auto-oxidation of pyrogallol. One unit of the activity of SOD was defined as the quantity of the enzyme, which inhibited the pyrogallol auto-oxidation by 50%, and the results are interpreted based on total protein content as the activity of SOD units/mg protein.
Catalase (CAT) assay
The CAT was done according to the method of Aebi [Citation28] with slight modification. A 0.1 mL of each liver homogenate was combined with 4.9 mL of distilled water. Then, 1 mL of the mixture was incorporated into hydrogen peroxide (H2O2) and phosphate buffer mixture at 100°C. The rate of breakdown of H2O2 was proportional to the reduction in absorbance at 600 nm. The difference was expressed as catalase units/mg protein per unit of absorbance.
Malondialdehyde (MDA) assay
The malondialdehyde levels in the liver homogenates were done according to the method of Varshney & Kale [Citation29]. Lipid peroxidation assay was assayed by measuring the MDA – thiobarbituric acid reactive (TBA) adduct formed as a pink coloration of the reaction. The quantification was measured spectrophotometrically at 532 nm and was considered as the index of lipid peroxidation. The results were expressed as nmol of MDA/g tissue using the molar extinction coefficient of the chromophore (1.56 × 105/m/cm).
Statistical analysis
The statistical analyses were carried out using one-way analysis of variance (ANOVA) procedures. Statistical differences in the samples were tested at P < 0.05. Tukey’s test was used to differentiate among the mean values and expressed as mean ± standard errors. All the analyses were done with SPSS (version 15.0; SPSS Inc., Chicago, IL, USA) software. Three independent experiments were carried out to confirm the results pattern.
Results
Bioactive compounds of the extracts
Methanolic extracts from Roselle and beetroots demonstrated the presence of saponins, tannins, flavonoids, steroids and anthraquinones. In addition, Roselle extract had terpenoids, while beetroots extract had alkaloids. Roselle extract displayed higher TPC and TFC values of 274.4 mg GAE/g and 1140.9 mg GAE/g, respectively, while beetroot extract had 26.7 mg GAE/g and 403.6 mg GAE/g, respectively.
Differences in Roselle and beetroot DPPH scavenging activity were marginally lower at the lowest concentration of 100 μg/mL with 64.8% and 13.1%, respectively, compared to ascorbic acid (95.6%) but became pronounced at higher concentrations (1000 μg/mL) with 95.7% and 73.8%, respectively, compared to ascorbic acid 96.9%. The DPPH radical scavenging activity of Roselle extract was higher than beetroot extract at all concentrations. The methanolic extract of Roselle had a higher ferric reducing antioxidant power of 887.6 µmol/L while that of beetroot extract was 662.6 µmol/L (). The extracts of Roselle and beetroots demonstrated high level of antioxidant activities.
Table 1. In vitro antioxidant activities and bioactive compounds of the methanolic extracts of Roselle and beetroot.
Confirmation of the bacterium strain and antibacterial activities of the extracts of Roselle and beetroots
The strain was observed to be Gram negative bacilli that possessed a metallic sheen morphological characteristic. The biochemical properties include positive to indole and methyl red, and negative to citrate and Voges Proskauer. The strain had a positive fermentative ability to glucose, lactose and maltose and negative to inositol and sorbitol.
The sensitivity pattern of the extracts against E. coli was concentration dependent. Greater zones of inhibition were observed at 1000 mg/mL concentrations of both extracts at 24 and 48 h of incubation period. The zones of inhibition of Roselle were 25.0 and 27.0 mm while those of beetroots were 19.0 and 21.0 mm at 24 and 48 h, respectively. The gentamicin used as control was at 30.0–34.0 mm, while DMSO had no observable clear zone (). The extracts of Roselle and beetroots possess great antimicrobial activities against E. coli.
Table 2. Antibacterial screening of the methanolic extracts of Roselle and beetroots against E. coli.
Hepatoprotective activity of the extracts
Results from the acute stress study showed that the oral administration of the methanolic extract of Roselle and beetroots was safe at a concentration ≤5,000 mg/kg of the bodyweight of the rat because there were no toxic injury and mortality recorded at the end of the experiment.
