ABSTRACT
Erectile dysfunction (ED) is a prevalent male sexual condition marked by the inability to achieve and maintain a satisfactory penile erection for sexual activity. On a global scale, ED is widespread yet often undertreated. While it isn’t life-threatening, it can significantly impact a patient’s psychosocial well-being and the overall quality of life for both the affected individual and their partner. ED can affect men of all age groups, but its incidence tends to rise with advancing age. It may result from physiological, psychological, or hormonal disturbances in the nervous, cardiovascular, endocrine, or reproductive systems. Throughout history, various plant-based products, known as aphrodisiacs (stimulating sexual behavior), have been used to enhance male potency. The quest for such natural remedies continues, as botanical products are preferred for their minimal side effects. Consequently, numerous potent plants and plant-derived products with aphrodisiac properties have been identified and made available commercially. In modern times, extensive research is dedicated to identifying the active compounds in these plants to develop effective treatments for ED. This review seeks to compile and discuss various potent plants and plant-derived products that possess scientific credibility and have demonstrated effectiveness in addressing erectile dysfunction.
Introduction
Infertility is the term used to describe the condition when a year of repeated unprotected sexual activity does not result in conception. Globally, the World Health Organization estimates that approximately 8–12% of couples experience infertility, with male factors contributing to 50% of infertility cases [Citation1]. Male sexuality is regulated by a complex physiological mechanism that significantly impacts one’s overall quality of life. This mechanism involves the neurological, circulatory, and endocrine systems [Citation2,Citation3]. Any alterations in these systems, whether physical, psychological, or hormonal, can lead to sexual dysfunction. Conditions such as Parkinson’s disease, cardiovascular disorders, and diabetes frequently lead to sexual dysfunction [Citation4,Citation5]. Consequently, sexual dysfunction, rather than being a singular disorder, is a multifaceted issue that affects the quality of a typical life [Citation6–8].
One of the most prevalent yet undertreated sexual disorders is erectile dysfunction (ED). The National Institute of Health defines ED as the inability of the penis to achieve or maintain sufficient rigidity for a satisfactory sexual experience [Citation9]. ED is closely associated with the arterial blood vessels within the endothelial lining of the corpora cavernosa in the penis and indirectly linked to cardiovascular diseases [Citation10]. Although ED is not a life-threatening condition, it has a substantial impact on overall health, given its strong connection to serious medical conditions that can harm psychological well-being. ED significantly affects both the patient and their partner’s quality of life [Citation11]). According to the Massachusetts Male Ageing Study, more than half of individuals aged 40 to 70 experience ED [Citation12]. Globally, it is estimated that approximately 150 million people are affected by this condition, and by 2025, that number is expected to rise to around 320 million [Citation13,Citation14].
A comprehensive investigation into the incidence of ED revealed that 1–10% of males under 40 years of age experience ED. In the 40–49 age group, ED affects 2% to 9% of males. The prevalence of ED in the 60–69 age group ranges from 20% to 40%, and in males over 70, it can affect anywhere from 50% to 100% [Citation15–18]. The significance and widespread impact of ED are apparent as it affects a broad cross-section of the population, irrespective of age, posing a threat to the quality of life for both individuals and their partners.
In India, there is a notable scarcity of research on erectile dysfunction (ED) due to its tendency to be ignored or overlooked, primarily because of the stigma attached to it. Regrettably, prevailing attitudes toward ED remain largely unchanged. Younger individuals often assume that the issue will spontaneously resolve over time, while the older generation tends to regard it as an inevitable consequence of aging. However, these perspectives fail to acknowledge the clinical intricacies associated with this condition. According to one cross-sectional study, approximately 41% of ED cases in India were reported to occur in individuals aged 30–39 years [Citation19].
Mechanism of penile erection
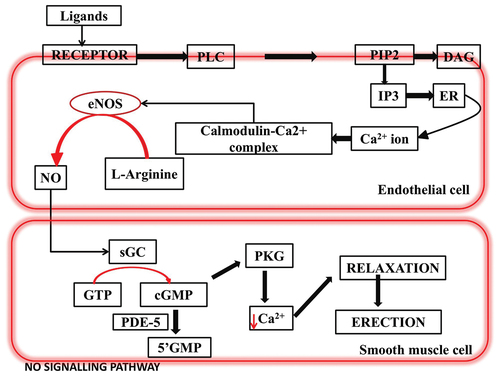
An individual’s sexual function is a highly intricate process that requires the harmonious coordination of multiple bodily systems, encompassing psychological, endocrinological, vascular, neurological, and circulatory aspects. This biopsychosocial process plays a pivotal role in an individual’s overall health and capacity for reproduction [Citation20]. Central to this process is the neurovascular mechanism of penile erection, which involves a complex interplay of hormones, neurotransmitters, and the sympathetic and parasympathetic nervous systems, with a key player being the vasodilator nitric oxide (NO) [Citation21]. NO, primarily synthesized by an enzyme located in the endothelium, serves as a major vasodilator in the penile erection mechanism [Citation22]). It exerts control over vascular wall function as a noncholinergic and noradrenergic vasodilatory neurotransmitter [Citation23].
Sexual arousal triggers the NO pathway through the dopamine-oxytocin-NO neural network, leading to the relaxation of cavernosal smooth muscles and the subsequent compression of subtunical veins in response to NO, which results in an increase in cyclic guanosine monophosphate (cGMP) (see ). NO further modulates the activity of Ca2+ and K+ channels on the cell membrane of the corpus cavernosum, ultimately leading to enhanced blood flow. Consequently, a substantial volume of blood is directed into the sinuses of the corpus cavernosum, impeding local venous return and causing penile erection [Citation24–28].
On the other hand, penile detumescence occurs as a result of adrenergic receptor activation in the arteries of the corpus cavernosum and the trabecular smooth muscles. This activation diminishes arterial blood flow and causes a collapse in the lacunar space, leading to the relaxation of the cavernous body’s drainage venules and, subsequently, the cessation of the erection [Citation20]. Therefore, it’s worth noting that the release of NO plays a crucial role in promoting penile erection. Testicular hormones also contribute to the augmentation of penile blood supply. Additionally, NO is involved in quality control of sperm and actively participates in spermatogenesis. Conversely, a reduction in NO activity could potentially be a factor contributing to erectile dysfunction [Citation24–26,Citation29,Citation30].
Pathophysiology and causes of ED
As previously outlined, typical sexual function is a multifaceted, well-coordinated process that relies on the interplay of psychological, endocrinological, vascular, and neurological systems. Consequently, erectile dysfunction (ED) in an individual can be classified into distinct categories: psychogenic, organic (which encompasses causes such as hormonal, arterial, cavernous, or drug-induced factors), or a combination of both psychogenic and organic causes. In certain cases, ED may be attributed to a blend of psychological and organic factors.
Psychogenic ED
Psychological well-being plays a central role in regulating normal sexual function. Various factors tied to the psychological aspects of erectile dysfunction (ED) have been identified, encompassing developmental, emotional, interpersonal, and cognitive variables. Psychogenic ED primarily arises from factors like relationship stress, performance anxiety, or other psychological disorders [Citation31,Citation32]. Severe mental health conditions, particularly when they result from a long-standing organic issue, can influence both erectile function and sexual desire. A healthy psychological state can assist patients in managing feelings of anxiety and depression, which is pivotal for controlling their sexual behavior. Prolonged negative psychological experiences can significantly inhibit one’s sexual life. While organic dysfunction is a significant contributor to sexual dysfunction (SD), it’s important to note that psychological issues are prevalent in nearly all patients experiencing SD, including conditions like ED, sensory dysfunction, and organic dysfunction [Citation33].
