ABSTRACT
Physical activity is important for the prevention of many diseases. However, strenuous physical activity makes individuals more prone to infections. This might be linked to immunosuppression and involves changing in cytokines levels especially IL-6 which act as immunomodulatory agent. The aim of this study is to test the correlation between exercise duration and the level of IL-6 in rats, detect the prevalence of bacteremia in different study groups and find a relation between IL-6 levels, intensity of physical exercises and the susceptibility to infection. Sixteen experimental rats were divided into four groups and have been swimming for different periods of time over three consecutive weeks. Blood samples were obtained at the end of each week before and after the last session of exercise. Blood cultures were done and ELISA for measuring plasma levels of IL-6. IL-6 levels decreased after exercise and this was more significant as the exercise duration increased (R = −0.19, P = 0.05). The more the exercise duration is, the more the susceptibility to infection (R = 0.47, P = 0.01). IL-6 levels in the blood are inversely proportional to the exercise duration which might explain the functional immuno-depression and thus, the increasing susceptibility to infections.
Introduction
Cytokines are signaling low molecular weight proteins produced by a broad range of cells as immune cells, endothelial cells, fibroblasts and various stromal cells to regulate the immunity, inflammation and hematopoiesis [Citation1]. They can be classified into family groups according to the types of secondary and tertiary structure or according to cells that secrete them [Citation2]. Interleukin- 6 (IL-6) is an immunomodulatory cytokine with many physiological actions. It is believed that physical exercises induce spikes of the pro-inflammatory and anti-inflammatory cytokines. Regular moderate physical exercises are required to maintain overall balanced health and psychological status, on the other hand, exhaustive exercise was linked to transient immune-suppression which may be due to the elevated levels of cortisol or inhibitory cytokines after strenuous exercises [Citation3]. In addition, cellular immunity is suggested to be suppressed in response to strenuous exercises [Citation4]. Blood samples isolated from individuals participating in extreme endurance events showed that there was transient bacteremia after the event as reported by S. Gill et al [Citation5]. Epidemiological studies show an association between intensity of physical exercise and severity of the body response to infections [Citation6].
Many explanations have been made to explain the possibility of compromised immune status as a result of exercise and one of these explanations was related to level of IL-6 [Citation7,Citation8]. In contrary, high expression of IL-6 might enhance immune response in athletes [Citation9]. This study was conducted to test the correlation between physical activity and the immediate changes in the levels of IL-6 in rats, detect the prevalence of bacteremia in different study groups and find a relation between IL-6 levels, intensity of exhaustive exercises and the susceptibility to infection.
Methodology
The experimental methodology was approved by the Medical Research Ethics Committee of the Institutional Review Board of Mansoura Faculty Of Medicine, Code Number: RP.21.02.96. Experimental animals part was done in Medical Experimental Research Center (MERC) in Mansoura University. ELISA and microbiology cultures were done in Microbiology and Immunology Department laboratories in Mansoura University.
Experimental Animals
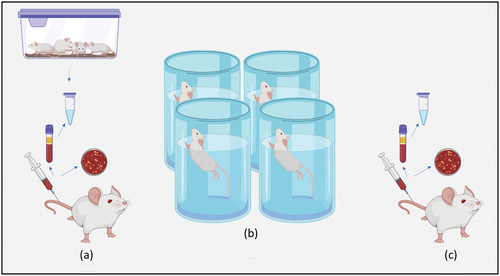
Sixteen male rats nearly the same age were enrolled in this study obtained from the same environment and weighing between 230–250 grams. Room temperature, humidity and lighting were maintained at 20–24°C, 50–60% and of 12 hours dark:12 hours light, respectively. Diet type and quantity, water and serving time were identical for all groups each day [Citation10]. Rats were divided into four groups as following:
Group I: four rats with basal daily activity without exercises.
Each of the following groups includes four rats with daily exercises in the form of continuous swimming for:
10 minutes in Group II.
20 minutes in Group III.
30 minutes in Group IV.