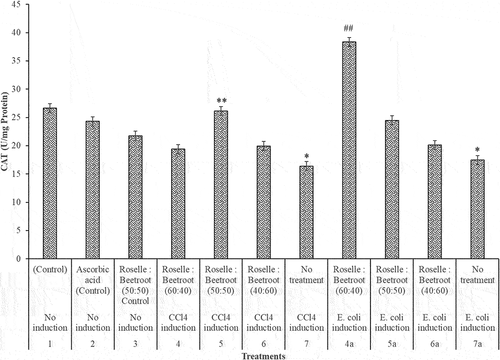
In the hepatoprotective study, the liver biomarkers (GSH, SOD, CAT and MDA) showed interesting results. In the evaluation of the level of the antioxidant markers (GSH, SOD and CAT) in the livers of the CCl4- and E. coli-induced rats and the control groups, there was a significant decrease in these enzymes after induction (P < 0.001). In the CCl4-induced group, treatment with Roselle and beetroots (50:50) was observed to be significantly higher at P < 0.05 in the three antioxidant markers. In the E. coli-induced group, the treatment with Roselle and beetroots (60:40) was significantly higher (P < 0.05) in GSH and CAT, while the ratio (50:50) performed best for SOD ().
Figure 1. GSH (mg/g protein) activities of the livers of rats administered with varying concentrations of methanolic extracts of Roselle and beetroots against induced CCl4 and E. coli. Values represent mean ± SE of three independent experiments (n = 4). * Indicates significant difference (decrease) relative to the control groups at P < 0.001, ** indicates significant difference (increase) relative to Group 7, the untreated group in which CCl4 was induced at P < 0.05 and ## indicates significant difference (increase) relative to Group 7a, the untreated group in which E. coli was induced at P < 0.05.
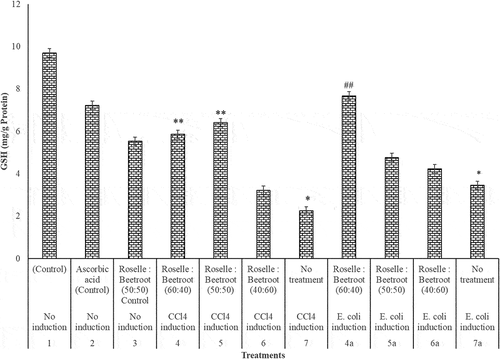
Figure 2. SOD (U/mg protein) activities of the livers of rats administered with varying concentrations of methanolic extracts of Roselle and beetroots against induced CCl4 and E. coli. Values represent mean ± SE of three independent experiments (n = 4). * Indicates significant difference (decrease) relative to the control groups at P < 0.001, ** indicates significant difference (increase) relative to Group 7, the untreated group in which CCl4 was induced at P < 0.05 and ## indicates significant difference (increase) relative to Group 7a, the untreated group in which E. coli was induced at P < 0.05.
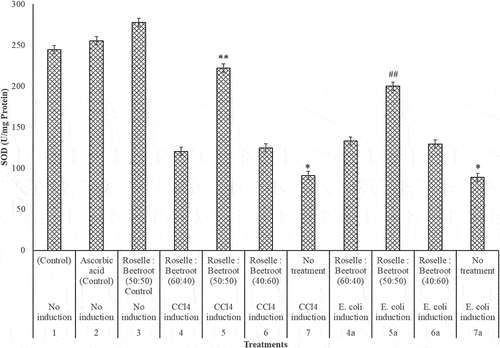
Figure 3. CAT (U/mg protein) activities of the livers of rats administered with varying concentrations of methanolic extracts of Roselle and beetroots against induced CCl4 and E. coli. Values represent mean ± SE of three independent experiments (n = 4). * Indicates significant difference (decrease) relative to the control groups at P < 0.001, ** indicates significant difference (increase) relative to Group 7, the untreated group in which CCl4 was induced at P < 0.05 and ## indicates significant difference (increase) relative to Group 7a, the untreated group in which E. coli was induced at P < 0.05.
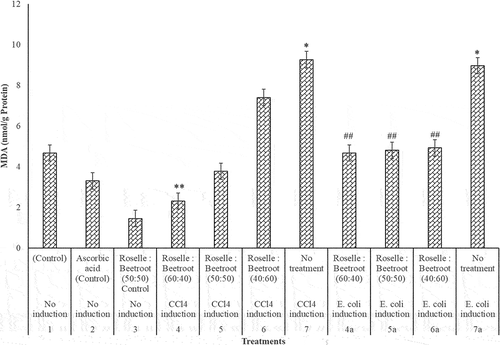
In the assessment of MDA in the CCl4- and E. coli-induced groups of the rats, there was a significant increase in the MDA levels in comparison to the control groups (P < 0.001). In the CCl4-induced rats, the combination of Roselle to beetroots (60:40) significantly decreased the level of the lipid peroxidation, while in the E. coli-induced group, all the ratios significantly reduced the lipid peroxidation in the livers of the rats (). The extracts of Roselle and beetroots were not toxic with remarkable hepatoprotective activities.