Organic ED
Organic ED is further divided into neurological or neurogenic, endocrinological, vascular, cavernosal, or drug-induced.
Neurogenic ED
To achieve proper erectile function, a healthy central, peripheral, and autonomic nervous system is essential [Citation34]). Contrary to the roles of monoamine neurotransmitters observed in animal models, research involving humans has highlighted the critical influence of these transmitters on sexual desire [Citation35]. In men with untreated hypertension, sexual dysfunction (SD) may occur due to the involvement of monoamine pathways within the central nervous system. This mechanism could potentially explain the higher prevalence of impotence in unmanaged hypertensive individuals compared to those with normal blood pressure. Moreover, antihypertensive medications that impact neurotransmitters, both centrally and peripherally, can negatively affect libido and alter the neural regulation of erectile mechanisms, consequently leading to SD [Citation36]. It remains uncertain whether this lack of impact is a result of temporary drug exposure or if hypertensive patients inherently encounter difficulties in achieving penile erection [Citation37].
Disruptions to the spinal cord, whether due to injury, surgery, or trauma, can lead to a disorientation of the overall sexual function process. Erectile dysfunction (ED) is also associated with various neurological conditions, including Alzheimer’s disease, Parkinson’s disease, stroke, and multiple sclerosis [Citation38]).
Vasculogenic ED
A harmonious balance between the blood entering and exiting the penile veins is essential for the occurrence of a healthy penile erection. When a sequence of neurovascular events triggers an increased blood flow into the penis, an erection is initiated [Citation39] [Citation40]. Consequently, any physiological or pathological changes that lead to injuries or disturbances affecting the hemodynamics of the corpus cavernosa can result in erectile dysfunction (ED) [Citation41].
Furthermore, various associated factors can contribute to the development of penile arterial insufficiency. These factors encompass conditions such as high blood pressure, smoking, hyperlipidemia, arterial atherosclerosis, pelvic irradiation, and diabetes mellitus [Citation42]. These conditions can interfere with the normal blood flow required for a healthy penile erection
Hormonal ED
Hormones are integral to a closed-loop feedback system that plays a pivotal role in the optimization of sexual function. In male sexual dysfunction (SD), the reproductive regulatory system, comprising the hypothalamus-pituitary and intratesticular hormones, is a significant contributor. The secretion of FSH (Follicle-Stimulating Hormone) by anterior pituitary is instrumental in testicular development. FSH also regulates the quantity of spermatogenic cells, the binding of androgen binding protein (ABP) to testosterone, and the testosterone levels within spermatogenic cells [Citation3,Citation43].
In the penis, the expression of PDE5 (Phosphodiesterase 5) and NO synthase is influenced by the hormone testosterone. Testosterone, a vital steroid hormone in men, exerts a profound influence on libido and spermatogenesis. Maintaining standard testosterone levels is crucial for the preservation of secondary sexual characteristics and a healthy sex drive. Testosterone stimulates sperm maturation and the synthesis of proteins in the reproductive organs and muscles. Any deviations or deficiencies in hormonal levels can lead to a decrease in sexual desire, subsequently resulting in sexual dysfunction, erectile dysfunction (ED), and a decline in men’s reproductive capacity [Citation43,Citation44]. Both primary and secondary hypogonadism have been linked to ED. Prolactin also plays a role in sexual function. As prolactin levels rise, it inhibits gonadotropin-releasing hormones, leading to a decrease in luteinizing hormone, which is crucial for testosterone synthesis [Citation45,Citation46].
To develop effective strategies for enhancing or treating infertility, further research into the roles of hormones in spermatogenesis and libido is essential.
Drug-induced ED
Medications can also have a significant impact on an individual’s sexual health, potentially leading to complications such as erectile dysfunction (ED). Among the common drugs associated with the development of ED are psychotropic medications and antihypertensive drugs like thiazides and statins. It can be challenging to differentiate between sexual dysfunction caused by medications and that caused by other underlying medical conditions, anxiety, or depression. Antidepressants and antipsychotics, including drugs like risperidone and olanzapine, have also been linked to varying degrees of erectile dysfunction [Citation41,Citation47–49].
Medications that block cholinergic transmission can potentially impact the parasympathetic branch of the autonomic nervous system. This interference may result in inadequate relaxation of smooth muscle in the corpora cavernosa, which could disrupt the normal sequence of events, leading to increased blood flow through the arteries while simultaneously compressing the veins. Sedation, mood alterations, and decreased libido are potential side effects of these centrally acting drugs [Citation50].
Mechanisms underlying the pathophysiology of erectile dysfunction
Phosphodiesterase 5 enzyme activity
Penile erection is a response to sexual arousal, which initiates with the activation of the nitric oxide (NO) signaling pathway. This activation results in the production of a second messenger, 3′-5′-cyclic guanosine monophosphate (cGMP). cGMP, in turn, phosphorylates essential proteins required for muscle relaxation. Additionally, cGMP reduces the concentration of intracellular calcium ions (Ca2+). The relaxation of the trabecular and arterial smooth muscles, facilitated by these processes, enhances blood flow to the penis, leading to a firmer and erect state (see ) [Citation24–27,Citation51].
Figure 2. Mechanisms underlying the pathophysiology of erectile dysfunction. Here the blue arrows represent the stimulatory pathways and the red arrows represent the inhibitory pathways.
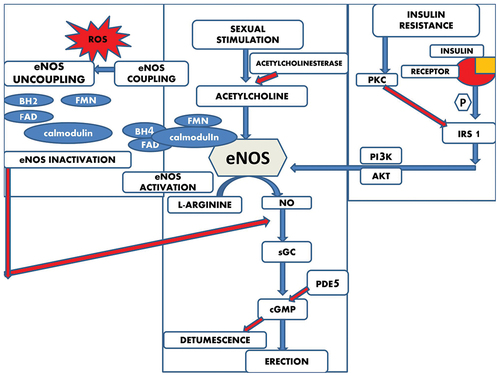
However, the phosphodiesterase 5 (PDE5) enzyme exerts inhibitory feedback on cGMP, causing the constriction of trabecular and arterial smooth muscles, which results in penile detumescence. The reduction in cGMP levels due to PDE5’s catalytic activity is a significant factor in the development of erectile dysfunction (ED). Therefore, inhibiting the catalytic activity of PDE5 leads to increased cGMP levels and, subsequently, penile erection [Citation52].
Nitric oxide synthase uncoupling
Oxidative stress occurs when there is an imbalance between the levels of reactive oxygen species (ROS) and antioxidants [Citation53]. In general, co-factors like tetrahydrobiopterin (BH4), flavin adenine dinucleotide, flavin mononucleotide, and calmodulin are associated with endothelial nitric oxide synthase (eNOS). These co-factors work in conjunction with molecular oxygen to produce nitric oxide (NO) using the substrate L-Arginine.
However, in the presence of oxidative stress, BH4 becomes oxidized to BH2 (dihydrobiopterin), which leads to the decoupling of eNOS (see ). This uncoupling results in eNOS producing more superoxide anions instead of NO, causing a decrease in the amount of NO. These superoxide anions then combine with other reactive oxygen species (ROS) to generate the stable oxidant known as peroxynitrite (ONOO-). This, in turn, leads to increased oxidative stress and ultimately contributes to the development of erectile dysfunction (ED) [Citation54].
