ABSTRACT
The presence of Enterobacteria in food in large numbers indicates fecal contamination. This study was carried out to determine the susceptibility of Enterobacteriaceae strains isolated from ready-to-eat foods from the Damietta market. A total of 40 random ready-to-eat food samples were collected from different localities at Damietta Governorate, Egypt. These samples were examined for Enterobacteriaceae count, and identification of members by conventional biochemical methods and antimicrobial susceptibility was determined by disc diffusion method. Enterobacteriaceae isolates were detected among 34/40 (85%) examined Ready-to-eat food samples. A total of 139 isolates were identified. The most frequent genus was Klebsiella followed by Enterobacter. Other members such as Citrobacter spp., Proteus spp., Providencia spp., and E. coli spp. were also identified. The isolates showed different susceptibility to 12 tested antimicrobials. Most of the tested isolates showed high resistance percentages to cefepime and ampicillin. Imipenem, ciprofloxacin, amikacin, gentamicin, and cefotaxime were the most effective antimicrobials. In addition, 29% of the isolates were multiple-drug resistant (MDR). The high prevalence of members of Enterobacteriaceae in different food sources is a reliable index of food contamination. So, it is recommended to reconsider restrictions on hygiene and sanitary control in the current food acts and regulations.
Introduction
Ready-to-eat foods can be described as the condition of food being ready for consumption immediately, it could be raw or cooked, and can be consumed without further processing [Citation1]. The increase in the consumption of ready-to-eat fast foods is due to a change in social styles characterized by increased mobility, large numbers of itinerary workers and less family-oriented activities [Citation2]. Good manufacturing practices for foods purchased outside the home, such as appropriate food handling and hygienic measures, have thus been transferred from people and families to food vendors, who infrequently enforce such procedures [Citation3].
In developed countries, the annual percentage of people suffering from infectious diseases transmitted through food reaches 30%, while the problem is likely to be more dramatic in developing countries [Citation4]. A study revealed that more than 2 million people die every year in developing countries due to foodborne diseases, which are among more than 13 zoonoses implicated in over 2 billion illnesses around the world [Citation5]. In Africa, people with gastrointestinal symptoms (diarrhea, vomiting, abdominal cramps) rarely go to health facilities; therefore, the prevalence of food poisoning is underestimated [Citation4]. Over 91 million African people are affected by food-borne diseases according to the report by the World Health Organization [Citation6].
Enterobacteriaceae have medical and economic importance; their presence in large numbers indicates fecal contamination, unqualified processing, and post-processing contamination of food [Citation7]. Enterobacteriaceae members affecting plants and animals worldwide result in huge financial losses, particularly in the food industry [Citation8]. In addition, hospitalizations with Enterobacteriaceae and the need of specific antimicrobial agents are costly [Citation9]. Enterobacteriaceae species (spp.) have been linked to many outbreaks. This family includes a number of important foodborne pathogens such as Salmonella, pathogenic E. coli and Shigella spp. Other family members are considered as opportunistic pathogens in clinical cases (Klebsiella spp. and Citrobacter spp.) [Citation10]. E. coli, Klebsiella pneumoniae, Enterobacter spp. and Citrobacter spp. were involved in gastrointestinal diseases such as gastroenteritis, food poisoning, cholera-like syndrome, diarrhea, in addition to other diseases such as cystitis, pyelonephritis, appendicitis, pyelitis, peritonitis, lobar pneumonia, and septicemia [Citation11]. Escherichia coli O157: H7 is a major cause of food-borne diseases and young dairy cattle are a reservoir for it. Infection with Enterohemorrhagic E. coli (EHEC) strains usually associated with food-borne outbreaks from milk and dairy products, causing hemorrhagic colitis (bloody diarrhea) and hemolytic uremic syndrome in human [Citation7].
Enterobacteriaceae foodborne pathogens such as Salmonella spp., Proteus spp. and some serotypes of E. coli are the leading pathogens that cause food poisoning outbreaks [Citation12]. Also, Klebsiella pneumoniae is most common cause of nosocomial infections and mostly causes pneumonia, urinary tract, central nervous system, hepatic, wound and/or blood infections as well as associated with intestinal infections [Citation13,Citation14]. Salmonella spp. associated with enteric fever in humans and fatal diseases, gastroenteritis, and other extra-intestinal complications. Enterobacter spp. are widely distributed in nature found in the soil, water, dairy products, and in the intestines of humans and animals [Citation15].
The presence of Enterobacteriaceae in dairy products induces undesirable changes rendering the product of inferior quality, unmarketable, and unsuitable for human consumption. Furthermore, their presence is always considered as a reliable index of fecal contamination. Therefore, the presence of Enterobacteriaceae and coliforms are routinely involved in determining the hygienic quality of foods, especially dairy products [Citation16].
