ABSTRACT
The current study was carried out to investigate the effects of levamisole Hcl. (LMS) supplementation on the performance, immunological response, and various hematological, biochemical, and histopathological changes in broilers. A total of 120 one-day-old Ross (308) broiler chicks were randomly assigned to one of two feeding treatments, each of which has 3 replicates (20 chicks per each replicate). The first (control) group was fed a basal diet with no supplements, whereas the second (LMS) group was given LMS 25 mg/kg live body weight via drinking water every day for 37 days. The results showed that LMS supplementation enhanced final body weight, weight gain, and average FCR, over the entire experimental period, and increased PCV percent, Hb percent, and WBC count. Furthermore, phagocytic activity increased (P < 0.05) by roughly 15.3% at the 5th week of chicken age compared to the control. At 3 and 5 weeks of the experiment, the LMS-treated group’s intestinal villi improved considerably (P < 0.05) in length, width, goblet cell number, and crypt depth compared to the control group. In conclusion, including LMS in the diet increased broiler chick growth, performance, and immunological response. As a result of its growth-promoting and immunostimulant properties, we recommended its usage in chicken farms.
Introduction
Growth promoting agents are compounds that influence the physiological equilibrium between anabolism and catabolism in order to boost the rate of growth. They typically act by either enhancing anabolism or decreasing catabolism [Citation1]. Immunostimulants are substances that, when combined with an antigen, stimulate nonspecific immunological processes or specific immune mechanisms. Immunostimulants are a class of biological and synthetic chemicals that stimulate animals’ nonspecific defense mechanisms, allowing them to acquire generalized protection [Citation2]. Egyptian chicken farming has evolved from an agricultural activity to an industry in recent years. Egypt’s broiler chicken meat output has increased in response to rising customer demand for low-cost animal protein. At the same time, the lack of government involvement in defining the rules for industry development, or, more accurately, the lack of a National strategy for poultry industry development, has resulted in a slew of issues such as a massive live bird market, inefficient production practices, and so on [Citation3]. The administration of health-risking compounds such as growth promoters and veterinary medications (antibiotics) is a recurring issue in animal production since these drugs are frequently employed to improve productivity and minimize breeding costs [Citation4]. Due to increasing concerns about the transmission and multiplication of resistant bacteria in human via the food chain, the European Union has outlawed the use of antibiotic growth promoters (A.G.P.) in animal feed since 2006. As a result, new commercial additives taken from nature are being researched as part of potential future alternative feed plans [Citation5]. There are several experimental non-therapeutic alternatives to antibiotic growth promoters (A.G.Ps.) as feed additives in animal production, including as enzymes, probiotics, prebiotics, herbs, essential oils, immunostimulants, and organic acids [Citation6]. The most prevalent application of LMS is as a broad-spectrum anthelmintic. LMS, on the other hand, has been shown in a number of clinical and experimental studies to be effective as an immunomodulator in the treatment of a variety of disorders [Citation7, Citation8,and Citation9]. Despite the fact that it has been used to improve human immunity in infectious diseases, leprosy, and cancer, the mechanism of action of LMS is mostly unknown [Citation10].
LMS has been demonstrated to stimulate lymphocyte proliferation in both mice and chickens, implying that it has the potential to produce a cellular immunological response, as evidenced by its ability to trigger a high level of IFN-gamma (IFN-γ.). Furthermore, LMS not only increases humoral immunity but also induces cell-mediated immunity, resulting in long-term immunological responses to NDV vaccination [Citation11].
Following immunization, LMS can also stimulate the immune system by functioning as a multifunctional modulator, influencing the cell-mediated response of T cells while also encouraging activated B cells to produce antibodies [Citation12]. LMS helps to normalize the dendritic cell4/dendritic cell8 (CD4/CD8) ratio in autoimmune diseases while also improving cellular immunity [Citation13].
[Citation14] found that giving LMS to broiler chicks at different dose levels increased performance parameters such cumulative feed intake, cumulative feed efficiency, and cumulative body weight gain. Several studies have demonstrated that LMS improves both humoral and cellular immune responses in healthy chickens [Citation15]. LMS may have a good effect on broiler performance and immunity, and it can be used frequently to improve immune response and promote productivity in broilers, particularly in the face of continual immune system challenges such as those found in tropical environments [Citation16].
In this work, experiments were carried out to assess the effects of LMS as a growth promoter in broiler chicks. In addition, the drug’s hematological, biochemical, and histopathological effects were studied. Furthermore, immunological experiments were conducted to assess the immunostimulant effects of this drug on antibody titer against Newcastle disease vaccination, phagocytic activity, and phagocytic index.
Material and methods
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of ICLAS-2015. All procedures and experiments were accepted by the local ethics committee for animal use from the Faculty of Veterinary Medicine, Alexandria University-Institutional Animal Care and Use Committee (AU-IACUC).
