ABSTRACT
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a toxic environmental compound that causes a deleterious effect on lipid metabolism. This study was conducted to evaluate the protective effect of ethanolic Eruca sativa leaves extract against dioxin-induced lipid metabolic abnormalities in male Rattus rattus. For 5 weeks, Eruca sativa leaves extract (500 mg/kg) was co-administered with dioxin (100 ng/kg) daily via gastric intubation. The concomitant treatment with both dioxin and Eruca sativa extract nearly approached the concentration of serum cholesterol, high-density lipoprotein, γ-glutamyltransferase, in addition to testicular concentration of phospholipids, and the activities of both alkaline phosphatase and acid phosphatase activities. In addition, we have noticed a decline in serum concentration of triglycerides, very low- and low-density lipoprotein, total lipid, phospholipid, alkaline phosphatase, acid phosphatase, and lactate dehydrogenase as well as testicular cholesterol, triglyceride, total lipid, γ- glutamyltransferase, and lactate dehydrogenase activities have shown significant (p < 0.05) decreases. Notably, these alterations were not achieved to the control value relative to the dioxin-treated rate. In conclusion, the protective effects of Eruca sativa leaves extract are attributed to its antioxidant effect and fatty acid content.
KEYWORDS:
Introduction
Dioxins (TCDD) are environmental pollutants belonging to persistent organic pollutants and produced as a by-product of several industrial processes such as waste incineration, smelting, chlorine bleaching of paper pulp, and the manufacturing of some herbicides and pesticides [Citation1]. Dioxins accumulate in the food chain, and their sources of human exposure are inhalation of polluted air and consumption of contaminated food [Citation2]. The most toxic dioxin is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [Citation3]. Once TCDD enters the body, it tends to accumulate in adipose tissue with half-lives of 7–11 years [Citation4] because of the high lipophilicity.
TCDD is reported to be responsible for biological adverse effects including cytotoxicity, immunotoxicity, developmental toxicity, reproductive disorders, endocrine disruption, fatty liver, cardiovascular diseases, genotoxicity, teratogenesis, tumor promotion, and cancer [Citation5]. The toxic mechanisms of dioxins have been extensively explored through many experiments. Dioxins are activators of the aryl hydrocarbon receptor (AhR), which stimulate expressions of target genes and proteins [Citation6]. The AhR-induced signaling pathway is responsible for the physiological effects associated with dioxin [Citation7]. Dioxin exposure has been linked to alterations in sperm functions, spermatogenesis, and steroidogenesis [Citation8–10]. Several studies reported alterations of lipids related to dioxin exposure [Citation11–13].
Medicinal plants have gotten a lot of attention because they have potential therapeutic effects used for the treatment of human diseases since they are natural medicines, contain safe phytochemicals, and are healthier than synthetic medicines [Citation14]. Eruca sativa, a member of the Brassicaceae family, is one of the edible medicinal plants and is widely used in folk medicine [Citation15]. A wide range of phytochemicals has been recognized in the Eruca sativa such as fatty acids, phenolic acids, terpenes, alkaloid, glycosides, saponins, sterols, carbohydrates, vitamin A, and vitamin C, which has been associated with a wide range of many pharmacological activities such as antiplatelet and antithrombotic [Citation16], anti-inflammatory and antimicrobial [Citation17], antioxidant [Citation18], hyperlipidemic and antidiabetic [Citation19].
Previous study of El-Gayar et al. [Citation20] mentions that dioxin alters the balance of oxygen free radicals and antioxidant defense system. The current study was designed to evaluate the protective effect of Eruca sativa ethanolic leaves extract on lipid metabolic abnormalities induced by dioxin in male rats.
Materials and methods
Chemicals
TCDD (Cas No. 1746–01-6) purchased from the agent of Sigma Aldrich (Saint Louis, Missouri, USA), Ethanol purchased from (Al-Gomohria Company for chemicals, Abou-Zaabal, Egypt), and the corn oil was obtained from a local store. All other chemicals were of analytical grade.