Figure 4. MDA (nmol/g protein) activities of the livers of rats administered with varying concentrations of methanolic extracts of Roselle and beetroots against induced CCl4 and E. coli. Values represent mean ± SE of three independent experiments (n = 4). * Indicates significant difference (increase) relative to the control groups at P < 0.001, ** indicates significant difference (decrease) relative to Group 7, the untreated group in which CCl4 was induced at P < 0.05 and ## indicates significant difference (decrease) relative to Group 7a, the untreated group in which E. coli was induced at P < 0.05.
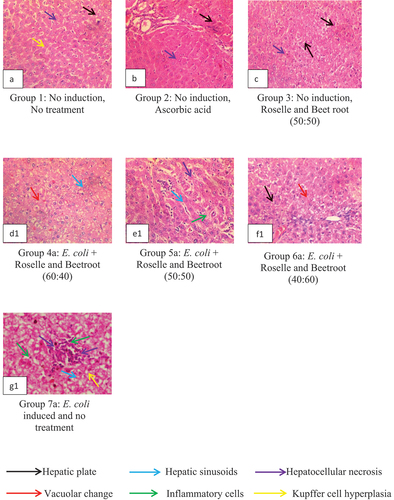
Histopathological study further reveals hepatoprotective activities of the extracts
The liver histology of the rats in the control and treatment groups are shown in . The study on CCl4-induced stress is presented in , while the study on E. coli-induced stress is presented in .
Figure 5. Representative Plates A – G: Liver histology showing hepatoprotective activities of the methanolic extracts of Roselle and beetroot against carbon tetrachloride (CCl4)-induced rats as observed in magnification (×400).
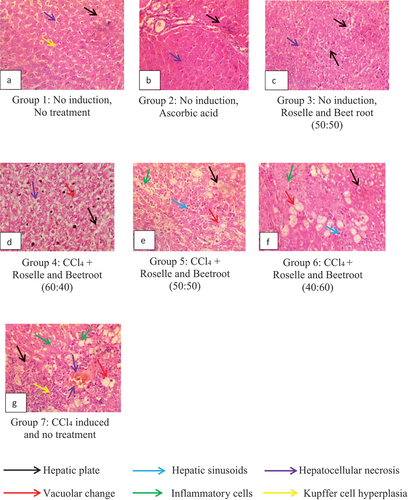
Figure 6. Representative Plates A – G1: Liver histology showing hepatoprotective activities of the methanolic extracts of Roselle and beetroot against E. coli-induced rats as observed in magnification (×400).
Animals with no induction and no treatment (Group 1) showed closely packed hepatic plates (black arrow) with single-cell hepatic sinusoids (blue arrow) and mild Kupffer cell hyperplasia (yellow arrow) (Representative Plate A). However, animals administered with ascorbic acid (Group 2) and the extracts in the ratio 50:50 (Group 3) in Representative Plates B and C, respectively, showed more closely packed hepatic plates (black arrow) with hepatic sinusoids (blue arrow), no obvious Kupffer cell hyperplasia and absence of necrosis. Thus, the Roselle and beetroot combination showed hepatoprotective potentials comparable to the group given ascorbic acid.
The animals were administered with varying concentrations of methanolic extracts of Roselle and beetroot (60:40, 50:50 and 40:60), respectively, and induced with CCl4 (Groups 4, 5 and 6), which showed varying degrees of hepatoprotective capacities. Groups 4, 5 and 6 as seen in Representative Plates D, E and F, respectively, showed multiple foci of moderate thinning of hepatic plates (black arrow) and multiple foci of marked vacuolar change of hepatocytes (red arrow) with the nuclei of affected hepatocytes completely displaced to the periphery. There were local extensive foci of dense inflammatory aggregates (green arrow) associated with hepatic sinusoids (blue arrow). In Group 7 (Representative Plate G), the rats induced with CCl4 without any treatment appeared to have multiple hepatocellular necrosis and inflammatory aggregates.
E. coli-induced animals in Groups 4a, 5a and 6a administered with varying concentrations of the extracts of Roselle and beetroot (60:40, 50:50 and 40:60), respectively, showed closely packed hepatic plates with hepatic sinusoids (blue arrows) with scanty inflammatory aggregates. Animals administered with Roselle and beetroot in the ratio (60:40) proportion (Group 4a, Representative Plate D1) showed multiple foci of marked vacuolar change (red arrow) of hepatocytes and moderate congestion of the hepatic sinusoids (blue arrow). Animals given Roselle and beetroot in the ratio (50:50) (Group 5a, Representative Plate E1) displayed multiple foci of moderate thinning of hepatic plates with slightly dilated sinusoids (blue arrow), random foci of single-cell hepatocellular necrosis (purple arrow) and multiple aggregates of mononuclear inflammatory cells (green arrow). Animals administered with Roselle and beetroot in the ratio 40:60 (Group 6a) exhibited moderate vacuolar change (red arrow) of periportal hepatocytes (Representative Plate F1). Group 7a, Representative Plate G1, which was administered with E. coli without any treatment, had multiple densely packed inflammatory aggregates, hepatocellular necrosis and Kupffer hyperplasia, which indicate hypertrophy related to inflammatory conditions. Altogether, treatments with varying combination ratios of Roselle and beetroots demonstrated interesting hepatoprotective activity in the rats.