In Egypt, the risk of bacterial occurrence in the food chain exists. There have been numerous reports of outbreaks of foodborne illness linked to eating raw or inadequately treated food. Patients in several governorates suffered from serious illnesses that were caused by the highly pathogenic Enterobacteriaceae members that were recovered from dairy and meat products with a high prevalence [Citation17,Citation18].
The present work aims to study the prevalence of Enterobacteriaceae members in different ready-to-eat foods and determine the antimicrobial susceptibility of different isolates.
Materials and methods
Collection of samples
A total of 40 random ready-to-eat food samples were collected from small dairies, groceries, and supermarkets from different localities at Damietta Governorate, Egypt. These samples included: 5 arish cheese, 5 Domiati cheese, 10 yogurt, 16 ice cream and 4 luncheon. Samples were obtained in their containers as sold to the consumer or in sterile containers and dispatched directly to the laboratory in an insulated icebox at 4°C to be examined [Citation19].
This study was approved by the Research Ethics Committee of Faculty of Pharmacy with code (2023 – 183).
Preparation of serial dilution
A weight of 25 g of each prepared sample (ice cream, yogurt, arish cheese, Domiati cheese) was added separately to 225 ml of warm sterile 2% sodium citrate solution (40°C) in sterile polyethylene bag. They were thoroughly homogenized in a stomacher for 3 min to prepare a dilution of 1:10 from which sequential decimal dilutions were prepared in sterile peptone water (Hi Media, India) [Citation20]. For luncheon, 25 g of sample were added to 225 ml of sterile peptone water and homogenized thoroughly by using sterile blender for 2.5 min, from which tenfold serial dilutions were prepared up to 106 [Citation21].
Enterobacteriaceae count
One ml portion from the prepared ten-fold serial dilutions of each sample was carefully transferred into duplicate plates and mixed with 15 ml of Violet-Red Bile glucose agar (VRBG -agar) (Oxoid, UK) using pour-plating technique. The plates were overlaid by 5 ml of the same medium after solidification for ensuring anaerobic condition. The plates were incubated at 37°C for 48 h. Deep purple colonies surrounded by a purple halo were counted, and results were recorded [Citation20].
Isolation and Identification of Enterobacteriaceae organisms
Different colonies were picked up from countable plates and streaked onto nutrient agar (Oxoid, UK) slants and incubated at 37°C for 24 h. A presumptive identification of Enterobacteriaceae isolates were achieved by the following biochemical tests: motility test, indole test, methyl red test, Voges Proskauer test, citrate utilization test, urease test, hydrogen sulfide (H2S) test, nitrate reduction test, gelatin liquefaction test, ornithine decarboxylase test, lysine decarboxylase test, o-nitrophenyl-β-D- galactosidase test and sugar fermentation tests (lactose, sucrose, dulcitol, salicin, arabinose, inositol, xylose), the tests were performed according to Bergey’s manual of determinative bacteriology [Citation22].
Serotyping of E. coli and Klebsiella pneumoniae
The methods were performed at Food Analysis Center, Faculty of Veterinary Medicine, Benha University as the following:
Serotyping of E. coli
Different biochemically confirmed E. coli isolates were serologically identified by rapid diagnostic E. coli antisera sets (DENKA SEIKEN Co., Japan) for diagnosis of different enteropathogenic types [Citation23]. Enteropathogenic identification was performed according to the following steps: two separate drops of saline were placed on a glass slide additionally to one portion of a colony from the suspected culture previously emulsified with the saline solution to give a smooth fairly dense suspension. A loopful of saline was added in order to control suspension and then mixed; another loopful of undiluted antiserum was added to the other suspension and titled back and forward for 1 min. Furthermore, agglutination was observed using indirect lighting over a dark background. When the positive colony agglutination is identified with one of the pools of polyvalent serum, a further portion was inoculated onto a nutrient agar slant and incubated at 37°C for 24 h to allow growing as a culture for testing with mono-valent sera. A heavy bacterial suspension from each slope culture was prepared in saline and is then tested using the diagnostic sera to identify the O-antigen.
Serotyping of Klebsiella pneumoniae
Serological identification of capsular antigen of Klebsiella pneumoniae isolates was performed using Quellung test ‘Neufeld reaction’ [Citation24]. The suspected isolates were serologically identified for antigens K1 and K2. The test was carried out according to the producer instruction using specific antigens purchased from Statens Serum Institute, Copenhagen, Denmark.