Preparation of the ethanolic Eruca sativa leaves extract (ES)
Ethanolic extract was prepared according to the method of Banso [Citation21]. 1 kg powder of Eruca sativa shade dried leaves was extracted with 8.0 L of 95% ethanol, the mixture was shaked by using a magnetic stirrer for 3 hr/day and allowed to stand for 21 hr for 3 days, the mixture was filtered on Whatman filter paper # 45; hence, they were re-extracted with 9 L 95% ethanol and refiltered. The soluble ethanol extract was concentrated to dryness under reduced pressure at 60°C. Solvent elimination of the extract was weighed and finally given 135.0 g. The percentage yield was calculated using this formula: (weight of extract/original weight × 100 giving 12.16% yields) of green fatty crude Eruca sativa leaves ethanol extract. The plant extract was stored at 4°C in the refrigerator. The extract was suspended in distilled water before administration.
Determination of total phenolic and flavonoid content
The total phenolic and flavonoid content was determined according to the method [Citation22].
Determination of total phenolic content
Briefly, 500 µl of the extract was transferred into a test tube and oxidized with the addition of 250 µl of Folin-Ciocalteau reagent. After 5 min, the mixture was neutralized with 1.25 ml of 20% aqueous Na2CO3 solution. After 40 min, the absorbance was measured at 725 nm against the solvent blank. The total phenolic content was calculated using a gallic acid calibration curve and expressed as mg of gallic acid equivalent (GAE) per gram of sample.
Determination of flavonoid content
Briefly, 100 µl of the extract was mixed with 50 µl of 5% NaNO2. After 6 min, 500 µl of a 10% AlCl3 solution was added. After 7 min, 250 µl of 1 M NaOH was added, and the mixture was centrifuged at 5000 g for 10 min. The absorbance of the supernatant was measured at 510 nm against the solvent blank. The total flavonoid content was expressed as mg of catechin equivalent (CE) per gram of sample.
Determination of total antioxidant activity by DPPH and ABTS
Free radical scavenging capacity was determined according to a method [Citation23].
Determination of total antioxidant activity by DPPH
Freshly prepared (0.004% w/v) methanol solution of 2,3-Diphenyl-1-picryl-hydrazyl (DPPH) radical. A 40 µl aliquot of methanolic solution of the extract was added to 3 ml of DPPH solution. The decrease in absorbance at 515 nm was determined continuously at 1 min intervals until the absorbance stabilized. The absorbance of the DPPH radical without antioxidant (control) and the reference compound were also measured. The percentage of inhibition of the DPPH radical was calculated according to the formula:
Inhibition (%) = 100 × (A control−A sample)/A control
Also, the antioxidant activity was determined by means of a calibration curve prepared with Trolox acid and expressed as mg of Trolox equivalent (TE) per gram of sample.
Determination of total antioxidant activity by ABTS
The stock solutions of ABTS* reagent was prepared by reacting equal quantities of a 7.4 mM aqueous solution of ABTS* with 2.45 mM potassium persulfate for 24 h at room temperature in the dark. The working solution was then prepared by diluting 1 mL ABTS* solution with 60 mL of ethanol: water (50:50, v/v) to obtain an absorbance of 1.0 ± 0.02 units at 732 nm. Various concentrations of sample (100–1000 µg/ml) were prepared in 10% ethanol. 50 µl of the samples were allowed to react with 950 µl of the ABTS* solution for 10 min, the absorbance was taken at 732 nm. The percentage of inhibition of the ABTS* free radical was calculated by the following equation:
Inhibition (%) = 100 × [(A control-A sample)/A control]
The standard curve was prepared using Trolox. Results were expressed in terms of mg Trolox equivalents (TE)/g sample).
Determination of fatty acids using GC-MS
Esterification of fatty acids was made as described by Marchetti and Errazu [Citation24]. Fatty acid methyl esters were analyzed on Agilent Technologies 7890B GC equipped with Zebron ZB-FAME capillary column (60 m × 0.25 mm internal diameter × 0.25 μm film thickness; Agilent Technologies, Little Falls, CA, USA) and a flame ionization detector. The injector and detector temperatures were set at 250°C and 285°C, respectively. The column temperature is programmed; initial temp. 100°C for 3 min; rising at 2.5°C/min to 240°C and held for 10 min. The flow rate of carrier gas (H2) was 1.8 ml/min. A sample of 1.0 µl was injected, using split mode (split ratio, 1:50). Components of fatty acids were identified by comparing their retention times with their fatty acids methyl ester standard (GC-MS) spectra from a library (MassHunter GC/MS Acquisition B.07.03.2129).