Medicated and chemical agents
Levamisole (Levamison®) is produced by ADWIA Pharmaceuticals Co., Egypt. All the diagnostic kits used for assaying the hepatic (total serum protein, serum albumin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, serum triglycerides, and cholesterol) and renal function related serum parameters (serum urea and creatinine) were obtained from Bio-diagnostic Company, Egypt. Other chemicals for hematological studies (RBCs and WBCs counts, hemoglobin percentage and packed cell volume value), histopathological and immunological studies (heamagglutination inhibition test for detection of Newcastle antibodies and phagocytic activity and index) used during the experiment were obtained from EL-Gomhoria Company, Egypt.
Birds and experimental design
One hundred and twenty-one-day old Ross (308) broiler chicks were obtained from El-Doha Company for Parents, Dakahlia, Egypt, and were randomly assigned to two equivalent treatments, each with three replicates and 20 birds for each replicate. The control group received only the basal ration, without any additives. The LMS-treated group received 25 mg of LMS/kg of live body weight via drinking water (the concentration of LMS in the water to give the required dose per kilogram of body weight was calculated by determining the water consumption and body weight of each bird on the day of medication) [Citation10], also according to manufacturer recommendations; the experiment lasted for 37 days.
Chicks were reared in floor pens smeared with a thick litter system of wood shavings to a height of around 5 cm. According to [Citation17], all chicks were fed the same basal starter diets (until 21 days of age) and grower rations (from 22 to 37 days of age) based on corn and soybean. The broiler chickens were subjected to a photoperiod of one hour of darkness, which was left for the chicks on the first day of age to be adapted to electricity cut, followed by 24 hours of light from the second day till the end of the experiment. The temperature program was 33°C, 30 o C, 27°C, and 24°C at the first, second, third, and fourth & fifth weeks of the experiment, respectively, and the humidity average during the trial was 65% [Citation18].The feed and water were added ad-libitum during the experiment. The physical and chemical compositions of the experimental basal diets are shown in . All chicks were vaccinated against Newcastle Disease (Izovac Hitchner IB, Clon 79, and Clon 30 vaccines were administered through the oculonasal route at 7, 17, and 27 days, respectively), and Gumboro disease (Izovac Gumboro intermediate vaccine was administered through the oculonasal route at 9 and 19 days).
Table 1.: The physical and chemical composition of basal diet.
Performance parameters
Average body weight (ABW), average body weight gain (AWG), feed intake, and feed conversion ratio [FCR) were evaluated according to the method described by [Citation19].
Blood /serum sampling and analysis
On the 3rd and 5th weeks, five birds per replicate were euthanized using isoflurane 5% and slaughtering was performed. Four blood samples were collected from the wing veins of five birds per replicate. The first blood sample was collected on heparin [100 IU/5 mL of blood] for hematological studies. The other blood samples were collected without anticoagulant and centrifuged at 3000 rpm. For 15 minutes for separation of the serum, which was kept frozen at −20°C for biochemical analysis. PCV%, erythrocytic and total leucocytic counts were determined by the method described by [Citation20]. The Hb concentration was determined using the colorimetric method described by [Citation21]. Serum ALT and AST were calculated using the methods described by [Citation22], serum ALP was calculated using the methods described by [Citation23], serum total cholesterol and triglycerides were calculated using the methods described by [Citation24], total bilirubin was calculated using the methods described by [Citation25], serum total proteins and albumin were calculated using the methods described by [Citation24], serum urea and urea were calculated using [Citation26],described a method for measuring serum globulin levels.
Immune response evaluations
Hemagglutination inhibition test for detection of Newcastle antibodies
Blood samples were collected on days 14, 21, 28, and 35 of age from the brachial veins of five birds per replicate. The microtechnique of the hemagglutination inhibition test was done according to [Citation27]. According to [Citation28], a geometric mean titer (GMT] was calculated.
Determination of phagocytic activity and phagocytic index
At the 35th day of age, blood samples were collected from the brachial vein of five birds per replicate from each group of experimental birds in clean, dry vials containing anticoagulant (0.1 ml sodium citrate 3.8% [one part/4 parts blood)) for determination of phagocytic activity and phagocytic index. Phagocytic activity was determined according to [Citation29]. According to the following equations, Phagocytic activity (PA] = Macrophages containing yeast/Total number of Macrophages x100. Phagocytic index (PI) = Number of cells phagocytized/Number of phagocytic cells.
Immune organ relative weight (index)
At 21 and 35 days of chicken age, five chicks per replicate from each group were euthanized with isoflurane 5% and slaughtered. After necropsy, the spleen, thymus, and bursa were dissected out, grossly examined, and weighted. The organ weight index of each organ was calculated [Citation30] as: organ index weight = organ weight/live body weight x 100.