Insulin signalling pathway
Insulin plays a crucial role in regulating endothelial cell function. When insulin binds to an insulin receptor on these cells, it initiates a series of events. This includes the phosphorylation of insulin receptor substrate 1 (IRS-1). Phosphorylated IRS-1 then triggers downstream proteins to become phosphorylated, ultimately leading to the activation of endothelial nitric oxide synthase (eNOS). Activated eNOS is responsible for the synthesis of nitric oxide (NO), which, in turn, leads to vasorelaxation, the relaxation of blood vessels.
However, in cases of insulin resistance, there is a disruption in this process. Insulin resistance leads to the activation of protein kinase C (PKC), which subsequently reduces the phosphorylation of IRS and inhibits the activity of downstream protein kinase PI3K. As a result, the production of NO is inhibited, which can contribute to the development of erectile dysfunction (ED) [Citation55] ().
Management of ED through herbal therapy
Various medications are available for the management of erectile dysfunction (ED). Sildenafil citrate, tadalafil, and vardenafil are among the most commonly prescribed ED medications. These drugs function by inhibiting the enzyme phosphodiesterase isotype 5 (PDE5), leading to vasodilation of arteries, which in turn reduces blood pressure and enhances coronary blood circulation [Citation56,Citation57]. The inhibition of PDE5 by these inhibitors induces smooth muscle relaxation by preventing the degradation of cyclic GMP, resulting in sustained blood flow and the ability to achieve a penile erection. However, there have been reports of severe adverse effects associated with the long-term use of these medications [Citation57].
Due to concerns about side effects, the cost of treatment, and an increased awareness among individuals, there is a growing demand for natural remedies to manage various sexual dysfunctions. Numerous research studies have explored the potential use of nutraceuticals as an alternative approach to effective ED management [Citation58,Citation59]. Plant-based products are considered a safer option compared to commercially available pharmaceuticals, as they tend to have very few, if any, unwanted side effects [Citation60]. In the modern age, people are increasingly conscious of the products they use, and many individuals prefer organic natural remedies for their health concerns [Citation61].
Nature has bestowed upon us an invaluable treasure trove of medicinal plants, and the discovery of their therapeutic properties marks the inception of modern medicine. The utilization of these natural resources has enabled the laboratory synthesis of chemical compounds, facilitating the production of therapeutic substances at an accelerated pace [Citation62]). Yet, it remains astonishing that only a mere 1% of these natural medicinal resources have been explored to date. Consequently, scientists are now delving into the investigation of historical medical databases based on ancient texts, traditional practices, and experience-based claims to tap into this vast reservoir of knowledge [Citation60]. Plant products are known to have several beneficial effects on blood flow such as it decreases cholesterol in the blood [Citation63], maintains the enzymes necessary for glutathione (an important antioxidant) metabolism [Citation64], and antioxidant activities [Citation65].
This review represents a humble attempt to delve into the existing literature in order to gain insights into the use of medicinal plants for the management of sexual dysfunction. It explores some of the plants that have been reported to be effective in addressing erectile dysfunction (ED).
The information presented in this review was gathered from online search databases, including Google Scholar and PubMed. The search utilized specific key terms, such as ‘plant aphrodisiac’, ‘male infertility’, and ‘fertility-enhancing plants’. Only articles that exclusively described plants and their derivatives as aphrodisiacs or fertility enhancers were included in this review. Moreover, the articles were limited to studies conducted on male subjects. Due to the limited number of relevant articles, this review considered articles published from the 1990s onward to ensure the inclusion of a sufficient body of research in the analysis.
Allium tuberosum Rottler ex Spreng. (family: Alliaceae)
Allium tuberosum is widely used in cooking and is a common ingredient in many dishes. It contains several major constituents, including alkaloids, steroidal saponins, and sulfur-containing compounds. In a study, it was demonstrated that the butanol extract of its seeds exhibited notable aphrodisiac activity, leading to improved sexual behavior in the individuals who were treated with it [Citation66]. Additionally, in vitro and in vivo studies of A. tuberosum have shown that this plant enhances sexual activities by inducing vasorelaxation in the smooth muscles of the corpus cavernosum [Citation29].
Alpinia calcarata Roscoe (family: Zingiberaceae)
Alpinia calcarata is a rhizomatous perennial herb known for its diverse range of properties, including antibacterial, antioxidant, anti-inflammatory, anti-diabetic, and aphrodisiac activities. Phytochemical analysis of this plant extract has revealed the presence of alkaloids, flavonoids, tannins, polyphenols, and steroid glycosides [Citation67]. Studies have investigated the impact of this plant on male sexual competence and fertility. The research found that the hot water extract of the rhizome, when used at increasing concentrations, resulted in elevated testosterone levels and promoted rapid penile erections [Citation68].
Anacyclus pyrethrum (L.) (family: Asteraceae)
This perennial herb has a long history of medicinal use, with therapeutic potential found in its flowers and roots. Phytochemical analysis of the leaves, flowers, and roots has revealed the presence of catechic tannins, alkaloids, and reducing compounds. Additionally, the extract of this plant contains triterpene compounds, mucilaginous substances, coumarins, and garlic tannins. The roots of this plant are considered aphrodisiacs, attributed to the presence of bio-active compounds known as N-alkylamines. The roots have been found to contain nearly seven Alkylamides [Citation69,Citation70].
In an experiment examining the aphrodisiac qualities of this plant’s extract, it was observed that the experimental model displayed improved sexual and orientation behavior, penile erections, and other key parameters related to sexual well-being. Furthermore, the animal model exposed to this plant extract showed an increase in sperm count and an elevation in fructose concentration in a dose-dependent manner [Citation71]).
Bulbus natalensis Baker (family: Asphodelaceae)
Bulbus natalensis (Baker) is an evergreen plant that lacks a stem. Phytochemical analysis of this plant has revealed the presence of various compounds, including saponins, cardiac glycosides, tannins, alkaloids, and anthraquinones. Studies have demonstrated that the aqueous extract of this plant significantly improves intromission and mounting frequency, as well as hormonal levels of luteinizing hormone (LH) and testosterone. It also enhances parameters such as penile erection and sexual behavior. The aphrodisiac properties of this plant are highly dependent on the dosage relative to body weight. The extract of this plant exerts its aphrodisiac effects by either dilating the blood vessels of the reproductive organs, increasing androgen production, or a combination of both of these effects [Citation72].
Butea frondosa Koenig ex Roxb (family: Fabaceae)
Butea frondosa is a tall, deciduous tree known for its significant aphrodisiac activity. This plant has a long history of use as a remedy for erectile dysfunction and various male sexual disorders. A dose-dependent effect was observed with the aqueous extract of the plant’s bark, resulting in a promising improvement in intromission and mounting frequency. It also led to a decrease in ejaculatory latency and a substantial reduction in intromission and mounting latency and refractory interval in the male animal model. Additionally, when rats consumed a methanolic extract of the plant’s bark, regardless of their age, they engaged in more sexual activity. This enhanced sexual activity was attributed to the potential inhibition of RHO-kinase II by the plant bark, along with an increase in the smooth muscle-to-collagen ratio in the penile tissue of male rats. Both young and old rats exhibited increased sperm production and a reduction in the number of defective sperm [Citation73].