Exhaustive exercise in animals is usually done by treadmill running or forced swimming as in this study. We call these exhaustive exercises when we find the animals lacking coordinated movement or drowning to the bottom of the pool which happened in groups III and IV [Citation11].
Forced swimming is considered strenuous exercise as the case in group II; where the rats didn’t reach the exhaustion point where they start to drown, which is the cut point for calling the exercise exhaustive, but they are still doing a strenuous activity.
Two weeks before starting, training for 5–10 minutes was applied once daily for group II, III, and IV to ensure endurance.
Blood withdrawal procedures
A total of eighty-four venous blood samples were drawn from the lateral tail vein at the end of each week for three weeks in a row [Citation12]. Before the last session of exercise in each week, two ml blood was drawn by a sterile syringe with 0.2 ml of heparin from each experimental subject of groups II, III and IV (). Five minutes later, groups: II, III, and IV started swimming each according to its protocol (). After finishing the exercise, two ml blood was withdrawn in the same manner from the experimental groups. For the control group I, blood samples were withdrawn at the corresponding time ().
IL-6 level by ELISA (Bioassay Technology Laboratory, Shanghai, China)
One ml blood was centrifuged, the plasma was separated, stored in eppendorf and were frozen at – 20°C [Citation13]. IL-6 levels were detected by ELISA according to the manufacturer’s instruction. The sensitivity is: 0.052 ng/L. The plate reader was MagellanTM (Tecan, MedicalExpo)
Microbiology cultures
One ml blood was inoculated in blood culture bottles (Micrognost; Biotest), incubated at 37°C and sub-cultured daily for 6 successive days on blood and MacConkey agar plates (Oxoid, UK). Bacterial growth was identified by standard microbiology methods [Citation14].
Statistical analysis
Data were analyzed using SPSS, version 16. Independent t-test, chi-squared tests and repeated measures ANOVA were used to detect significant differences (P < 0.05). Repeated measures ANOVA was used to assess the significance of the data obtained throughout the first two weeks and the whole three weeks. Quantitative data were presented as means and standard deviation and also described as numbers and percentages.
Results
Detection of post-exercise IL6 plasma levels by ELISA
By the end of the experiment, the exercising groups: II, III and IV, in comparison to the control group, they exhibited a non-significant reduction in post-exercise IL-6 plasma levels (P = 0.058), however, the reduction in IL-6 levels was significant till the end of the second week (P = 0.01). By comparing the control group I and group II, group I reported statistical significance 0.007 and 0.037 higher IL-6 level in the second and the third week, respectively, than group II after exercise. By comparing group I and group III, group I reported statistical significance 0.002 and 0.054 higher IL-6 level in the second and the third week, respectively, than group III after exercise.
By comparing group I and group IV, group I reported high statistical significance 0.007, 0.0007 and 0.040 IL-6 level in the first, second and the third week, respectively, than group IV after exercise, as shows in .
Table 1. Comparison between group I and Group II, Group III, Group IV in circulating IL-6 levels after exercise.
There was no statistically significant difference between group II and III and IV in IL-6 level after exercise. There was no statistically significant difference between group III & IV in IL-6 level after exercise except in the second week p = 0.05.
By comparing IL-6 levels before and after exercise in group II, group III and group IV a statistically significant difference was reported in the third week (0.004), second week (0.02), and first and the second weeks (0.01 and 0.01), respectively, as shown in .
Table 2. Comparison between the circulating IL-6 levels in Groups II, III and IV before and after exercise.
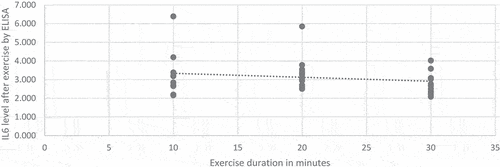
Negative correlation was reported between duration of the exercise and IL-6 level. The level of IL6 decreases when the exercise duration increases, however, the relationship is weak and not statistically significant (R = −0.196, P = 0.058) as shown in . But, this negative correlation was significant by the end of the second week (P = 0.01)
Microbiology cultures and infection prevalence
There were no manifestations or findings of apparent infection in all exercising groups throughout the three weeks; as all pre-exercise cultures were negative, with optimal performance during the exercise. Only bacteremia was detected in a number of post-exercise blood samples.