Histopathological techniques
Parts of the liver, kidney, spleen, intestines, bursa, and thymus from five slaughtered birds per replicate [at 21 and 35 days of chicken age) from each group were collected, fixed in 10% neutral buffered formalin solution, washed, and routinely processed through paraffin. Paraffin sections of 5 microns thick were prepared, stained with hematoxylin and eosin, and examined histopathologically according to the method described by [Citation31]. Measurement of the length and width of the intestinal villi was carried out by using a micrometer eyepiece and a light microscope at lens 10x according to the method described by [Citation32].
Statistical analysis
The data were analyzed using independent samples T-test with feeding groups serving as treatment means and replicates within each treatment group. There were two groups: the first group was the control group, and the second group was the LMS treated group. SPSS version 23 was used to analyze the data. The significant effect was set at (P ˂ 0.05]. Data was presented as mean ± standard errors.
Results
Growth performance parameters
LMS supplementation enhanced body weight gain and FCR (P ˂0.05) of broiler chickens () during starter and grower-finisher periods compared to control. However, However, compared to the control, LMS supplementation enhanced final body weight, weight gain, and average FCR (P˂ 0.05) throughout the whole experimental period by about 7.0%, 7.1% and 9.9%, respectively.
Table 2. Growth performance parameters of broiler chickens as affected by dietary supplementation of levamisoleHcl. aN = 60.
Hematological findings
As shown in , LMS supplementation in broiler chick’s diet increased PCV values, Hb content and WBC count (P < 0.05) at 3rd and 5th weeks of age compared to the control group, but had no effect on RBC count.
Table 3. Hematological parameters of broiler chicken as affected by dietary supplementation of levamisoleHcl. aN = 15.
Biochemical findings
displays the results of serum biochemical alterations. At the 3rd and 5th weeks of broiler chicken age, LMS supplementation reduced (P ˂ 0.05) serum urea, creatinine, total cholesterol, triglycerides, and total bilirubin concentrations. However, as compared to the control, LMS had no effect on serum total protein, albumin, globulin concentrations, or liver enzyme activity.
Table 4. Serum biochemical changes of broiler chickens as affected by dietary LMS supplementation. aN = 15.
Immunological findings
Antibody titer and phagocytosis
In , the effect of LMS supplementation of broiler chicks on antibody titers against New Castle disease is presented. Variable results were observed as LMS supplementation increased antibody titers at the 14th, 21st, and 35th days of broiler age while having no effect on the titer at day 28th compared to the control. Moreover, it was indicated that LMS supplementation increased (P ˂ 0.05) phagocytic activity by about 15.3% while having no effect on phagocytic index compared to the control.
Table 5. Antibody titer and phagocytosis of broiler chickens as affected by dietary supplementation of LMS aN = 15.
Immune organs relative weight
illustrates the immune organs relative weight (index) of broiler chickens, with LMS treated group exhibiting increased bursa and thymus gland relative weights (P˂0.05) at 3rd or 5th weeks of broiler chickens age compared to control.
Table 6. Shows the immune organs index of broiler chickens as affected by dietary LMS supplementation. aN = 15.
Immune organs morphology
illustrate histopathological alterations in the spleen, bursa, and thymus gland, respectively, at the 3rd and 5th weeks of broiler chick age as affected by LMS supplementation.
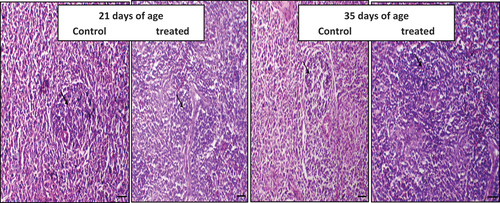
Figure 1. The effect of daily oral administration of levamisoleHcl. (2.5 mg./ml. in drinking water) in broiler chicks on bursa of Fabricius: (a): Bursa of Fabricius of chicken of control chicken (at 21 days of the experiment). Figure (b): Bursa of Fabricius of a chicken treated with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 21 days of the experiment) (c): Bursa of Fabricius of chicken of control chicken (at 35 days of the experiment). (d): Bursa of Fabricius of a chicken supplemented with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 35 days of the experiment) .
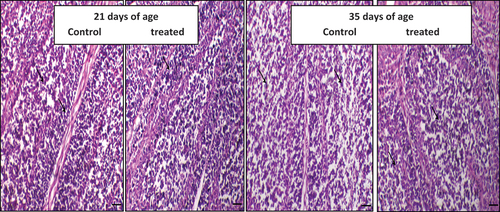
Figure 2. The effect of daily oral administration of levamisoleHcl. (2.5 mg./ml. in drinking water) in broiler chicks on thymus: (a): Thymus of chicken of control chicken (at 21 days of the experiment). (b): Thymus of a chicken treated with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 21 days of the experiment) (c): Thymus of a chicken of control chicken (at 35 days of the experiment). (d): Thymus of a chicken treated with levamisole (at a dose of 2.5 mg./ml. in drinking water) (at 35 days of the experiment).