Discussion
The bioactive compounds observed in the plants agree with the reports of the presence of saponin, tannin, flavonoid and anthraquinone in aqueous and methanolic extracts of Roselle and beetroots [Citation30,Citation31]. The absence of alkaloids among the screened phytochemicals in the Roselle extract is contrary to the findings of Obouayeba et al. [Citation32] who observed the presence of alkaloids. However, Onkar et al. [Citation33] reported the absence of alkaloids in beetroot extract. These differences in the observations could be attributed to the conditions of the plants before collection, as most phytochemicals are secondary metabolites that are produced in response to different environmental conditions [Citation17]. The solvents used for the extraction of bioactive compounds from plant materials are essential and related to the preferential and deferential solubility of the phytochemicals contained in the plants [Citation17,Citation34]. This is following previous works of researchers who reported the use of methanol to achieve high recovery of plant chemical compounds [Citation3,Citation17]. The occurrence of bioactive compounds in polar fractions elucidates the reasons why polar solvents such as water and alcohol are used in the preparation of herbal formulations indigenously [Citation3,Citation17]. The presence of flavonoids, tannins and other phytochemicals including anthraquinones, which broadly exhibit health beneficial bioactivities supports the use of Roselle and beetroot as nutritional supplements and as an ethnomedicine in diverse herbal medicinal practices throughout the world [Citation3,Citation31,Citation35].
Total phenolic and flavonoid contents can be used as chemical markers for assessing the antioxidant potentials of Roselle and beetroot extracts [Citation3]. Roselle extract had higher phenolic and flavonoid contents than the beetroot extract. Although both extracts demonstrated in vitro antioxidant activities, Roselle extract had better antioxidant potential than the beetroot extract. This may be because of higher phenolic contents of Roselle as compared to that of beetroot or possession of different structural polyphenol phytochemicals by the Roselle extract [Citation3,Citation36]. In addition, Tsai et al. [Citation37] correlated the antioxidant activity of Roselle extracts to their anthocyanin contents, while Falade et al. [Citation38] indicated that Roselle possessed high ascorbic acid content, which is a well-known natural antioxidant and excellent reducing agent. The antioxidant potentials of beetroot are dependent on the concentration of the phenolic compounds it possesses. The phenolic compounds in plant extract are more associated with biomolecules such as proteins, polysaccharides, terpenes, chlorophyll, lipids and inorganic compounds [Citation6].
The DPPH radical scavenging activity of Roselle extract was higher than beetroot extract at all concentrations. Differences in the values of Roselle and beetroot DPPH scavenging activity were marginally low at the lowest concentration but became pronounced at higher concentrations in comparison to ascorbic acid. This finding is similar to the report of Gulcin et al. [Citation39] who observed that the percentage DPPH scavenging ability of Roselle is concentration dependent. Beetroot juice exerts antioxidant activities by scavenging free radical species, two commercially obtained beetroot juices scavenged free radicals in vitro through DPPH and ABTS assays by 100% and 92%, respectively, [Citation10,Citation40]. The FRAP result indicated that both plant extracts possess antioxidant activity and were able to reduce ferric iron (Fe3+) to ferric ion (Fe2+) by chelating metal ions. The result demonstrated that plant extract with FRAP ability would significantly decrease in vivo oxidative modification of lipid [Citation17,Citation35]. Furthermore, Al-Hashimi [Citation35] suggested that the chelating ability of plant extract is dependent upon the localization of functional hydroxyl groups in their polyphenols. The antioxidant activity of beetroot extract was attributed to the betalain content, which comprises betacyanins and betaxanthins [Citation6]. These constituents demonstrated significant antioxidant activities in an in vitro system, which indicates them as quality candidates for health-promoting effects in humans [Citation3,Citation6,Citation30,Citation41]. Barhe & Tchouya [Citation42] inferred that over 95% of the antioxidant capacity of plant extract is due to the phenolic components. They further demonstrated a positive correlation between the total content of phenolic compounds and the antioxidant activity. In general, the in vitro antioxidant results indicated that Roselle and beetroot extracts possess the ability to inhibit free radicals’ formation. This is an indication that the antioxidant capacities of Roselle and beetroot juices in DPPH and FRAP are great at suppressing oxidative stress-related issues generated by E. coli and the CCl4 intoxication. Roselle and beetroot supplementation can be used as a beneficial strategy to support endogenous antioxidant defenses, which can assist in the protection of cellular components from oxidative damage [Citation3,Citation10].