No positive growth was detected in the blood samples obtained before exercise in the exercising groups; II, III and IV in all weeks. The Cultures of the control group came negative. No positive bacterial growth was detected in group II in the three weeks after exercise. Two (50%) samples gave positive bacterial growth after exercise in the second week only in group III. Quarter of the blood samples showed bacterial growth in the first week and all samples were positive for bacterial growth in the second in group IV after exercise. Quarter of the blood samples showed bacterial growth in the third week in group IV after exercise. As shown in .
Table 3. The number of positive and negative bacterial growth before and after exercise in groups: II, III and IV.
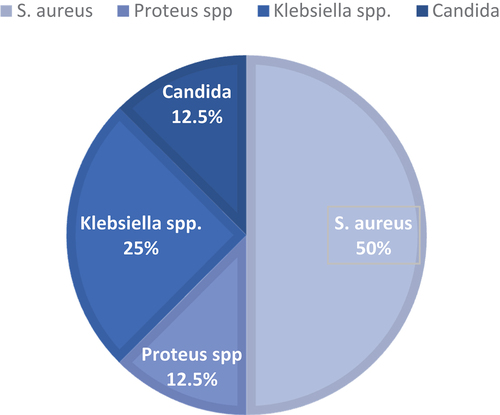
Four bacterial species were isolated from the blood samples; as shown in .
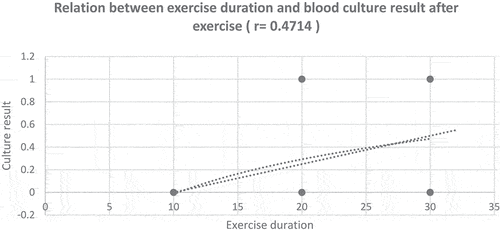
Infection susceptibility increases with the reduction in IL6 levels (R = −0.245, P = 0.09) and when the exercise duration increases (R = 0.4714, P = 0.0087), .
Discussion
Exercise immunology is considered a relatively new area for research; however, some studies were published and reported that immune system is very responsive to exercise and affected by the degree of physiological stress. Cytokines are critical mediators that contribute to chemical language for the immune system through signals transduction [Citation15].
In this study, IL6 level was decreased after exercise. This became evident when we compared the swimming groups of rats to group with basal daily activity. It also appeared by comparing the members of the exercised groups before and after exercise. In addition, the lengthening of the training time also leads to reduction in IL6 level. This was clearly seen with the group of rats that swam for half an hour more than the group that exercised for twenty minutes and those who spent ten minutes. Also, a negative correlation was reported between the level of IL6 and the exercise duration (R = −0.196). These results were in parallel with many studies [Citation16–18]. On the contrary, others have concluded that IL-6 level was increased dramatically after exercise [Citation3,Citation7,Citation19,Citation20]. This difference is likely to be due to different sample, exercise severity or time of measurement. When IL6 level was measured in muscles after severe exercise, a very high level reported due to muscle damage that contributes to upregulation of cytokines expression [Citation21–23]. Another study reported that IL-6 level is affected by the availability of energy substrates which may explain the increase in IL-6 concentrations observed among the athletes [Citation24].
Interestingly, in the current study, IL6 reduction with exercise was statistically significant in the beginning, especially the second week, but its statistical significance decreased later! This could be explained in the study of Charlotte Keller et al; they propose that not only the IL6 levels are affected, by being reduced by exercise, but also the level of expression of IL6 receptors in the skeletal muscles where they increase suggesting that after prolonged training period the basal levels of IL6 receptors mRNA are elevated due to sensitization of skeletal muscles to IL6 [Citation25].