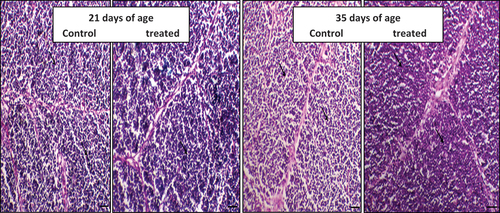
Figure 3. The effect of daily oral administration of levamisoleHcl. (2.5 mg./ml. in drinking water) on broiler chicks on spleen: (a): Spleen of chicken of control chicken (at 21 days of the experiment). (b): Spleen of a chicken supplemented with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at day 21 of the experiment) (c): Spleen of a chicken of control chicken (at 35 days of the experiment). (d): Spleen of a chicken treated with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 35 days of the experiment) .
Intestinal histomorphometric measurements
Ileum histomorphometric measurements are presented in and . Statistical analysis of the obtained data revealed that LMS supplementation increased (P < 0.05) villi height, villi width, crypt depth, villi height: crypt depth ratio (VH: CD ratio) and goblet cell number at both periods, except that the VH: CD ratio was reduced (P ≥ 0.05) at the 5th week of broiler age compared to control.
Figure 4. The effect of daily oral administration of levamisoleHcl. (2.5 mg./ml. in drinking water) in broiler on intestinal villi: (a): Intestinal villi of chicken of control chicken (at 21 days of the experiment). (b): Intestinal villi of a chicken treated with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 21 days of the experiment). (c): Intestinal villi of a chicken of control chicken (at 35 days of the experiment). (d): Intestine of a chicken treated with levamisole Hcl. (at a dose of 2.5 mg./ml. in drinking water) (at 35 days of the experiment) .
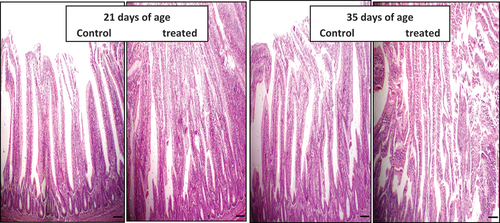
Table 7. Intestinal histopathology (ileum section) of broiler chickens as affected by dietary supplementation of LMS *N = 15.
Discussion
The effects of LMS as a growth promoter and immunostimulant in broiler chicks were examined in this study. The findings of this study show that LMS could be employed as a growth promoter and immunostimulant in broiler chickens to accomplish maximum performance. The current study found that LMS increased average weight gain and feed conversion ratio in broiler chicks during the starting and growing stages. This could be attributed to the current study’s findings of increased villus length and width in the jejunum. Thus, LMS supplementation will be extremely beneficial to young birds’ intestinal development, especially when antibiotics are not provided. Improvements in intestinal villi length, width, crypt depth, and goblet cell numbers provide possibilities for nutrient absorption, allowing for faster growth. This increase in average body weight gain as a result of LMS supplementation could be related to the fact that LMS, as an immunostimulant, has been shown to raise the body weight of stressed birds due to its immunomodulatory effects [Citation33]. Furthermore, [Citation34],shown that giving LMS can improve the histo-morphometric characteristics of the small intestinal wall of broiler chicks [especially at its anthelmintic dose). This could be the mechanism underlying the previously documented beneficial benefits of LMS on broiler body weight and performance. The findings were consistent with those of [Citation14], who discovered that oral administration of LMS to broiler chickens at various dose levels had a positive impact on performance parameters such as decreased cumulative feed intake, improved cumulative feed efficiency, and cumulative body weight gain. Likewise, Li, Wang, and Gatlin III [2006] revealed that feeding LMS at a concentration of 100 mg/kg to fish boosted growth and feed efficiency when compared to fish fed the basal ration. Interestingly, these findings are consistent with those of [Citation34], who observed an increase in muscularis width in the duodenum of several LMS supplemented groups. Thicker muscularis can be accompanied by stronger movements that aid in the passage of nutrients to the jejunum. The observed results were inconsistent with those recorded by [Citation35], who found no significant difference in mean body weight in broiler chicks fed with LMS at a dose level of 2 gm/kg of feed when compared to the control group. These could be as a result of a different dose or method of supplementing LMS. Furthermore, [Citation36],discovered a non-significant change in the weekly average body weight of broiler chickens. On days 21 and 35 of the experiment, there was a substantial rise in WBCs count but no significant difference in PCV percent, Hb percent, or RBCs count of chicks treated with LMS compared to the control group. This could be because LMS enhances dendritic cell maturation, which leads to enhanced expression of major histocompatibility complex [MHC] molecules. T-cells are activated by antigen and MHC complexes. T-cell activation causes the release of interferon gamma, which boosts the immunological response [Citation37]. The obtained result is in agreement with those recorded by [Citation38], who observed that the number of white blood cells in the blood increased on the first day after LMS was administered but then recovered to pre-dose values for the remainder of the experiment. The time of maximum LMS absorption in buffalo heifers could be linked to increases in RBC, PCV, and WBC after day one of LMS administration. Furthermore [Citation39],,discovered