Chlorophytum borivilianum Santapau & Fernandes (family: Liliaceae)
Chlorophytum borivilianum, commonly known as Safed Musli, is a traditional Indian herb that is considered rare and is critically endangered. It has a long history of use in traditional Indian literature, which also mentions its aphrodisiac properties. This herb is highly effective as an aphrodisiac, revitalizer, and spermatogen. Researchers have identified various compounds in its roots, including saponins, triterpenoids, sapogenins, fructans, calcium, magnesium, potassium, and polysaccharides, along with mucilage [Citation74]). Numerous studies involving plant extracts have demonstrated their effectiveness in improving the sexual behavior of animals used in experiments. This plant is recommended for increasing sexual desire, delaying ejaculation, increasing sperm count, and treating erectile dysfunction [Citation75,Citation76].
Curculigo orchioides Gaertn (family: Amaryllidaceae)
Curculigo orchioides, also known as kali musli, is a traditional herb in the Indian system of medicine. It has been utilized in various formulations for metabolism enhancement and as an aphrodisiac. The rhizome of this plant is known for its aphrodisiac properties and possesses immune-stimulant, anti-diabetic, and hepato-protective properties [Citation77]. Research on the ethanolic extract of the rhizomes has shown a significant improvement in sexual behavior, including the stiffness of the erect penis, mount frequency, and mating performance. It has also demonstrated an anabolic effect, resulting in increased weight of the reproductive organs and improved spermatogenic potential in animal models. The rhizome extract has a substantial positive impact on sexual behavior and overall fertility in male animals [Citation78,Citation79].
Dactylorhiza hatagirea D. Don (family: Orchidaceae)
Dactylorhiza hatagirea is a valuable orchid known for its exceptional medicinal properties. The primary components of its tubers include starches, albumens, glucosides, mucilages, ash, and a trace amount of volatile oil [Citation80]. Studies have examined the effects of this plant tubers on spermatogenesis and various parameters associated with sexual behavior. These studies have reported an increase in body weight and the weight of reproductive organs. Clinical data also suggest that the tubers of this plant can elevate testosterone levels in adult males, which, in turn, may lead to increased sexual desire and arousability [Citation81].
Eurycoma longifolia Jack (family: Simaroubaceae)
Eurycoma longifolia is a tall, slender, evergreen plant that is commonly found in Southeast Asia. It is important to note that this species is protected in the region. In sexually active male rats, Elongifolia has been shown to stimulate orientation behavior toward receptive females. The extract of roots and bark of this plant are traditionally used to treat erectile dysfunction and various other sexual issues. Additionally, it has been observed that the accessory reproductive glands, such as the seminal vesicles and prostate, increase in size when compared to control groups [Citation51,Citation82,Citation83].
Fadogia agrestis schweinf. ex Hiern (family: Rubiaceae)
Fadogia agrestis is a shrub that is primarily found in the Nigerian region and is widely used as an aphrodisiac in the management of erectile dysfunction (ED). The plant’s phytochemical constitution is mainly composed of saponins and alkaloids, with minor components including flavonoids and anthraquinones. Studies involving animal models have shown that extracts from this plant can significantly alter sexual behavior patterns, leading to an increase in mounting frequency. It’s worth noting that this plant extract also affects serum testosterone levels, which could potentially explain the improvement in sexual behavior [Citation84].
Ferulago orientalis (L.) (family: Apiaceae)
Ferulago orientalis, a native member of the Apiaceae family, is primarily distributed in the regions of southwestern Asia and southeastern Europe. In local parlance, it goes by the name ‘kasnisi’ in Turkey. Since ancient times, various species within the Ferulago genus, including F. orientalis, have been employed for a multitude of medicinal purposes, serving as diuretics, expectorants, carminatives, sedatives, antispasmodics, laxatives, antiseptics, analgesics, and stimulants [Citation85,Citation86]. Numerous cross-sectional studies have delved into the in vitro and in vivo research pertaining to the aphrodisiac properties of this plant. An in vitro study focused on erectile dysfunction in rats with streptozotocin-induced diabetes, specifically examining the vasorelaxant attributes of F. orientalis extracts. The findings revealed that the root extract of F. orientalis induced an impressive 98.12% relaxation of the corpus cavernosum (CC) [Citation85]. Furthermore, in a related in vitro investigation, root extracts of F. mughlae and F. sandrasica exhibited substantial CC relaxation rates of 97.80% and 97.55%, respectively [Citation87]. Additionally, an in vivo study explored the impact of extracts from the aerial parts and roots of four Ferulago species on rats with streptozotocin-induced diabetic erectile dysfunction. It was observed that the highest degree of CC relaxation was recorded in the CC responses derived from the methanolic root extract of F. bracteata [Citation88]. Extensive research on various Ferulago species has consistently supported the notion that these plants may hold promise in the management of erectile dysfunction.
Ginseng C.A. Meyer (family: Araliaceae)
Ginseng, often referred to as the ‘King of Herbs’, has a long history of use in traditional Chinese medicine as an aphrodisiac. In animal studies, ginseng has been demonstrated to enhance sexual behavior, including sexual desire and copulation. This property of ginseng can be attributed to the presence of ginsenosides Re and RB1. Additionally, ginseng contains other pharmacologically active constituents such as trilinolein, which is a mixture of linolenic acid and triacylglycerol. Both ginsenosides and trilinolein have been reported to be effective in nitric oxide-mediated vasorelaxation, which is relevant to sexual function [Citation89,Citation90].
Lepidium meyenii Walp (family: Brassicaceae)
Lepidium meyenii, commonly known as Maca, has been recognized as both food and medicine for centuries. It is nutrient-dense, containing ample proteins, amino acids, fats, and essential minerals such as magnesium, calcium, iodine, manganese, among others. Maca also contains various secondary metabolites including alkaloids, steroids, glucosinolates, and isothiocyanates, which contribute to its medicinal properties [Citation91,Citation92]. Maca is renowned for its aphrodisiac properties and its ability to improve fertility and sexual performance. It is also known to possess anti-proliferative properties, increase vitality, and enhance stress tolerance [Citation93]. In a double-masked clinical study involving 50 subjects with mild symptoms of erectile dysfunction, maca extract significantly increased the ‘International Index of Erectile Function-5’ (IIEF-5) as well as the ‘psychological performance-related satisfaction score’ (SAT-P) [Citation94].
Mucuna pruriens (L.) (family: Leguminaceae)
Velvet bean, scientifically known as Mucuna pruriens, has a long history of use in traditional medicine. Its seed extract has been extensively studied for the treatment of erectile dysfunction (ED). The ethanolic extract of M. pruriens seeds has been found to improve sexual performance in both healthy animal models and in models of diabetes-induced ED. Additionally, the extract has demonstrated effectiveness in protecting and restoring penile tissue from oxidative stress in animal models. There are also reports of M. pruriens extract stimulating aphrodisiac activity and potentially reversing spermatogenic loss in infertile men [Citation95].
Tribulus terrestris (L.) (family: Zygophyllaceae)
Tribulus terrestris, also known as Gokshur, has a rich history of use as a single therapeutic agent and as a significant or minor component in various formulations and food compounds. It has been a prominent aphrodisiac in traditional systems of medicine. The extract of this plant is known for its effectiveness against infertility, low sex drive, and erectile dysfunction (ED). Individuals exposed to this plant have reported increased sexual activity. This increased sexual activity is likely a result of androgen stimulation from the testes and the release of nitric oxide from the cavernosal nerve endings [Citation96,Citation97].