The health benefits of medicinal plants have been linked to their bioactive content, especially polyphenols [Citation17]. The antibacterial activity of Roselle and beetroot extracts can be attributed to the action of the bioactive compounds against the pathogen in this study. This was observed to be concentration dependent. This is in accordance with the work of Garcia-Alonso et al. [Citation43], which demonstrated that plant polyphenols are solely responsible for their antibacterial activity. In addition, flavonoids are hydroxylated phenolic substance known to be synthesized by plants in response to microbial infection [Citation35]. They have been observed in vitro to be effective antimicrobial agents against a wide array of microorganisms due to their ability to form complex with extracellular and soluble proteins [Citation35].
Several studies have reported an increase in the MDA level and a decrease in GSH, SOD and CAT levels when oxidative stress is generated. In this study, pretreatment of the rats with extracts from Roselle and beetroots improved the liver biomarkers by significantly decreasing the MDA level and increasing the GSH, SOD and CAT levels after the generation of oxidative stress with E. coli and CCl4. In the E. coli-induced groups, further studies are required to ascertain the generation of oxidative stress in the liver by oral administration within 72 hours. However, several studies have reported oxidative stress in E. coli-induced pyelonephritis in the kidney by urethral catheterization administration and the production of toxins and hemolytic uremic syndrome [Citation14]. In the CCl4-induced groups, the equal concentration of Roselle and beetroot extracts performed best in SOD and to some extent in the CAT, which supported the claim of increased bioactivity in plant combination therapy, increased efficacy of multi-plant herbal remedies [Citation17,Citation44,Citation45]. Thus, antioxidant activity or the inhibition of the generation of free radicals is important in the protection against CCl4-induced liver injury [Citation46–48]. The in vivo CCl4-induced liver injury models are useful to investigate the hepatoprotective and antioxidant potentials of plant-derived biologically active compounds [Citation49].
Evidence of the in vivo antioxidant ability of beetroot has been established in a study where rats were given beetroot pomace extract for 7 days and administered 2 mL/body weight (b.w.) of CCl4. After the administration, the liver homogenate of the rats treated with beetroot extracts displayed a considerably lower level of lipid peroxidation. This indicates that the beetroot extracts sustained the endogenous antioxidant activities such as reduced glutathione and catalase enzymes at normal cellular concentrations after the oxidative stress generated by CCl4 a RONS generator [Citation7,Citation10,Citation50]. There is a strong indication that beetroot and Roselle extracts can respond to an in vivo cellular influence and the combination exhibited an up-regulation of the antioxidant defense mechanisms in the tissues of the experimental animals. The SOD is a major enzyme present in the liver and other organs in the body, which provides protection by lowering the intensities of oxidants mutated to hydrogen peroxide (H2O2) broken down to water and oxygen by other enzymes [Citation7,Citation51]. However, pretreating the rats with varying concentrations of Roselle and beetroot improved the SOD levels. The concentrations of Roselle and beetroot treatment upgraded the enzyme inhibited by CCl4 and E. coli. Catalase controls the secondary metabolites produced by SOD, converts H2O2 molecules to water, which aids in the neutralization of the toxic effects in a biological system [Citation7,Citation52]. This agrees with our findings that show a significant improvement in the catalase activities in the hepatic tissues of the rats pretreated with a combination of the extracts of Roselle and beetroot. Lipid peroxidation is directly related to oxidative stress in the biological/living systems. High lipid peroxidation in an organ or tissue is indicative of oxidative stress in such organ or tissue [Citation7]. In this study, the MDA indicative of lipid peroxidation was high in the rats induced with CCl4 and E. coli that was reduced significantly in the groups pretreated with different combinations of Roselle and beetroot extracts and the control given ascorbic acid alone. This agrees with several studies where rats were induced with CCl4 and the pretreatment of beetroot juice only, riboflavin, Tanacetum parthenium and Roselle extracts significantly reduced it [Citation7,Citation46,Citation50,Citation53]. Reduced glutathione (GSH) is a potent antioxidant with high redox potential, and it also serves as a co-factor for several oxidative stress detoxifying enzymes. SOD is a distinct antioxidant, which aids in the transformation of superoxide to hydrogen peroxide purified through catalase and glutathione-peroxidase (GSH-px). The GSH-px utilizes GSH as a substrate in the elimination of free radicals with the assistance of SOD [Citation7,Citation46]. There was a positive effect of the combinations of Roselle and beetroot by improving endogenous antioxidant status of the animals and prevention of oxidative damage caused by the free radicals generated by CCl4 and E. coli. This could be attributed to the bioactive compounds present in the Roselle and beetroot extract that are betalains, phenolic compounds (chlorogenic, caffeic and cinnamic acids) and anthocyanins [Citation3,Citation7]. Vulic et al. [Citation50] reported a dose-dependent neutralization of oxidative stress by CCl4 through the administration of 2 and 3 mL/kg b.w. of dried beetroot powder. This is indicating that the combination of Roselle and beetroot extracts possessed antioxidants, which were significant in an in vivo administration. This can be attributed to the combined bioactive compounds of the two plants.