that hemoglobin concentrations and mean corpuscular hemoglobin concentration [MCHC) were presented at higher and lower doses of LMS supplementation at 200 mg and 50 mg/kg feed, respectively; however, they were not different from the un-supplemented control ration. WBC, on the other hand, demonstrated changes from the control diet, fish fed 100 mg of LMS per kg feed showed high values. The experimental data contradicted [Citation40]. They recorded that total WBC counts in sheep supplemented with LMS were considerably lower [P ˂0.05], but still within the normal range. This could be due to a difference in animal species, environmental condition or dose amount. On days 21 and 35 of the study, there were no significant variations in serum total protein, albumin, globulin, and A/G. ratio in chicks treated with LMS compared to the control group. This suggests that LMS increased the immune response mostly without affecting the liver or renal functioning. The findings were consistent with those of [Citation41], who reported that in lambs given LMS at a dose of 7.5 mg/kg, non-significant variations in serum total protein and total bilirubin were identified when compared to control values. Our findings contradicted those of [Citation42]. They concluded that feeding LMS at a dose of 250 mg/kg to common carp resulted in increased total protein and globulins at 57 and 70 days of feeding time; the same results was recorded for the index albumin: globulin at 70 days. Besides [Citation16], found that two important liver enzymes (ALT and AST) were significantly increased in the LMS treated (vaccine + LMS 10 mg/kg body weight) but not in group which was treated with (vaccine + LMS 3 mg/kg body weight), indicating that high doses of LMS [10 mg/kg) are supposed to damage the liver. In addition [Citation41],,found that LMS treatment dramatically enhanced serum albumin while decreasing total cholesterol in ewes. Furthermore, the obtained results contradicted the findings of [Citation38], who observed that after LMS supplementation, total serum protein was up on day 7, whereas globulin was raised on days 7 and 14. LMS is given to buffalo heifers at a single dose of 15 mg/kg body weight. On day 14 of the experiment, there was a considerable decrease in albumin concentration. In addition [Citation43], showed a drop in total plasma protein levels, followed by a fall in albumin and globulin levels, in Sahiwal heifers after reduced doses of LMS supplementation. This could be due to a difference in animal species, environmental condition or dose amount. On days 21 and 35 of the study, there was no change in ALT, AST, or ALP activity in chicks supplemented with LMS. This suggests that LMS increased the immune response mostly without affecting liver or kidney functions, which were supported by our histopathological findings, which revealed that hepatic tissue in chicks treated with LMS had normal histological structure. Several medications, including levamisole, Imidazole, Theophylline, and Amino Guanidine, have been demonstrated to have immunomodulatory effects, and LMS is a potent inhibitor of tissue nonspecific ALP. This action is related to the structure-inhibition caused by phenyl rings and imidazole [Citation44]. The use of LMS at low doses and for long periods at a time has been proven to reduce blood AST, ALT, and CK enzyme activity levels in patients with liver damage [Citation45]. The obtained results were consistent with those of [Citation46], who reported that the values of major biochemical parameters (including liver enzymes and metabolites) examined were within normal ranges for swine and corresponded to the ages of the swine. Furthermore [Citation47], revealed that cows administered LMS had higher total protein levels and lower ALT and AST levels in their blood plasma. Furthermore, our findings contradicted the findings of [Citation48], who found that the levels of liver enzymes increased in birds treated with LMS [0.2 ml/kg-BWt) when compared to the usual level in control chicks. On days 21 and 35 of the experiment, there was a substantial drop in blood cholesterol without a significant change in serum triglycerides or total bilirubin in chicks treated with LMS compared to the control group. The obtained results were consistent with those reported by [Citation41], who found that LMS supplementation can result in a significant reduction in total cholesterol in ewes. These findings could be attributed to treatments that shield thyrocytes from free radicals. The obtained results were inconsistent with those obtained in a study conducted by [Citation49], who observed that an increase in circulating cholesterol levels in cows treated with thiaben-dazole was associated with improved cellulose use. On days 21 and 35 of the experiment, there were decrease in serum urea and creatinine levels in chicks supplemented with LMS compared to the control group. The obtained results were in contrast to those reported in a study conducted by [Citation38], who reported that plasma urea concentrations first decreased after oral administration of LMS to buffalo heifers. The observed high urea levels could have been caused by either increased amino acid deamination or increased protein intake in the treated animals (Citation50]. On