Zosima absinthifolia (Vent.) (family: Apiaceae)
Numerous natural remedies, many of which originate from plant sources, have been suggested as potential treatments for male sexual dysfunction and erectile dysfunction, often displaying aphrodisiac properties [Citation60]. Among these remedies is the perennial herb Zosima absinthifolia (Vent.), belonging to the Apiaceae family, and native to regions including Iran, Turkey, Iraq, and other Caucasian nations. Z. absinthifolia exhibits a wide range of pharmacological effects, encompassing antidiabetic, antibacterial, antifungal, antioxidant, anti-inflammatory, and various other attributes [Citation98]. Umbelliferone, a compound found in Z. absinthifolia, has been found to manifest aphrodisiac effects in rats suffering from diabetes-induced erectile dysfunction. Researchers delved into the relaxation responses induced by umbelliferone when nitric oxide synthase (NOS) and cyclic guanosine monophosphate (cGMP) inhibitors were present. They utilized Corpus cavernosum (CC) strips from both control rats and those with diabetes-induced dysfunction. Their investigation uncovered that umbelliferone significantly ameliorated the notably reduced erectile responses observed in diabetic rats compared to the control group [Citation99]. In diabetic rats, umbelliferone demonstrated its potency by enhancing antioxidant levels, inhibiting nitric oxide production and lipid peroxidation, and preventing testicular damage. Moreover, it showed the ability to enhance the expression of peroxisome proliferator-activated receptor gamma (PPARγ) and testicular gonadotropin, along with its receptors and markers of steroidogenesis. Thus, umbelliferone could emerge as a promising preventative measure against testicular damage and the sexual dysfunction associated with persistent hyperglycemia [Citation100].
Some other plants and their products with their mode of action that might be beneficial for the treatment of ED have been depicted in the .
Table 1. List of plants or plant products and the mechanism they target to treat erectile dysfunction.
Conclusion
The intricate interplay of neural, hormonal, vascular, and psychological systems plays a crucial role in maintaining normal reproductive health. Even a minor disruption within any of these systems can potentially lead to dysfunction. Nevertheless, despite the proliferation of synthetic compounds through medical research, the preference for crude natural products remains steadfast among the populace. Natural products carry a distinct advantage over their synthetic counterparts due to their minimal or often non-existent side effects. In our pursuit, we sought out significant and valuable medicinal plants and plant-derived products that have historically been employed to address sexual complications. While these plants have found application in a variety of sexual health issues, it is imperative to emphasize that more comprehensive studies are necessary to unravel the intricate molecular mechanisms that underlie these processes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agarwal A, Baskaran S, Parekh N, et al. Male infertility. Lancet. 2021;397(10271):319–33. doi: 10.1016/S0140-6736(20)32667-2
- Wyllie MG. The underlying pathophysiology and causes of erectile dysfunction. Clin Cornerstone. 2005;7(1):19–26. doi: 10.1016/S1098-3597(05)80045-6
- Chen L, Shi GR, Huang DD, et al. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed Pharmacother. 2019;112:108585. doi: 10.1016/j.biopha.2019.01.046
- Terentes-Printzios D, Ioakeimidis N, Rokkas K, et al. Interactions between erectile dysfunction, cardiovascular disease and cardiovascular drugs. Nat Rev Cardiol. 2022;19(1):59–74. doi: 10.1038/s41569-021-00593-6
- Rahmanian E, Salari N, Mohammadi M, et al. Evaluation of sexual dysfunction and female sexual dysfunction indicators in women with type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019;11(1):1–7. doi: 10.1186/s13098-019-0469-z
- Gerra G, Manfredini M, Somaini L, et al. Sexual dysfunction in men receiving methadone maintenance treatment: clinical history and psychobiological correlates. Eur Addict Res. 2016;22(3):163–175. doi: 10.1159/000441470
- Malviya N, Malviya S, Jain S, et al. A review of the potential of medicinal plants in the management and treatment of male sexual dysfunction. Andrologia. 2016;48(8):880–93. doi: 10.1111/and.12677
- Ujah GA, Nna VU, Agah MI, et al. Effect of quercetin on cadmium chloride‐induced impairments in sexual behaviour and steroidogenesis in male Wistar rats. Andrologia. 2018;50(2):e12866. doi: 10.1111/and.12866
- Teoh JB, Yee A, Danaee M, et al. Erectile dysfunction among patients on methadone maintenance therapy and its association with quality of life. J Addict Med. 2017;11(1):40–46. doi: 10.1097/ADM.0000000000000267
- Goel B, Maurya NK. Aphrodisiac herbal therapy for erectile dysfunction. Archives Of Pharmacy Practice. 2020;11(1).
- Adam DR, Alem MM. Erectile dysfunction: pharmacological pathways with understudied potentials. Biomedicines. 2022;11(1):46. doi: 10.3390/biomedicines11010046
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151(1):54–61. doi: 10.1016/S0022-5347(17)34871-1
- Aytaç MK. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–56. doi: 10.1046/j.1464-410x.1999.00142.x
- Zhang L, Bao B, Guo J, et al. Current status and prospects of diabetes mellitus induced erectile dysfunction: a bibliometric and visualization study. Front Endocrinol. 2023;14:1168744. doi: 10.3389/fendo.2023.1168744
- Pinnock CB, Stapleton AM, Marshall VR. Erectile dysfunction in the community: a prevalence study. Med j Aust. 1999;171(7):353–357. doi: 10.5694/j.1326-5377.1999.tb123691.x
- Braun M, Wassmer G, Klotz T, et al. Epidemiology of erectile dysfunction: results of the ‘cologne male survey’. Int J Impot Res. 2000;12(6):305–311. doi: 10.1038/sj.ijir.3900622
- Nicolosi A, Moreira ED Jr, Shirai M, et al. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61(1):201–6. doi: 10.1016/S0090-4295(02)02102-7
- Nicolosi A, Glasser DB, Kim SC, et al. Sexual behaviour and dysfunction and help‐seeking patterns in adults aged 40–80 years in the urban population of Asian countries. BJU Int. 2005;95(4):609–14. doi: 10.1111/j.1464-410X.2005.05348.x
- Mutha AS. An observational study to evaluate the prevalence of erectile dysfunction (ED) and prescribing pattern of drugs in patients with ED visiting an andrology specialty clinic, Mumbai: 2012-14. J Clin Diagn Res. 2015;9(7):C08. doi: 10.7860/JCDR/2015/14520.6174
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20(1):17–29. doi: 10.1038/sj.ijir.3901581
- Mitidieri E, Cirino G, di Villa Bianca RD, et al. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. doi: 10.1016/j.pharmthera.2020.107493
- Lue TF, Wood AJJ. Erectile dysfunction. N Engl J Med. 2000 Jun 15;342(24):1802–13. doi: 10.1056/NEJM200006153422407
- Souza IL, Ferreira ED, Vasconcelos LH, et al. Erectile dysfunction: key role of cavernous smooth muscle cells. Front Pharmacol. 2022;13:895044. doi: 10.3389/fphar.2022.895044
- Ryan JG, Gajraj J. Erectile dysfunction and its association with metabolic syndrome and endothelial function among patients with type 2 diabetes mellitus. J Diabetes Complications. 2012;26(2):141–7. doi: 10.1016/j.jdiacomp.2011.12.001
- Leoni LAB, Fukushima AR, Rocha LY, et al. Physical activity on endothelial and erectile dysfunction: a literature review. Aging Male. 2014;17(3):125–130. doi: 10.3109/13685538.2014.923836
- Liu C, Lu K, Tao T, et al. Endothelial nitric oxide synthase polymorphisms and erectile dysfunction: a meta-analysis. J Sex Med. 2015;12(6):1319–28. doi: 10.1111/jsm.12896
- Koon CS, Sidi H, Kumar J, et al. The phosphodiasterase 5-inhibitors (PDE-5i) for erectile dysfunction (ED): a therapeutic challenge for psychiatrists. CDT. 2018;19(12):1366–1377. doi: 10.2174/1389450118666170215164747
- Zainol M, Sidi H, Kumar J, et al. Co-Morbid Erectile Dysfunction (ED) and antidepressant treatment in a patient–A management challenge? CDT. 2017;20(2):182–191. doi: 10.2174/1389450118666170315110902
- Tang X, Olatunji OJ, Zhou Y, et al. In vitro and in vivo aphrodisiac properties of the seed extract from Allium tuberosum on corpus cavernosum smooth muscle relaxation and sexual behavior parameters in male Wistar rats. BMC Complement Altern Med. 2017;17(1):1–0. doi: 10.1186/s12906-017-2008-5
- Pruss CM, Twofoot MT, Ross R, et al. The positive impact of exercise on endothelial and sexual dysfunction in sedentary overweight individuals. J Sex Med. 2014; 11:148–149.