Animals and dosing
Animals
Thirty male rats (Rattus rattus) weighing about (160 ± 10 g) were obtained from the Institute of Ophthalmic Disease Research, Cairo, Egypt. They were housed in stainless steel cages in an artificially illuminated and thermally controlled room (22–25°C and 12 h light/dark cycle). They were fed on a normal laboratory rodent diet and given water ad libitum for one week of acclimation.
Experimental protocol
Rats were classified into five groups, six rats each. The normal control (NC) group was fed on a standard diet without any supplementation. The corn oil (CO) group was treated orally with corn oil at a dose of 0.2 ml/kg BW. In the ES group, rats were given ES alcoholic extract orally at a dose of 500 mg/kg BW [Citation25]. The TCDD group rats received dioxin orally 100 ng/kg BW/day [Citation26] diluted in 2 ml corn oil. The ES + TCDD group was treated with 500 mg/kg of BW ES and 100 ng /kg/day TCDD. Treatment was continued daily for 5 weeks, at the end of the experimental period, the rats were fasted about 12 hr and anesthetized with halothane and sacrificed. Blood samples were collected and sera were separated by centrifugation at 860×g for 20 min at 4°C, sera were kept at –20°C for the assay of biochemical parameters. Testes were removed, washed with 0.9% Na Cl solution, and then wiped on a piece of filter paper. Testes were washed with 50 mM (sodium phosphate buffer saline pH 7.4) in ice-containing 0.1 mM EDTA to remove any RBCs or clots, then stored at – 20°C for the assay of biochemical parameters.
Estimated biochemical parameters
The following parameters were determined using kits purchased from Bio-Diagnostic kit Co. (Dokki, Giza, Egypt): total cholesterol (CHOL), triglycerides (TG), high-density lipoprotein (HDL-c), low-density lipoprotein (LDL-c), very low-density lipoprotein (VLDL-c), Total lipids (TL) phospholipids (PLs), alkaline phosphatase (ALP), acid phosphatase (ACP), γ- glutamyltransferase (γ-GT), and gactate dehydrogenase (LDH)were determined in serum and testes according to the manufacturer’s instructions.
Statistical analysis
The obtained data were analyzed using a statistical package for social science, version 23 (SPSS Software, SPSS Inc., Chicago, USA) and expressed as means ± standard error (Mean± SE). The significance was performed using analysis of variance (one-way ANOVA) and followed by Scheffe multiple-comparisons test. For data with Gaussian distribution with non-homogeneity of variances, statistical analyses were performed using analysis of variance (One Way ANOVA) followed by Dunnett’s T3 test. For parameters with non-Gaussian distribution, Kruskal–Wallis test was employed followed by Mann-Whiney U test for multiple comparisons. Differences were considered significant at p ˂ 0.05.
Results
shows the total phenolic (TPC), total flavonoid (TFC) as well as antioxidant activity either by DPPH or ABTS. The results illustrated that phenolic content from Eruca sativa leaves extract revealed that, it has a good source of phenolic and flavonoids 12.522 mg GAE/g and 9.938 mg CE/g extract, respectively. Also, the antioxidant activity of ES displayed a decrease in the DPPH and ABTS with a 63.2% and 90.3% scavenging activity, respectively.
Table 1. Total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (AOA) by DPPH and ABTS of ELSE.
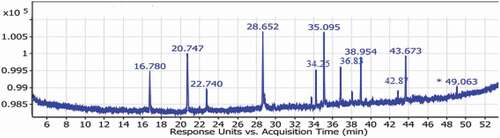
and show GLC analysis of the fatty acids methyl esters which represent the identification of 11 fatty acids in which lauric, palmitic, myristic, stearic, oleic, linoleic, linolenic, and arachidonic acids are the major component. Moreover, it was shown that the total unsaturated fatty acids represent the major constituents of the total mixture (46.1%) which are monounsaturated fatty acids (14.25), whereas the total saturated fatty acids (36.55%).