days 14, 21, and 35 of the experiment, there was a marked increase in antibody titer against Newcastle disease. There was no change in the titer on day 28. T lymphocyte differentiation enhances dendritic cell maturation, restores monocyte and macrophage functions, and boosts neutrophil mobility and chemotaxis [Citation51–53]. A study has found that LMS enhances activation of T cells and increases antibody production by using either a DNA vaccine or an inactive vaccine co-administered with LMS. This is another possible method of LMS to stimulate the immune system. LMS can increase the proliferation of lymphocytes both in mice and chickens [Citation54,Citation55]. The obtained results were in harmony with [Citation56], who found that LMS orally administrated [30 mg/kg Bwt) resulted in a higher level of antibody titer against Newcastle vaccination than control groups in broilers. The obtained results were in compatible with those reported in a study carried out by [Citation57], who observed that using LMS at low (5 mg/kg] and middle (10 mg/kg) doses resulted in significantly higher NDV antibody titers, while using LMS at a high (25 mg/kg) dose resulted in no significantly higher response. Furthermore [Citation16], discovered that the group supplemented with (Vaccine+LMS-10 mg/kg bwt.) had the highest antibody titer, followed by the group treated with (Vaccine+LMS-3 mg/kg bwt.). Our results showed that there was no significant difference in the phagocytic percentage and phagocytic index between chicks treated with LMS compared to the control group on day 35 of the experiment. This result may be due to LMS therapeutic efficacy depending on different factors such as the dose administered, timing, and immune status of animal or human patients [Citation9]. The obtained results were incompatible with [Citation58], who concluded that fish exposed to various concentrations of LMS had significantly higher total phagocytic activity and phagocytic index than the control group. Also, they observed that both concentrations of LMS improved immunity, especially in nonspecific immune system components. On 14–56 days after LMS exposure, leucocrit levels, total leucocyte counts, abundance of neutrophils, monocytes, and lymphocytes, total phagocytic activity, phagocytic index, and total protein level in the blood were significantly improved in LMS treated fish compared to control fish. In the present study, there was a significant increase in bursa weight and percentage without a significant difference in thymus and spleen weight and percentage on day 21 of the experiment. On day 35 of the experiment, there was no significant difference in bursa, thymus, and spleen weight and percentage between chicks treated with LMS compared to the control group. The obtained results were agreed with [Citation10], who recorded that the most noticeable effect of LMS was related to its highest dose (25 mg/kg), which was the only dose of the drug that significantly increased follicular cortex thickness as compared to the control group. The most prominent effect of this dose was on the number of follicles per plica, which increased three times in comparison to that of control chicken.
Three notable limitations affected this study. The first issue was the time factor, as the study needed another week to investigate the effects of the drug on day 42. Another limitation was the sample size. Finally, the work lacked funding and some equipment.
Conclusion
It can be concluded that LMS supplementation of broiler chickens improved growth performance, feed efficiency parameters, and intestinal health as well as positively affected the immune response of broiler chickens through improvement of antibody production against viral diseases and relative weight or histomorphometric parameters of immune organs.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of ICLAS-2015. All procedures and experiments were accepted by the local ethics committee of animal use from the Faculty of Veterinary Medicine, Alexandria University-Institutional Animal Care and Use Committee (AU-IACUC).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in [repository name e.g ‘figshare’] at http://doi.org/[doi], reference number [reference number].
Additional information
Funding
References
- Aziz MA. Growth promoting agents in Hand book of veterinary pharmacology. 5th. Vol. 468 (ZAFER office); 2006. 476.
- Bairwa MK, Jakhar JK, Satyanarayana Y, et al. Animal and plant originated immunostimulants used in aquaculture. J Nat Prod Plant Res. 2012;2(3):397–400.
- Ahlam El Nagar, Ibrahim A. Case study of the Egyptian poultry sector. Ministry of agricultural and land reclamation, economic affairs sector. Department of Agricultural Economics; 2021. University of Zagazig
- Toffolatti L, Gastaldo LR, Patarnello T, et al. Expression analysis of androgen-responsive genes in the prostate of veal calves treated with anabolic hormones. Domest Anim Endocrinol. 2006;30(1):38–55.
- Jaiswal S, Chaturvedani A, Raza M, et al. Review: natural growth promoters, alternative to antibiotic growth promoters on poultry. Int J Environ Sci Technol. 2017;6:254–259.
- Adil S, Banday M, Bhat G, et al. Alternative strategies to antibiotic growth promoters-A review. Vet Scan| Online Veteri Med J. 2011;6(1):76.