- Carson C, Dean J, Wylie M. Management of erectile dysfunction in clinical practice. New York: Springer Medical Publishing; 2006.
- Muneer A, Kalsi J, Nazareth I, et al. Erectile dysfunction. BMJ. 2014;348(jan27 7):g129–g129. doi: 10.1136/bmj.g129
- Aversa A, Bruzziches R, Pili M, et al. Phosphodiesterase 5 inhibitors in the treatment of erectile dysfunction. Curr Pharm Des. 2006;12(27):3467–84. doi: 10.2174/138161206778343046
- Goksu C, Deveer M, Sivrioglu AK, et al. Peripheral atherosclerosis in patients with arterial erectile dysfunction. Int J Impot Res. 2013;26(2):55–60. doi: 10.1038/ijir.2013.35
- Ji S, Zang Z, Ma H, et al. Erectile dysfunction in patients with plaque psoriasis: the relation of depression and cardiovascular factors. Int J Impot Res. 2016;28(3):96–100. doi: 10.1038/ijir.2016.6
- Viigimaa M, Doumas M, Vlachopoulos C, et al. Hypertension and sexual dysfunction: time to act. J Hypertens. 2011;29(2):403–7. doi: 10.1097/HJH.0b013e328342c659
- Gowani Z, Uddin SMI, Mirbolouk M, et al. Vascular erectile dysfunction and subclinical cardiovascular disease. Curr Sex Health Rep. 2017;9(4):305–312. doi: 10.1007/s11930-017-0137-y
- Nehra A, Moreland RB. Neurologic erectile dysfunction. Urologic Clini North Am. 2001;28(2):289–308. doi: 10.1016/S0094-0143(05)70139-7
- Mariya OA, Oleg SM. Correction of hemodynamic and endothelial dysfunction by Telmisartan in hypertensives with heart failure and hyperinsulinemia. J Clin Hypertens. 2013.
- Jackson G. Erectile dysfunction and cardiovascular disease. Arab J Urol. 2013;11(3):212–6. doi: 10.1016/j.aju.2013.03.003
- Aversa A, Rossi F, Francomano D, et al. Early endothelial dysfunction as a marker of vasculogenic erectile dysfunction in young habitual cannabis users. Int J Impot Res. 2008;20(6):566–573. doi: 10.1038/ijir.2008.43
- Jackson G. The importance of risk factor reduction in erectile dysfunction. Curr Sex Health Rep. 2007;4(3):114–117. doi: 10.1007/s11930-007-0012-3
- Algeffari M, Jayasena CN, MacKeith P, et al. Testosterone therapy for sexual dysfunction in men with type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetic Med. 2018;35(2):195–202. doi: 10.1111/dme.13553
- Shi GJ, Li ZM, Zheng J, et al. Diabetes associated with male reproductive system damages: onset of presentation, pathophysiological mechanisms and drug intervention. Biomed Pharmacother. 2017;90:562–74. doi: 10.1016/j.biopha.2017.03.074
- Traish AM, Munarriz R, O’Connell L, et al. Effects of medical or surgical castration on erectile function in an animal model. J Andrology. 2003;24(3):381–7. doi: 10.1002/j.1939-4640.2003.tb02686.x
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. doi: 10.1530/EJE-11-0447
- Thomas A, Woodard C, Rovner ES, et al. Urologic complications of nonurologic medications. Urologic Clini North Am. 2003;30(1):123–31. doi: 10.1016/S0094-0143(02)00111-8
- Do C, Huyghe E, Lapeyre-Mestre M, et al. Statins and erectile dysfunction: results of a case/non-case study using the French Pharmacovigilance System Database. Drug Saf. 2009;32(7):591–7. doi: 10.2165/00002018-200932070-00005
- Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. 2011;26(3):130–40. doi: 10.1097/YIC.0b013e328341e434
- Conaglen HM, Conaglen JV. Drug-induced sexual dysfunction in men and women. Aust Prescr. 2013;36(2):42–45. doi: 10.18773/austprescr.2013.021
- Zanoli P, Zavatti M, Montanari C, et al. Influence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotent male rats. J Ethnopharmacol. 2009;126(2):308–13. doi: 10.1016/j.jep.2009.08.021
- Saikia Q, Hazarika A, Mishra R. A review on the pharmacological importance of PDE5 and its inhibition to manage biomedical conditions. J Pharmacol Pharmacother. 2022;13(3):246–57. doi: 10.1177/0976500X221129008
- Halliwell B. Free radicals and antioxidants–quo vadis? Trends Pharmacol Sci. 2011;32(3):125–30. doi: 10.1016/j.tips.2010.12.002
- Masuku NP, Unuofin JO, Lebelo SL. Promising role of medicinal plants in the regulation and management of male erectile dysfunction. Biomed Pharmacother. 2020;130:110555. doi: 10.1016/j.biopha.2020.110555
- Tabit CE, Chung WB, Hamburg NM, et al. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11(1):61–74. doi: 10.1007/s11154-010-9134-4
- Lui JL, Shaw NM, Abbasi B, et al. Adverse reactions of PDE5 inhibitors: an analysis of the World Health Organization pharmacovigilance database. Andrology. 2023;11(7):1408–1417. doi: 10.1111/andr.13430
- Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96(3):257–80. doi: 10.1111/j.1464-410X.2005.05614.x
- Edo GI, Ugbune U, Ezekiel GO, et al. Medicinal plants used for the treatment of sexual dysfunction; ethnobotanical study and phytochemical analysis. Acta Ecologica Sinica. 2023a. doi:10.1016/j.chnaes.2023.05.008.