Table 2. GLC-MS analysis of Fatty acids methyl esters of ELSE.
shows serum and testicular total cholesterol (CHOL), triacylglycerol (TG), total lipid (TL), and phospholipids (PLs), concentrations in the control and different treated rat groups. The data showed a significant (p < 0.05) increase in the estimated parameters in TCDD group compared to the control group except for HDL-c, which showed a significant (p < 0.05) decrease. However, comparing the group of rats treated with Es+ TCDD to that treated with TCDD, the data showed a significant (p < 0.05) decrease in the estimated parameters except for HDL-c showed a significant (p < 0.05) increase. Comparing the group of rats treated with Es+ TCDD with the control group, the data showed that serum CHOL and HDL-c, as well as testicular PLs, tend to stay within the normal range, whereas serum TG, LDL-c, VLDL-c, TL, PLs, and testicular CHOL, TG, and TL as well as testicular CHOL, TG, and TL are still significantly higher than control levels. Also, the administration of Es only did not show any significant change in the estimated parameters compared to the control group or that received CO group.
Table 3. Serum and testicular total cholesterol (CHOL), triacylglycerol (TG), phospholipids (PLs), and total lipids (TL), concentrations in the control and different treated rat groups.
shows the testicular and serum activities of alkaline phosphatase (ALP), acid phosphatase (ACP), γ- glutamyltransferase (γ- GT), and lactate dehydrogenase (LDH) activities of the control group and the different treated rat groups. The data showed a significant (p < 0.05) increase in serum ALP, ACP, γ- GT, and LDH,as well as in testicular LDH in the TCDD-treated rat groups compared to the control group. These increases were associated with a significant (p < 0.05) decrease in the testicular ALP, ACP, and γ- GT in rat groups treated with TCDD compared to the control group. Comparing the groups of rats treated with Es+ TCDD with the TCDD group, the data showed a significant decrease in all serum ALP, ACP, γ- GT, and LDH as well as in testicular LDH activities. While the activities of testicular ALP, ACP, and γ- GT in the Es+ TCDD group increased significantly (p < 0.05) compared to the TCDD group. The activities of serum ALP, ACP, LDH as well as testicular γ- GT and LDH were significantly (p < 0.05) higher than the control group. While activities of serum γ- GT and testicular ALP and ACP in Es+ TCDD group were no significant (p > 0.05) when compared to the control group or CO group.
Table 4. Serum and testicular concentration of lactate dehydrogenase (LDH), alkaline phosphatase (ALP), acid phosphatase (ACP), and γ- glutamyl transferase (γ- GT) in the control and in the different treated rat groups.
Discussion
The current study aimed to evaluate the protective effect of Eruca sativa ethanolic leaves extract on lipid metabolic abnormalities induced by dioxin in male rats. The results indicate that TCDD induces hyperlipidemia of both serum and testes including CHOL, TG, PLs, and TL as well as LDL-c and VLDL-c, while HDL-c was significantly decreased. These results are similar to the findings of Magesh et al. [Citation27]. The increase in the serum CHOL concentration in the TCDD group may be due to hepatic overproduction of specific cytochrome P450 enzymes, such as CYP3A [Citation28]. In addition, the inhibition of phosphoenol pyruvate carboxykinase (PEPCK) activity leads to the hypersensitivity of the liver to excessive amounts of CHOL [Citation29]. During adipocyte differentiation, decreased expression of peroxisome proliferator activated receptor gamma (PPARγ) and lipoprotein lipase (LPL) may contribute to an increase in serum CHOL [Citation28,Citation30]. An increase in CHOL may be due to an excess in HMG CoA reductase [Citation31]. The down regulation of LDL receptors may also lead to this result [Citation32]. Also, inhibiting the synthesis and secretion of bile acids causes a rise in cholesterol [Citation33].