Comparable liver biomarker levels in rats administered with only extracts and ascorbic acids can be linked to the higher concentration and doses of the extracts as 1000 mg of the extract per kg body weight of the rat was administered daily for 14 days while a dose of 50 mg of the ascorbic acids per kg body weight of the rat was administered. The higher concentration of the extracts resulted in a comparable performance of the extracts with ascorbic acids. In a related development, the influence of dose and concentration of medicinal plants on antioxidant and hepatoprotective activities has been reported by several authors, most reporting a concentration-dependent activity [Citation3,Citation7,Citation39,Citation54]. The components of the white blood cells and Kupffer cells are recognized to be activated by stimuli such as the inducement of CCl4 and E. coli. In addition, previous studies indicate that the oxidative damage produced by the derivatives of CCl4 may activate Kupffer cells in the liver, which is liable to the release of TNF-α from the liver inflammation [Citation13,Citation53]. Previous studies indicate that the protection of the liver may be partly due to TNF-α inhibition [Citation7,Citation46,Citation53,Citation55]. Improvements in treatment with the different concentrations of Roselle and beetroot were evident in the histopathological results. Treatments with ascorbic acid and Roselle to beetroot in equal proportions revealed the presence of intact hepatic plates and sinusoids and the absence of Kupffer hyperplasia and necrosis, while the different concentrations of Roselle and beetroot gave varying hepatoprotective potentials in the liver sections. The result from this study agrees with the report of hepatoprotection against the CCl4-induced damage to the liver and evident as well in the E. coli-induced damage [Citation7,Citation46,Citation53,Citation55]. Vulic et al. [Citation50] reported significant improvement in hepatic catalase activities in rats treated with dried beetroot pomace. In addition, they reported that the dried beetroot pomace possesses hepatoprotection activities and shields from other oxidative-associated ailments. This study shows that antioxidant and anti-inflammatory activities of the varying concentrations of Roselle and beetroot are liable for the regulation of the liver function at the structural and biochemical conditions. Roselle and beetroot crude extracts contain more than one of these antioxidants in abundance with synergistic activity and this accounts for the extract’s comparable activity with the ascorbic acid.
Conclusions
In conclusion, calyces of Roselle and beetroots are high in bioactive compounds with remarkable antioxidant potentials. In addition, both extracts showed safety properties signifying that no form of toxicity is associated with the plant consumption. The extracts demonstrated interesting hepatic protection ability against oxidative stress in the liver. This study has provided evidence that the combination of Roselle and beetroot are excellent sources of antioxidants, which protect cellular components of the body from oxidative stress in both in vitro and in vivo assays. This indicates that Roselle and beetroot when used as supplements or taken as drinks may act as prophylaxis in the management of diseases generated by oxidative stress in the liver. However, further studies are necessary to investigate the mechanisms of action of the combined plant extracts in bioprotection against E. coli-induced stress.
Authors Contributions
KB designed the research; SO, and KB conducted the research; KB, SO and MA wrote the first draft of the manuscript, KB and AIS interpreted, corrected, and technically sound final versions of the manuscript; Project administration was by KB; AIS and KB supervised the study. All authors critically reviewed and approved the final version of the manuscript for submission.