- Jikah AN, Edo GI. Moringa oleifera: a valuable insight into recent advances in medicinal uses and pharmacological activities. J Sci Food Agric. 2023;103(15):7343–61. doi: 10.1002/jsfa.12892
- Pratap SA, Rajender S. Potent natural aphrodisiacs for the management of erectile dysfunction and male sexual debilities. Front Biosci. 2012;4(1):167–180. doi: 10.2741/s259
- Edo GI, Samuel PO, Ossai S, et al. Phytochemistry and pharmacological compounds present in scent leaf: a review. Food Chem Adv. 2023c;3:100300. doi: 10.1016/j.focha.2023.100300
- Hassan F, Edo GI, Nwosu LC, et al. An inventory of medicinal plants used as sedative, analgesic and blood tonic in Abeokuta, Ogun State, Nigeria. Acta Ecologica Sinica. 2023;43(3): 459–68 .
- Edo GI, Samuel PO, Jikah AN, et al. Proximate composition and health benefit of Roselle leaf (Hibiscus sabdariffa). Food Chem. 2023b;3:100437. doi: 10.1016/j.focha.2023.100437
- Onyibe PN, Edo GI, Nwosu LC, et al. Effects of vernonia amygdalina fractionate on glutathione reductase and glutathione-S-transferase on alloxan induced diabetes wistar rat. Biocatal Agric Biotechnol. 2021;36:102118. doi: 10.1016/j.bcab.2021.102118
- Nwosu LC, Edo GI, Özgör E. The phytochemical, proximate, pharmacological, GC-MS analysis of Cyperus esculentus (Tiger nut): A fully validated approach in health, food and nutrition. Food Biosci. 2022;46:101551. doi: 10.1016/j.fbio.2022.101551
- Guohua H, Yanhua L, Rengang M, et al. Aphrodisiac properties of Allium tuberosum seeds extract. J Ethnopharmacol. 2009;122(3):579–82. doi: 10.1016/j.jep.2009.01.018
- Rahman MA, Islam MS. Alpinia calcarata Roscoe: A potential phytopharmacological source of natural medicine. Pharmacogn Revi. 2015;9(17):55. doi: 10.4103/0973-7847.156350
- Ratnasooriya WD, Jayakody JR. Effects of aqueous extract of Alpinia calcarata rhizomes on reproductive competence of male rats. Acta Biol Hung. 2006;57(1):23–35. doi: 10.1556/ABiol.57.2006.1.3
- Subasri G, John SA. Screening of phytochemical compounds, trace metals and antimicrobial activity of anacyclus pyrethrum. Int J of Adv in Sci Res. 2016;2(1):32–37. doi: 10.7439/ijasr.v2i1.2891
- Cherrat A, Amalich S, Regragui M, et al. Polyphenols content and evaluation of antioxidant activity of anacyclus pyrethrum (L.) lag. From timahdite a Moroccan Middle Atlas region. Int J Adv Res. 2017;5(3):569–77. doi: 10.21474/IJAR01/3546
- Sharma V, Thakur M, NS C, et al. Effects of petroleum ether extract of anacyclus pyrethrum DC. On sexual behavior in male rats. Zhong xi yi jie he xue bao=. J Chin Integr Med. 2010;8(8):767–773. doi: 10.3736/jcim20100807
- Yakubu MT, Afolayan AJ. Effect of aqueous extract of bulbine natalensis (Baker) stem on the sexual behaviour of male rats. Int JAndrology. 2009;32(6):629–36. doi: 10.1111/j.1365-2605.2008.00910.x
- Ramachandran S, Sridhar Y, Sam SK, et al. Aphrodisiac activity of Butea frondosa Koen. ex Roxb. extract in male rats. Phytomedicine. 2004;11(2–3):165–8. doi: 10.1078/0944-7113-00343
- Thakur GS, Bag M, Sanodiya BS, et al. Chlorophytum borivilianum: a white gold for biopharmaceuticals and neutraceuticals. CPB. 2009a;10(7):650–666. doi: 10.2174/138920109789542084
- Thakur M, Chauhan NS, Bhargava S, et al. A comparative study on aphrodisiac activity of some ayurvedic herbs in male albino rats. Arch Sex Behav. 2009b;38(6):1009–1015. doi: 10.1007/s10508-008-9444-8
- Kenjale R, Shah R, Sathaye S. Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytother Res. 2008;22(6):796–801. doi: 10.1002/ptr.2369
- Thakur M, Chauhan NS, Sharma V, et al. Effect of curculigo orchioides on hyperglycemia-induced oligospermia and sexual dysfunction in male rats. Int J Impot Res. 2012;24(1):31–37. doi: 10.1038/ijir.2011.43
- Chauhan NS. Curculigo orchioides: the black gold with numerous health benefits. Zhong xi yi jie he xue bao=. J Chin Integr Med. 2010;8(7):613–623. doi: 10.3736/jcim20100703
- Chauhan NS, Rao CV, Dixit VK. Effect of curculigo orchioides rhizomes on sexual behaviour of male rats. Fitoterapia. 2007;78(7–8):530–4. doi: 10.1016/j.fitote.2007.06.005
- Thakur M, Dixit VK. Aphrodisiac activity of dactylorhiza hatagirea (D. Don) Soo in male albino rats. Evid Based Complement Alternat Med. 2007;4(s1):29–31. doi: 10.1093/ecam/nem111
- Pant S, Rinchen T. Dactylorhiza hatagirea: a high value medicinal orchid. J Med Plants Res. 2012;6(19):3522–3524. doi: 10.5897/JMPR12.097
- Ang HH, Sim MK. Eurycoma longifolia Jack and orientation activities in sexually experienced male rats. Biol Pharm Bull. 1998;21(2):153–5. doi: 10.1248/bpb.21.153
- Ang HH, Cheang HS, Yusof AP. Effects of Eurycoma longifolia Jack (Tongkat Ali) on the initiation of sexual performance of inexperienced castrated male rats. Exp Anim. 2000;49(1):35–38. doi: 10.1538/expanim.49.35
- Yakubu MT, Akanji MA, Oladiji AT. Aphrodisiac potentials of the aqueous extract of fadogia agrestis (Schweinf. Ex hiern) stem in male albino rats. Asian J Andrology. 2005;7(4):399–404. doi: 10.1111/j.1745-7262.2005.00052.x
- Karakaya S, Yilmaz Oral D, Gur S, et al. Effect of aerial part and root extracts from Ferula orientalis L. growing in Turkey on erectile dysfunction in streptozotocin-ınduced diabetic rats. Biol Divers Conserv. 2019a;12(1):1–6. doi: 10.5505/biodicon.2019.29392
- Badalamenti N, Ilardi V, Rosselli S, et al. The ethnobotany, phytochemistry and biological properties of genus ferulago–A review. J Ethnopharmacol. 2021;274:114050. doi: 10.1016/j.jep.2021.114050
- Karakaya S, Delimustafaoglu-Bostanlik F, YILMAZ ORAL Dİ, et al. Effect of aerial part and root extracts from Ferulago mughlae pe?men and ferulago sandrasica pe?men quézel growing in turkey on erectile dysfunction in streptozotocin-induced diabetic rats. Jrp. 2019b;23(2):235–241. doi: 10.12991/jrp.2019.129
- Karakaya S, Oral DY, GÜ S, et al. Effect of extracts of the aerial parts and roots from four Ferulago species on erectile dysfunction in rats with streptozotocin-induced diabetes. Tjps. 2019c Sep;16(3):317. doi: 10.4274/tjps.galenos.2018.26879
- Dou DQ, Ren J, Chen Y, et al. [Study on the chemical constituents of the roots of commercial ginseng]. Zhongguo Zhong Yao Za Zhi. 2003;28(6):522–524.