Excess testicular TG may be due to their accumulation in tissues after exposure to TCDD an explanation in consistent with Matsumura [Citation34]. In addition, the obtained increase in TG may be attributed to the exceeded VLDL-c synthesis and/or decline if its clearance Magesh et al. [Citation27]. The excess serum total lipid concentration in dioxin-treated rats may be due to an increase in the mobilization of free fatty acid from the peripheral fat depots due to the action of hormone-sensitive lipases [Citation35]. Furthermore, a decrease in CHOL utilization in the dioxin group for the synthesis of male sex steroid leads to an increase in lipid components in the testes [Citation36]. The administration of the ethanolic extract of Es leaves in concomitant with TCDD rats significantly decreases the concentration of serum and testes CHOL, TG, PLs, TL, LDL-c, and VLDL-c, while the concentration of HDL-c was increased. Similar results were obtained by Hussein et al. [Citation37]. The obtained results may be due to the rich source of polyphenols, flavonoids, and polyunsaturated fatty acids. ω-3 fatty acid is suggested to increase serum HDL-c and decrease CHOL, TG, LDL-c, and VLDL-c through inhibiting hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG COA) [Citation38]. The estrogenic effect of flavonoid increases may be linked to LDL receptor activation, which enhances CHOL transport to the testes [Citation39]. The isothiocyanates in Es extract can impair cytochrome P450 activity and upregulate the detoxifying enzyme systems (γ-GT) causing the reduced synthesis of CHOL and TG by the liver and increased fecal bile acids and excretion of CHOL [Citation28,Citation40]. Alternatively, the decline of TG by Es may be due to a decrease in its endogenous synthesis and increased lipid protein lipase activity that promotes the clearance of TG [Citation41]. Furthermore, the decrease in serum CHOL, TG, LDL, and VLDL may be due to the fact that ES extract contains PUFAs, which increases the levels of antioxidant enzymes and antioxidant substrates like GSH, resulting in annular lipid fluidity and plasma cholesterol clearance [Citation42].
Regarding the activities of the enzyme, the results of the current study showed that TCDD significantly increases the concentrations of ALP, ACP, and γ-GT in the serum. These results are in agreement with [Citation43], which may be attributed to degenerative changes and lytic activity in the testicular parenchyma [Citation44]. The depletion of the reduced glutathione and antioxidant enzymes as a result of dioxin exposure leads to free radical generation causing tissue damage [Citation45]. The observed increase in testicular LDH activity with increased its concentration in the blood reflects the deterioration of the germinal epithelium of the testicular seminiferous tubules in which the activities of these enzymes are regulated by testosterone [Citation46]. The protective role of Es ethanolic extract may be achieved by the noticeable improvement in serum ALP, ACP, γ-GT, and LDH, which goes in parallel with the suppressive effect on TCDD due to the effect of various antioxidant compounds such as flavonoids, Zn, and Cu present in ES extract, which are thought to be an essential component of free radical scavenging SOD and also impair lipid peroxidation [Citation20,Citation47,Citation48].
Limitation of the study
Although this study considered all relevant blood and testicular lipid metabolism parameters, there were some potential limitations. First, unavailability of resources for measuring dioxin levels in the blood of control and treated groups as well as a concentration of dioxin food and feed were unavailable. Furthermore, the relationships between the dioxin level and the estimated parameters is discussed. Despite these limitations, this study contributed to understanding the effect of dioxin on blood lipid and testicular lipid components and demonstrated the protective effect of Es extract in preventing dioxin-induced lipid changes.
Conclusion
Dioxin treatment is associated with a positive change in blood lipid components, whereas pretreatment with Es extract may prevent oxidation stress caused by dioxin, regulate body metabolism, and maintain lipid values within the normal range. Our recommendations are to assess dioxin and its relation to infertility in both sexes, as well as how to prevent this effect with a safe natural product extract.
Ethical Approval
All the experiments were conducted in agreement with the ‘Institutional Animal Ethics Committee at Mansoura University, Mansoura, Egypt regulation, which is in accordance with the ‘Handbook for the Care and Use of Laboratory Animals issued by the ‘National Academy of Sciences.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Hussam Ahmed El-Gayar, Eman Taha Salem, and Gamal Mohamed Edrees. The first draft of the manuscript was written by Hussam Ahmed El-Gayar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare no financial or commercial conflict of interest.