Acknowledgement
We acknowledge Late Mr. Okon, Department of Physiology, Mrs. Tawakalit Lawal, Department of Animal Science and Mr. Tosin Ale, Department of Pharmaceutical Chemistry of the University of Ibadan, Oyo State, Nigeria for their technical assistance. We would want to appreciate the Department of Medical Microbiology, University College Hospital (UCH), Ibadan, Oyo State, Nigeria for the provision of the strain used in this study and Mr. Ambrose Nwagbara of the Department of Chemical Pathology, UCH, Ibadan for the histopathological aspect of the liver sections. We acknowledge BioRender for the preparation of the graphical abstract.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
- Gondokesumo ME, Kusuma HS, Widowati W. α‐/β‐glucosidase and α‐amylase inhibitory activities of roselle (Hibiscus sabdariffa L.) ethanol extract. Mol Cell Biomed Sci. 2017;1:34. DOI:10.21705/mcbs.v1i1.3
- Peredo Pozos GI, Ruiz-López MA, Zamora Nátera JF, et al. Antioxidant capacity and antigenotoxic effect of Hibiscus sabdariffa L. Extracts obtained with ultrasound-assisted extraction process. Appl Sci. 2020;10(2):560.
- Banwo K, Sanni A, Sarkar D, et al. Phenolics-Linked antioxidant and anti-hyperglycemic properties of edible Roselle (Hibiscus sabdariffa Linn.) Calyces targeting type 2 diabetes nutraceutical benefits in vitro. Front Sustain Food Syst. 2022;6:660831.
- Riaz G, Chopra RA. Review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharm. 2018;102:575–586.
- Izquierdo-Vega J, Arteaga-Badillo D, Sánchez-Gutiérrez M, et al. Organic acids from Roselle (Hibiscus sabdariffa L.)—A brief review of its pharmacological effects. Biomedicines. 2020;8(5):100.
- Wruss J, Waldenberger G, Huemer G, et al. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J Food Compos Anal. 2015;42:46–55.
- Iahtisham‐Ul‐Haq Butt MS, Randhawa MA, Shahid M. Hepatoprotective effects of red beetroot‐based beverages against CCl4 ‐induced hepatic stress in Sprague Dawley rats. J Food Biochem. 2019;e13057:1–11.
- Zhang A, Sun H, Wang X. Recent advances in natural products from plants for treatment of liver diseases. Eur J Med Chem. 2013;63:570–577.
- Kazimierczak R, Hallmann E, Lipowski J, et al. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: metabolomics, antioxidant levels and anti-cancer activity. J Sci Food Agric. 2014;94(13):2618–2629.
- Clifford T, Howatson G, West DJ, et al. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7:2801–2822.
- Kouakou TH, Konkon NG, Obouayeba AP, et al. Anthocyanin production in the calyx and callus of Roselle (Hibiscus sabdariffa L.) and its impact on antioxidant activity. J Pharmacog Phytochem. 2015;4(3):09–15.
- Abdelhafez OH, Fawzy MA, Fahim JR, et al. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE. 2018;13(8):e0202362.
- Nguyen Y, Sperandio V. Enterohemorrhagic Escherichia coli (EHEC) pathogenesis. Front Cell Inf Microbiol. 2012;2:1–7.
- Baronetti JL, Villegas NA, Aiassa V, et al. Hemolysin from Escherichia coli induces oxidative stress in blood. Toxicon. 2013;70:15–20.
- Sharma KD, Vinod KG, Surendra K, et al. Evaluation of antidiarrheal activity of ethanolic extract of Holarrhena antidysenterica seeds in rats. Vet World. 2015;812:1392–1395.
- Ojekunle O, Banwo K, Sanni AI. In vitro and In vivo evaluation of Weissella cibaria and Lactobacillus plantarum for their protective effect against cadmium and lead toxicities. Lett Appl Microbiol. 2017;64:379–385.
- Banwo K, Alao MB, Sanni AI. Antioxidant and antidiarrhoeal activities of methanolic extracts of stem bark of Parkia biglobosa and leaves of Parquetina nigrescens. J Herbs Spices Med Plants. 2020;26(1):14–29.
- Hossain MA, Al-Hdhrami SS, Weli AM, et al. Isolation, fractionation, and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in Sultanate of Oman. Asian Pacific J Trop Biomed. 2014;4:S368–S372.
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287.
- Gulcin H, Hassan Y, Aboul E. Radical scavenging and antioxidant activity of tannic acid. Arabian J Chem. 2010;31:43–53.
- Sun Y, Rukeya J, Tao W, et al. Bioactive compounds and antioxidant activity of wolfberry infusion. Sci Rep. 2017;7:40605.
- Kwon YI, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15:107–118.
- Benzie IF, Strain JJ. The Ferric Reducing Ability of Plasma FRAP as a measure of “Antioxidant power”: the FRAP Assay. Anal Biochem. 1996;239:70–76.
- Joseph JA, Ayyappan UPT, Sasidharan SR, et al. Ameliorative effect of phytocee™ cool against carbon tetrachloride‑induced oxidative stress. Pharmacog Res. 2014;6(4):320–325.
- Zhai Q, Wang G, Zhao J, et al. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol. 2013;79:1508–1515.