Prolonged exertion increases the risk of infection due to physiologic stress, immunity suppression, decreased blood immune cell counts, lower granulocyte and monocyte phagocytosis and oxidative burst activity, and a diminished pro- and anti-inflammatory cytokine response [Citation26].
In the present study, a highly significant positive correlation was reported between duration of the exercise and infection (R = 0.4714). Susceptibility to infection was increased with IL6 level reduction. This was in agreement with Simpson et al. who reported that moderate exercise is immuno-enhancing but prolonged periods of intensive exercise training can depress immunity [Citation27]. Also, Hume et al demonstrated that a lack of IL-6 resulted in a concomitant increase in bacterial load and severity of infection in eye. His findings imply that IL-6 may play a role in the activation of PMNs during infection [Citation28]. IL-6 also correlates with neutrophil activation because it stimulates production of elastase and prime neutrophils for the production of oxygen-free radicals [Citation29]. In a study conducted by Shi Y et al, they found that the increase of lactic acid during exhaustive exercises was related to the decrease in the production of neutrophil extracellular traps and reactive oxygen species released from neutrophils which protect the body from invasion of microorganisms [Citation30]. This could be due to the decreased levels of IL-6 as found in this study. This can explain the transient immune suppression and increased susceptibility to infection. Clinical studies of IL-6 inhibitors reveal that their use is associated with an increased rate of both serious and opportunistic infections because IL-6 has a prominent role in the synthesis and secretion of acute-phase proteins [Citation31]. IL-6 play an important role in the regulation of the innate and adaptive immune response and IL6 deficient mice showed higher mortality by weakened immune defense and higher bacterial burden [Citation32]. This finding was not true in other studies which reported that exercise is a powerful behavioral intervention that improve immunity and health outcomes and the practice of physical activities strengthens the immune system, suggesting a benefit against infectious diseases [Citation33,Citation34].
Conclusion
The outcomes of this study reveal an inversely proportional relation between exercise duration and plasma IL6 levels. Decreased IL6 levels after exhaustive exercises transiently suppress immune response which could be due to impaired response of the innate immunity represented by the neutrophils which are affected by IL-6 levels, subsequently, increasing the risk of bacterial infection. Therefore, additional infection control measures in training halls and gymnasiums and healthy diet including immune-boosting vitamins and elements as Zinc are required for high-intensity training athletes.
Futuristic studies including human studies with additional measurements of IL-6 mRNA expression levels, Cortisol, WBCs functions and other immune markers in the setting of physical activities may contribute to better understanding of the immunological role of IL6 during physical exercises.
Acknowledgments
The authors would like to thank the MMPME committee for elective courses and research, for their continued assistance throughout research conduct. We would like also to acknowledge the valuable support provided by Mansoura Medical Experimental Research Centre (MERC) administration, team of veterinarians, biochemists. Finally, we would like to thank the laboratory team of department of Microbiology and Immunology for providing assistance and consultation in some lab procedures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78.
- Zídek Z, Anzenbacher P, Kmonícková E. Current status and challenges of cytokine pharmacology. Br J Pharmacol. 2009;157(3):342–361.
- Suzuki K, Yamada M, Kurakake S, et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81(4):281–287.
- Nieman DC. Immune response to heavy exertion. J Appl Physiol. 1997;82(5):1385–1394.
- Gill S, Hankey J, Wright A, et al. The Impact of a 24-h Ultra-Marathon on Circulatory Endotoxin and Cytokine Profile. Int J Sports Med. 2015. DOI:10.1055/s-0034-1398535
- Nieman DC. Is infection risk linked to exercise workload? Med Sci Sports Exerc. 2000;32(7):S406–S411.
- Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48.
- Suzuki K. Cytokine Response to Exercise and Its Modulation. Antioxidants. 2018;7(1):17.
- Zehsaz F, Farhangi N, Monfaredan A, et al. IL-10 G-1082A gene polymorphism and susceptibility to upper respiratory tract infection among endurance athletes. J Sports Med Phys Fitness. 2015;55(1–2):128–134.