Additional information
Funding
References
- Matés JM, Segura JA, Alonso FJ, et al. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. 2010;49:1328–1341.
- Maqbool F, Mostafalou S, Bahadar H, et al. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016;145:265–273.
- Patrizi B, Siciliani de Cumis M. TCDD toxicity mediated by epigenetic mechanisms. Int J Mol Sci. 2018;19:4101.
- Srogi K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: a review. Environ Chem Lett. 2008;6:1–28.
- Dömötörová M, Stachová Sejáková Z, Kočan A, et al. PCDDs, PCDFs, dioxin-like PCBs and indicator PCBs in soil from five selected areas in Slovakia. Chemosphere. 2012;89:480–485.
- Tanos R, Patel RD, Murray IA, et al. Aryl hydrocarbon receptor regulates the cholesterol biosynthetic pathway in a dioxin response element-independent manner. Hepatology. 2012;55:1994–2004.
- Chopra M, Schrenk D. Dioxin toxicity, aryl hydrocarbon receptor signaling, and apoptosis—Persistent pollutants affect programmed cell death. Critical Rev in Toxicol. 2011;41(4):292–320
- Ha A, Rm K. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat Sertoli cells: possible role of mitochondrial fractions of Sertoli cells. Toxicol Appl Pharmacol. 2011;1:252.
- Edrees G, El-Gayar H, and Habibi E-S, et al. Protective Effect Of Petroselinum Crispum Or Eruca Sativa Extracts On Testes Against Dioxin Intoxication In Rats European j. biomed. pharm. sci. 2015;2:57–66.
- Nwanaji-Enwerem JC, Jenkins TG, Colicino E, et al. Serum dioxin levels and sperm DNA methylation age: findings in Vietnam war veterans exposed to agent Orange. Reprod Toxicol. 2020;96:27–35.
- Fader KA, Nault R, Ammendolia DA, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters lipid metabolism and depletes immune cell populations in the jejunum of C57BL/6 mice. Toxicol Sci. 2015;148:567–580.
- Doskey CM, Fader KA, Nault R, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters hepatic polyunsaturated fatty acid metabolism and eicosanoid biosynthesis in female Sprague-Dawley rats. Toxicol Appl Pharmacol. 2020;398:115034.
- Lu J, Liu M, Fan Y, et al. TCDD induced lipid accumulation by impairment of autophagic flux in THP-1 macrophages. Environ Sci Pollut Res. 2021;28:36053–36059.
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79.
- Yaniv Z, Schafferman D, Amar Z. Tradition, uses and biodiversity of rocket (Eruca Sativa, Brassicaceae) in Israel. Econ Bot. 1998;52:394–400.
- Fuentes E, Alarcón M, Fuentes M, et al. A novel role of Eruca sativa mill. (rocket) extract: antiplatelet (NF-κB inhibition) and antithrombotic activities. Nutrients. 2014;6:5839–5852.
- Kim B, Choi Y-E, Kim H-S. Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phytother Res. 2014;28:1359–1366.
- Eid AM, Jaradat NA, Al-Masri M, et al. Development and antimicrobial evaluation of eruca sativa oil nanoemulgel with determination of the oil antioxidant, sun protection factor and elastase inhibition. Curr Pharm Biotechnol. 2020;21:244–255.
- Piragine E, Flori L, Di Cesare Mannelli L, et al. Eruca sativa mill. seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phytother Res. 2021;35:1983–1990.
- El-Gayar H, El-Habibi E, Edrees G, et al. Licensed under creative commons attribution cc by role of alcoholic extracts of Eruca sativa or petroselinum crispum on dioxin-induced testicular oxidative stress and apoptosis. J Agric Food Chem. 2016;5:1415–1421.
- Banso A. Phytochemical and antibacterial investigation of bark extracts of acacia nilotica. JMPR. 2009;3:082.
- Zilić S, Serpen A, Akıllıoğlu G, et al. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (zea mays L.) kernels. J Agric Food Chem. 2012;60:1224–1231.
- Hwang E-S, Thi ND. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (porphyra tenera). Prev Nutr Food Sci. 2014;19:40–48.
- Marchetti JM, Errazu AF. Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy. 2008;32:892–895.