- Achike FI, Kwan CY. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin Exp Pharmacol Physiol. 2003;30(9):605–15. doi: 10.1046/j.1440-1681.2003.03885.x
- Li G, Ammermann U, Quirós CF. Contenido de glucosinolatos en semillas de maca, plántulas, plantas maduras y en varios productos derivados. Economic Botany. 2001;2001(2):255–262. doi: 10.1007/BF02864563
- Cui B, Zheng BL, He K, et al. Imidazole alkaloids from Lepidium meyenii. J Natural Prod. 2003;66(8):1101–3. doi: 10.1021/np030031i
- Li J, Chen L, Li J, et al. The composition analysis of maca (Lepidium meyenii Walp.) from Xinjiang and its antifatigue activity. J Food Qual. 2017;2017:1–7. doi: 10.1155/2017/2904951
- Zenico T, Cicero AF, Valmorri L, et al. Subjective effects of Lepidium meyenii (maca) extract on well‐being and sexual performances in patients with mild erectile dysfunction: a randomised, double‐blind clinical trial. Andrologia. 2009;41(2):95–9. doi: 10.1111/j.1439-0272.2008.00892.x
- Duangnin N, Phitak T, Pothacharoen P, et al. In vitro and in vivo investigation of natural compounds from seed extract of Mucuna pruriens lacking l-DOPA for the treatment of erectile dysfunction. Asian Pac J Trop Med. 2017;10(3):238–52. doi: 10.1016/j.apjtm.2017.03.001
- Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of Tribulus Terrestris extract (protodioscin) in normal and castrated rats. Life Sci. 2002;71(12):1385–96. doi: 10.1016/S0024-3205(02)01858-1
- Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncturevine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complementary Med. 2003;9(2):257–65. doi: 10.1089/10755530360623374
- Alikhanova NS, Novruzov EN. Chemical composition and biological activity of zosima absinthifolia (Apiaceae). KazNu Chem Bull. 2022;105(2):34–42. doi: 10.15328/cb1266
- Karakaya S, Yilmaz-Oral D, Kilic CS, et al. Umbelliferone isolated from Zosima absinthifolia roots partially restored erectile dysfunction in streptozotocin-induced diabetic rats. Med Chem Res. 2019d;28(8):1161–7. doi: 10.1007/s00044-019-02359-9
- Allam MA, Khowailed AA, Elattar S, et al. Umbelliferone ameliorates oxidative stress and testicular injury, improves steroidogenesis and upregulates peroxisome proliferator-activated receptor gamma in type 2 diabetic rats. J Pharm Pharmacol. 2022;74(4):573–584. doi: 10.1093/jpp/rgab083
- Ademosun AO, Adebayo AA, Oboh G. Anogeissus leiocarpus attenuates paroxetine-induced erectile dysfunction in male rats via enhanced sexual behavior, nitric oxide level and antioxidant status. Biomed Pharmacother. 2019a;111:1029–35. doi: 10.1016/j.biopha.2019.01.022
- Oboh G, Adebayo AA, Ademosun AO, et al. Aphrodisiac effect of hunteria umbellata seed extract: modulation of nitric oxide level and arginase activity in vivo. Pathophysiology. 2019;26(1):39–47. doi: 10.1016/j.pathophys.2018.11.003
- Fukuhara S, Tsujimura A, Okuda H, et al. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin‐induced diabetic rat: preliminary findings. J Sex Med. 2011;8(4):1061–71. doi: 10.1111/j.1743-6109.2010.02193.x
- Adefegha SA, Oyeleye SI, Dada FA, et al. Modulatory effect of quercetin and its glycosylated form on key enzymes and antioxidant status in rats penile tissue of paroxetine-induced erectile dysfunction. Biomed Pharmacother. 2018;107:1473–9. doi: 10.1016/j.biopha.2018.08.128
- Ogunro OB, Yakubu MT. Fadogia agrestis (Schweinf. Ex hiern) stem extract restores selected biomolecules of erectile dysfunction in the testicular and penile tissues of paroxetine-treated Wistar rats. Reproductive Sciences. 2023;30(2):690–700. doi: 10.1007/s43032-022-01050-6
- Ademosun AO, Oboh G, Adebayo AA, et al. Grapefruit peel extract mitigates paroxetine‐induced erectile dysfunction in rats through stimulation of erectile response, antioxidant status, and inhibition of key enzymes related with impaired penile erection. J Food Biochem. 2022;46(11):e14193. doi: 10.1111/jfbc.14193
- Ademosun AO, Adebayo AA, Oboh G. Orange peels modulate antioxidant markers and key enzymes relevant to erection in the penile tissue of paroxetine‐treated rats. Andrologia. 2019b;51(9):e13371. doi: 10.1111/and.13371
- Adefegha SA, Oboh G, Olopade EO. β-caryophyllene improves sexual performance via modulation of crucial enzymes relevant to erectile dysfunction in rats. Toxicol Res. 2021;37(2):249–260. doi: 10.1007/s43188-020-00061-2
- Martey ON, He X. Possible mode of action of mondia whitei: an aphrodisiac used in the management of erectile dysfunction. J of Pharmacology and Toxicology. 2010;5(8):460–468. doi: 10.3923/jpt.2010.460.468
- Ojo OA, Afon AA, Ojo AB, et al. Spondias mombim L.(Anacardiaceae): chemical fingerprints, inhibitory activities, and molecular docking on key enzymes relevant to erectile dysfunction and Alzheimer’s diseases. J Food Biochem. 2019;43(3):e12772. doi: 10.1111/jfbc.12772
- Ojo OA, Ojo AB, Maimako RF, et al. Exploring the potentials of some compounds from Garcinia kola seeds towards identification of novel PDE‐5 inhibitors in erectile dysfunction therapy. Andrologia. 2021;53(7):e14092. doi: 10.1111/and.14092
- Olawale F, Olofinsan K, Ogunyemi OM, et al. Deciphering the therapeutic role of kigelia africana fruit in erectile dysfunction through metabolite profiling and molecular modelling. IMU. 2023;37:101190. doi: 10.1016/j.imu.2023.101190
- Folawiyo MA, Omotuyi IO, Ajao FO, et al. Catechin from Anonna senegalensis is a potential inhibitor of erectile dysfunction: implication for its use in male sexual enhancement. Appl Biochem Biotechnol. 2023;195(8):4936–4964. doi: 10.1007/s12010-023-04557-z
- Ehigiator BE, Cobhams AE, Adikwu E, et al. Predicting the possible mechanism of aphrodisiac action of Pheonix dactylifera L. J Trop Dis. 2022;10:312.
- Eser N, Buyuknacar HS, Cimentepe OO, et al. The effect of Ferula elaeochytris root extract on erectile dysfunction in streptozotocin-induced diabetic rat. Int J Impot Res. 2020;32(2):186–194. doi: 10.1038/s41443-019-0137-8
- Yilmaz‐Oral D, Onder A, Gur S, et al. The beneficial effect of clove essential oil and its major component, eugenol, on erectile function in diabetic rats. Andrologia. 2020;52(6):e13606. doi: 10.1111/and.13606
- Saikia Q, Hazarika A, Kalita JC. Isoliquiritigenin ameliorates paroxetine-induced sexual dysfunction in male albino mice. Reprod Toxicol. 2023;117:108341. doi: 10.1016/j.reprotox.2023.108341