- Brunner C, Muscoplat C. Immunomodulatory effects of levamisole. J Am Vet Med Assoc. 1980;176(10 Spec No):1159–1162.
- Mulcahy G, Quinn P. A review of immunomodulators and their application in veterinary medicine. J Vet Pharmacol Ther. 1986;9(2):119–139.
- Symoens J, Rosenthal M. Levamisole in the modulation of the immune response: the current experimental and clinical state. J Reticuloendothel Soc. 1977 Mar;21(3):175–221. PMID: 142150.
- Hamedi S, Shomali T, Akbarian R, et al. Effect of Levamisole on active antibody titres and histomorphometric parameters of immune organs in broiler chickens. Bulg J Vet Med. 2017;20(2):174–182.
- Yin J, Jin H, Kang Y, et al. Efficacy of modified levamisole adjuvant on inactivated virus vaccine. Viral Immunol. 2006;19(3):525–535.
- Chawak M, Rajmane B, Rande A, Effect of levamisole on performance and immunomodulation against ranikhet disease in broilers under stress. Indian Journal of Animal Science (India). 1993;63(10):1060–1061.
- Sharma S, Ali FM, Saraf K, et al. Anti-helminthic drugs in recurrent apthous stomatitis: a short review. J Pharm Bioallied Sci. 2014;6(2):65.
- Porchezhian T, Punniamurthy N. Effect of oral Levamisole hydrochloride on feed intake and body weight of broiler chicks. J Anim Vet Adv. 2006a;5(10):847–848.
- Soppi E, Lassila O, Viljanen M, et al. In vivo effect of Levamisole on cellular and humoral immunity in normal chickens. Clin Exp Immunol. 1979;38(3):609.
- Shovon AK, Nazim U, Hridoy AF, et al. The effect of levamisole on growth performance, humoral immunity and blood biochemical profile in broiler chickens. J Bangladesh Agric Univ. 2020;18(Suppl. 1):858–863.
- NRC. Metabolic modifiers, Effect on the nutrient requirements of food producing animals. Washington D.C: National Academies Press;1994.
- Al-Shafay HM. Influence of some dietary factors on the performance and immunological parameters in broilers. Master Veteri Sci. 2007;22–25.
- Oliveira M, Rodrigues E, Marques R, et al. Performance and morphology of intestinal mucosa of broilers fed mannan-oligosaccharides and enzymes. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2008;60(2):442–448.
- Dacie J, Lewis S. Practical Haematology. London New York: Churchill Livingston; 1984.
- Winterobe MM. Clinical Haematology. 4th ed. Phildelphia: Lea and Febiger; 1965. p. 631–650.
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63.
- Kind P, King E. Colourimetric determination of alkaline phosphatase. J Clin Pathol. 1954;7:322.
- Friedman, young. Effects of disease on clinical laboratory tests. 3rd ed. AACC.press; 1997.
- Walter M, and Gerade H. colorimetric method for estimation of total bilirubin. Microchem J. 1970;15:215–231.
- Coles EH. Veterinary clinical pathology. London Toronto: W.B. Saunders Company, Philadelphia; 1974. p. 211–213.
- Takatasy GY. The use of 100 M in serological and virological micro methods. Acta Microbiol Acad Scient Hungaricae. 1955;3:191.
- Brugh MG. A simple method for recording and analyzing serological data. Avian Disease. 1978;22:362–365.
- Kawahara E, Ueda T, Nomura S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991;26(4):213–214.
- Matousek J. Effects on spermatogenesis in Guinea-pigs, rabbits and sheep after their immunization with sexual organ fluids of bulls. Reproduction. 1969;19(1):63–72.
- Harries ML. Carleton’s Histopathological Technique. 5th ed. New York Toronto: Oxford Univ., Press; 1989. p. 33–48.
- Rezaian M, Yaghoobfar A, Barin J. Effects of pellet and mesh diets on the activity of the microflora and morphology of the small intestine of broiler chicks. J Anim Vet Adv. 2007;6:723–727.
- Porchezhian T, Punniamurthy N. Effect of oral Levamisole hydrochloride on humoral immune response and serum proteins of broilers. J Anim Vet Adv. 2006b;5(10):873–874.
- Shomali T, Hamedi S, Solhdoost H, et al. Levamisole improves histomorphometric parameters of small intestinal wall of broiler chickens. Bulg J Vet Med. 2017;20:385–391.
- Rajput A, Kolte B, Shisodiya J, et al. Effect of vitamin A, vitamin C, vitamin E and Levamisole on performance of broilers. Vet World. 2009;2(6):225.
- Mani K, Sundaresan K, Viswanathan K. Effect of immunomodulators on the performance of broilers in aflatoxicosis. Ind Veteri J. 2001;78(12):1126–1129.
- O’Sullivan BJ, Thomas R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-κB. J Immunol. 2002;168(11):5491–5498.