- Alqasoumi S. Carbon tetrachloride-induced hepatotoxicity: protective effect of “rocket” Eruca sativa L. in rats. Am J Chin Med. 2010;38:75–88.
- Latchoumycandane C, Mathur PP. Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J Appl Toxicol. 2002;22:345–351.
- Magesh SB, Rajappa R, Ramkumar KM, et al., Acetyl-L-Carnitine restores abnormal lipid metabolism induced by 2,3,7,8-tetrac-hlorodibenzo-P-dioxin in mice. 2017;Biomed Pharmacol J. 10:569–576.
- Jin J, Wahlang B, Thapa M, et al. Proteomics and metabolic phenotyping define principal roles for the aryl hydrocarbon receptor in mouse liver. Acta Pharm Sin B. 2021;11:3806–3819.
- Nash JT, Szabo DT, Carey GB. Polybrominated diphenyl ethers alter hepatic phosphoenolpyruvate carboxykinase enzyme kinetics in male Wistar rats: implications for lipid and glucose metabolism. J Toxicol Environ Health. 2013;76:142–156.
- Choi EM, Suh KS, Jung -W-W, et al. Glabridin attenuates antiadipogenic activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in murine 3T3-L1 adipocytes. J Appl Toxicol. 2018;38:1426–1436.
- Prince PSM, Kannan NK. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2006;58:1373–1383.
- Bombick DW, Matsumura F, Madhukar BV. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in the low density lipoprotein (LDL) receptor activities in the hepatic plasma membrane of the guinea pig and rat. Biochem Biophys Res Commun. 1984;118:548–554.
- Fletcher N, Wahlström D, Lundberg R, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicol Appl Pharmacol. 2005;207:1–24.
- Matsumura F. Mechanism of action of dioxin-type chemicals, pesticides, and other xenobiotics affecting nutritional indexes. Am J Clin Nutr. 1995;61:695S–701S.
- Boverhof DR, Burgoon LD, Tashiro C, et al. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-mediated hepatotoxicity. Toxicol Sci. 2005;85:1048–1063.
- Mai X, Dong Y, Xiang L, et al. Maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses male reproductive functions in their adulthood. Hum Exp Toxicol. 2020;39:890–905.
- Hussein J, Salah A, Oraby F, et al. Antihepatotoxic effect of eruca sativa extracts on alcohol induced liver injury in rats. undefined [Internet]. 2010 cited 2022 Jun 9]; Available from 2022 Jun 9: https://www.semanticscholar.org/paper/Antihepatotoxic-Effect-of-Eruca-Sativa-Extracts-on-Hussein-Salah/18d40041d2ba369-0a20fdfc6bdb9cb0247de357e
- Courtney L Millar, Quinn Duclos, and Christopher N Blesso, Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL function, Advances in Nutrition, Volume 8, Issue 2, March 2017, Pages 226–239, https://doi.org/10.3945/an.116.014050
- Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis. 2007;193:86–93.
- Abdull Razis AF, Konsue N, Ioannides C. Isothiocyanates and xenobiotic detoxification. Mol Nutr Food Res. 2018;62:e1700916.
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324.
- Hashimoto M, Hossain MS, Shimada T, et al. Effects of docosahexaenoic acid on annular lipid fluidity of the rat bile canalicular plasma membrane. J Lipid Res. 2001;42:1160–1168.
- Aly HAA, Khafagy RM. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat Sertoli cells: possible role of mitochondrial fractions of sertoli cells. Toxicol Appl Pharmacol. 2011;252:273–280.
- Aly HAA, Azhar AS. Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod Toxicol. 2013;40:8–15.
- Lee D-H, Jacobs DR. Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants? Free Radic Res. 2009;43:533–537.
- Selvakumar E, Prahalathan C, Sudharsan PT, et al. Protective effect of lipoic acid on cyclophosphamide-induced testicular toxicity. Clin Chim Acta. 2006;367:114–119.
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24.
- ELSadek M. Chemical constituents of Eruca sativa and treatment activity against paracetamol inducing hepatic injury in experimental rats. 2014;Egyptian J Nutrition Health. 9:1–12.