- Sedlak J, Lindsay RH. Estimation of total, protein-bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;28:192–205.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474.
- Aebi HC. Catalase In vitro. Meth Enzymol. 1983;105:121–126.
- Varshney R, Kale RK. Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Rad Biol. 1990;58:733–743.
- Canadanović-Brunet JM, Savatović SS, Ćetković GS, et al. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J Food Sc. 2011;29(6):575–585.
- Mensah JK, Golomeke D. Antioxidant and antimicrobial activities of the extracts of the Calyx of Hibiscus sabdariffa Linn. Curr Sc Persp. 2015;1(2):69–76.
- Obouayeba AP, Djyh NB, Diabate S, et al. Phytochemical and antioxidant activity of Roselle (Hibiscus sabdariffa L.) petal extracts. Res J Pharm Biol Chem Sc. 2014;5(2):1453–1464.
- Onkar PR, Powar PV, Sharma PH, et al. Evaluation of phytochemical and pharmacological activity of beetroot extracts (Beta vulgaris). J Biochem Pharmacol. 2013;2(4):456–468.
- Builders PF, Kabele-Toge B, Builders M, et al. Wound healing potential of formulated extract from Hibiscus sabdariffa Calyx. Indian J Pharm Sci. 2013;6:45–52.
- Al-Hashimi AG. Antioxidant and antibacterial activities of Hibiscus sabdariffa L. extracts. Afr J Food Sci. 2012;6(21):506–511.
- Liuqing Y, Ying G, Ting Z, et al. Antioxidant capacity of extracts from calyx fruits of Roselle (Hibiscus sabdariffa L.). Afr J Biotechnol. 2012;1(17):4063–4068.
- Tsai PJ, Mcintosh J, Pearce P, et al. Anthocyanin and antioxidant capacity in roselle (Hibiscus sabdariffa L.) extract. Food Res Int. 2002;35:351–356.
- Falade OS, Otemuyiwa IO, Oladipo A, et al. The chemical composition and membrane stability activity of some herbs used in local therapy for anemia. J Ethnopharmacol. 2005;102:15–22.
- Gulcin I, Oktay M, Kırecci E, et al. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83:371–382.
- Wootton-Beard PC, Ryan L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J Funct Foods. 2011;3(4):329–334.
- El -Gamal AA, MS A, Raish M, et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflam. 2014;3:1–12.
- Barhe TA, Tchouya GRF. Comparative study of the antioxidant activity of the total polyphenols extracted from Hibiscus sabdariffa L., Glycine max L. Merr., yellow tea and red wine through reaction with DPPH free radicals. Arabian J Chem. 2016;9:1–8.
- Garcia-Alonso J, Ros G, Vidal-Guevara L, et al. Acute intake of phenolic-rich juice improves antioxidant status in healthy subjects. J Nutr Res. 2006;26:330–339.
- Wang Y, Changyun T, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. Food Drug J Analy. 2015;23(2):310–317.
- World Health Organization (WHO). General guidelines for methodologies on research and evaluation of traditional medicine. Geneva: World Health Organization; 2000.
- Mahmoodzadeh Y, Mazani M, Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4 - induced liver damage in rats. Toxicol Rep. 2017;4:455–462.
- Wu T, Li J, Li Y, et al. Antioxidant and hepatoprotective effect of Swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem. 2017;41:2242–2254.
- Srivastava A, Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010;118:411–417.
- Vitcheva V, Simeonova R, Krasteva I, et al. Protective effects of a purified Saponin mixture from Astragalus corniculatus bieb, in vivo hepatotoxicity models. Phyt Res. 2012;27(5):731–736.
- Vulić JJ, Ćebović TN, Čanadanović‐Brunet JM, et al. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J Func Foods. 2014;6:168–175.
- Indo HP, Yen H‐C, Nakanishi I, et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J Clin Biochem Nutr. 2015;56(1):1–7.
- Roleira FM, Tavares‐Da‐Silva EJ, Varela CL, et al. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem. 2015;183:235–258.
- Al-Harbi NO, Imam F, Nadeem A, et al. Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. Intl Immunopharmacol. 2014;21(2):383–388.
- Onoja SO, Madubuike GK, Ezeja MI. Hepatoprotective and antioxidant activity of hydromethanolic extract of Daniella oliveri leaves in carbon tetrachloride-induced hepatotoxicity in rats. J Bas Clin Physiol Pharmacol. 2015;26(5):465–470.
- Da-Costa-Rocha I, Bonnlaender B, Sievers H, et al. Hibiscus sabdariffa L. – a phytochemical and pharmacological review. Food Chem. 2014;165:424–443.