- Vento PJ, Swartz ME, Martin LB, et al. Food intake in laboratory rats provided standard and fenbendazole-supplemented diets. J Am Assoc Lab Anim Sci. 2008;47(6):46–50.
- Xia L, Li M, Zhang Y, et al. Exhaustive Exercise Does Not Affect Humoral Immunity and Protection after Rabies Vaccination in a Mouse Model. Virol Sin. 2018;33(3):241–248.
- Pattamaprapanont P, Muanprasat C, Soodvilai S, et al. Effect of Exercise Training on Signaling of Interleukin-6 in Skeletal Muscles of Type 2 Diabetic Rats. Rev Diabet Stud. 2016;13(2–3):197–206.
- Kalinichenko LS, Koplik EV, Pertsov SS. Cytokine profile of peripheral blood in rats with various behavioral characteristics during acute emotional stress. Bull Exp Biol Med. 2014;156(4):441–444.
- Fuller DD, Davis TE. Comparison of BACTEC plus Aerobic/F, Anaerobic/F, Peds Plus/F, and Lytic/F media with and without fastidious organism supplement to conventional methods for culture of sterile body fluids. Diagn Microbiol Infect Dis. 1997;29(4):219–225.
- Rose-John S. Interleukin-6 Family Cytokines. Cold Spring Harb Perspect Biol. 2018;10(2):a028415.
- Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
- Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R987–R991.
- Amin MN, El-Mowafy M, Mobark A. Naglaa Abass and Abdelaziz Elgaml. Exercise-induced downregulation of serum interleukin-6 and tumor necrosis factor-alpha in Egyptian handball players. Saudi J Biol Sci. 2021;28(1). DOI:10.1016/j.sjbs.2020.10.065
- Suzuki K, Nakaji S, Kurakake S, et al. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc Immunol Rev. 2003;9:48–57.
- Goh JM, Lim CL, Suzuki K. Effects of endurance-, strength-, and concurrent training on cytokines and inflammation. Concurr Aerobic Strength Train. 2019: 125–138.
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406.
- Peake JM, Della Gatta P, Suzuki K, et al. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25.
- Thomson AW. The cytokine handbook. Third ed. California: Academic Press; 1998.
- Soares V, Silveira de Avelar I, Espíndola Mota Venâncio P, et al. Acute Changes in Interleukin-6 Level During Four Days of Long-Distance Walking. J Inflamm Res. 2020;13(10):871–878.
- Keller C, Steensberg A, Hansen AK, et al. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol. 2005;99(6):2075–2079.
- Nieman DC. Exercise immunology: nutritional countermeasures. Can J Appl Physiol. 2001;26(S1):S45–S55.
- Simpson RJ, Kunz H, Agha N, et al. Exercise and the Regulation of Immune Functions. Prog Mol Biol Transl Sci. 2015;135:355–380.
- Hume EB, Cole N, Garthwaite LL, et al. A protective role for IL-6 in staphylococcal microbial keratitis. Invest Ophthalmol Vis Sci. 2006;47(11):4926–4930.
- Rosenbloom AJ, Pinsky MR, Bryant JL, et al. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA. 1995;274(1):58–65.
- Shi Y, Shi H, Nieman DC, et al. Lactic Acid Accumulation During Exhaustive Exercise Impairs Release of Neutrophil Extracellular Traps in Mice. Front Physiol. 2019;10:709.
- Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409.
- Albrecht LJ, Tauber SC, Merres J, et al. Lack of Proinflammatory Cytokine Interleukin-6 or Tumor Necrosis Factor Receptor-1 Results in a Failure of the Innate Immune Response after Bacterial Meningitis. Mediators Inflamm. 2016;2016:7678542.
- Silveira MP, Silva Fagundes KK, Bizuti MR, et al. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2021;21(1):15–28.
- Ilbäck NG, Friman G, Crawford DJ, et al. Effects of training on metabolic responses and performance capacity in Streptococcus pneumoniae infected rats. Med Sci Sports Exerc. 1991;23(4):422–427. PMID: 2056899.