- Sandhu M, Ahmad T. Haematological and serum biochemical profiles of Buffalo heifers as influenced by levamisole. Comp Clini Pathol. 2003;12(3):147–150.
- Yuji Sado R, de Almeida Bicudo AJ, Possebon Cyrino JE. Dietary Levamisole influenced hematological parameters of juvenile pacu, Piaractus mesopotamicus (Holmberg 1887). J World Aquacult Soc. 2010;41:66–75.
- Rashid BM, Yüksek N. The effects of immunostimulants (Zinc, Levamisole, Vitamin AD3E) use together with enterotoxemia vaccine on immunoglobulins in sheep. Turk J Veteri Res. 2019;3(2):65–75.
- Atessahin A, Karahan I, Pirincci I. Effects of therapeutic and toxic doses of Levamisole on thyroid hormones and some biochemical parameters in sheep. Cell Biochem Funct Cell Biochem Modulat Active Agent Disease. 2004;22(5):281–286.
- Maqsood S, Samoon M, Singh P. Immunomodulatory and growth promoting effect of dietary Levamisole in Cyprinus carpio fingerlings against the challenge of Aeromonas hydrophila. Turk J Fisher Aquat Sci. 2009;9(1):111–120.
- Asif Zia-ur-Rahman M, Naqvi Z, Mushtaq-ul-Hassan M, et al. Effect of Levamisole administered orally on hematological and biochemical profiles of Sahiwal heifers. J Veterinarski arhiv. 1995;65:185–192.
- Martins MJ, Negrão MR, Hipólito-Reis C. Alkaline phosphatase from rat liver and kidney is differentially modulated. Clin Biochem. 2001;34(6):463–468.
- Fu L-S, Chi C-S. Levamisole in steroid-sensitive nephrotic syndrome children with steroid-dependency and/or frequent relapses. Acta Paediat Taiwan. 2000;41(2):80–84.
- Valpotić H, Barić-Rafaj R, Mrljak V, et al. The influences of immune modulation with Levamisole and polyoxy-ethylene-polyoxypropylene copolymers on the immunohematological, serum biochemical parameters and intestinal histocytomorphology of weaned pigs. VETERINARSKI ARHIV. 2016;86(5):667–684.
- Biswal S, Das S, Mohanty D, et al. Effect of systemic and local immunomodulation therapies on conception rate in endometritic cows. Ind J Field Veterinari (The). 2014;10(2):1–4.
- Ulaiwi AH. Effect of levamisole, Vitamin E, and selenium against aflatoxicosis in broilers chicken. Vet World. 2018;11(2):248.
- Fox M, Jacobs D, Campling R, et al. Effect of thiabendazole treatment on feed intake, digestibility and selected blood values in lactating dairy cows. Vet Rec. 1985;116(10):257–260.
- Sykes A. An assessment of the value of plasma urea nitrogen and albumin concentrations as monitors of the protein status of sheep. BSAP Occas Public. 1978;1:143–154.
- Ali SH, Abdel-Fattah Y, Abdul-Muttalib S. Biochemical, immuno-modulatory and antioxidant properties of Levamisole at different storage conditions and administration routes. Pak J Biol Sci. 2012;15:986–991.
- Chandy ML, Soman C, Kumar SP, et al. Understanding molecular mechanisms in multivariant actions of Levamisole as an anti-helminthic, anti-inflammatory, antioxidant, anti-neoplastic and immunomodulatory drug. J Oral Maxillofac Surg Med Pathol. 2016;28(4):354–357.
- Dörücü M, İspir Ü, Türk C. Levamisolün Gökkuşağı Alabalığı (Oncorhynchus mykiss, W)’nın Bazı Kan Parametrelerine Etkisinin Araştırılması. Fen ve Mühendislik Bilimleri Dergisi. 2005;17(2):405–411.
- Jin H, Li Y, Ma Z, et al. Effect of chemical adjuvants on DNA vaccination. Vaccine. 2004;22(21–22):2925–2935.
- Kang Y, Jin H, Zheng G, et al. The adjuvant effect of Levamisole on killed viral vaccines. Vaccine. 2005;23(48–49):5543–5550.
- Habibi M, Ghahri H, Zadeh RS, et al. Effects of Levamisole on the immune response of broilers against Newcastle disease vaccines. Afr J Pharm Pharmacol. 2012;6(25):1860–1864.
- Cao J-Y, Dou S-L, Zhang S-D, et al. Effects of dexamethasone and levamisole on humoral immune response in chicks [J]. Chin J Vet Sci. 2002;5.
- Perera H, Pathiratne A. Enhancement of immune responses in Indian carp, Catla catla, following administration of Levamisole by immersion. Disease Asian Aquacult VI Fish Health Sect. 2008;129–142.