Antimicrobial susceptibility tests
According to the disc diffusion method [Citation25,Citation26], 131 out of 139 isolates (species with identified isolates <5 were excluded) were tested for susceptibility to 12 antimicrobial agents according to the criteria set by Clinical and Laboratory Standards Institute (CLSI 2017) [Citation27]. The tested antimicrobial discs were ampicillin (AM), amoxicillin-Clavulanic (AMC), cefazolin (CZ), cefoxitin (FOX), cefotaxime (CTX), cefepime (FEP), gentamicin (CN), amikacin (AK), imipenem (IPM), ciprofloxacin (CIP), tetracycline (TE), and sulfamethoxazole-trimethoprim (SXT), all discs were of Bioanalyze Turkey products. The cultures were prepared by suspending 4–5 identical pure colonies in 5 ml nutrient broth medium (Oxoid, UK) and were incubated at 37°C for 24 h. Following that, the optical density (OD) was adjusted by comparing to 0.5 McFarland suspension standard tube [Citation28]. Inoculation of Muller Hinton agar (Oxoid, UK) plates (4 mm depth) was accomplished by dipping a sterile swab into the bacterial culture and streaking the inoculum across the entire surface of the plates in three different directions to ensure even distribution. After that, the plates were dried for 20 min in a laminar flow hood at room temperature. Later, sterile forceps were used to apply antimicrobial discs (at least 25 mm apart, center to center) over the agar surface. The plates were inverted and incubated overnight at 37°C [Citation29]. By measuring to the nearest millimeter, the diameter of each inhibition zone surrounding discs was recorded. The isolates were reported as resistant, intermediate or susceptible from the respective interpretation charts of CLSI guidelines 2017 (Supplementary table 1) [Citation27].
Results
Enterobacteriaceae isolates were detected among 34 out of 40 (85%) examined ready-to-eat food samples. They were detected in 14 out of 16 (87.5%) examined ice cream samples with a mean count of 3.14 × 102 ± 6.4 x 10 and 8 out of 10 (80%) examined yogurt samples with a mean count of (3.12 x 103 ± 5.32 x 102). Arish cheese showed the lowest percentage of positive Enterobacteriaceae samples (3/5, 60%) with a mean count of (3.16 x 102 ± 1.09 x 102). Enterobacteriaceae were detected in all Domiati cheese and luncheon samples with a mean count of (2.5 x 103 ± 1.96 x 103 and 2.3 × 103 ± 3.39 x 102, respectively) ().
Table 1. Enterobacteriaceae count in the examined ready-to-eat food samples.
Biochemical results
A total of 139 Enterobacteriaceae isolates were obtained from different ready-to-eat food samples as identified by different biochemical tests as shown in . Seven genera comprising 16 species were detected. The most frequent genus was Klebsiella (48 isolates) including 36 Klebsiella pneumoniae, 9 Klebsiella oxytoca, 2 Klebsiella ozaenae, one Klebsiella terrigena, followed by Enterobacter genus (45 isolates) including 19 Enterobacter aerogenes, 12 Enterobacter agglomerans, 11 Enterobacter cloacae, 2 Enterobacter hafniae, and one Enterobacter sakazakii. Fifteen isolates of E. coli were obtained. Proteus genus (13 isolates) included 8 Proteus mirabilis, 5 Proteus vulgaris. Providencia rettgeri (9 isolates), Citrobacter genus (8 isolates including 7 citrobacter freundii and one citrobacter diversus isolates) and Serratia liquefaciens (1 isolate) were the least detected Enterobacteriaceae members.
Table 2. Results of biochemical identification of Enterobacteriaceae members.
Serotyping results
Serotyping results confirmed the presumptive identification of the isolates as the following:
For E. coli, four pathotypes were detected including: Enterohemorrhagic E. coli (EHEC) (8 isolates), Enterotoxigenic E. coli (ETEC) (3 isolates), Enteropathogenic E. coli (EPEC) (2 isolates), Enteroinvasive E. coli (EIEC) (2 isolates). Eight different serotypes were detected. These serotypes included O26:H11 (4 isolates), O127:H6 (3 isolates), O91:H21 (2 isolates), O159 (2 isolates) and only one isolate was detected for O17:H18, O111:H2, O103:H4, O55:H7 serotypes.
K1 and K2 were the only detected serotypes among Klebsiella pneumoniae isolates. K1 serotype was the most predominant among the isolates (30 isolates), 5 isolates were K2 and only one isolate was untypable. Several biotypes (B1:B5) were identified, 25 isolates were B1, 5 isolates were B3, 3 isolates were B4, 2 isolates were B2 and only one isolate was B5.
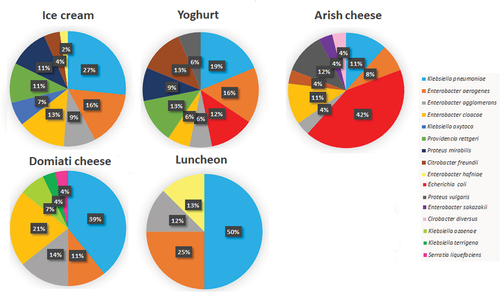
The distribution of Enterobacteriaceae members among the five different food types is shown in . It was found that the highest number of isolates was obtained from ice cream (45 isolates) followed by 32 isolates from yogurt, 28 isolates from Domiati cheese, 26 isolates from arish cheese and only 8 isolates were obtained from luncheon. Isolates of Klebsiella pneumoniae, Enterobacter aerogenes and Enterobacter agglomerans were obtained from all types of food specimens. E. coli and proteus vulgaris isolates were isolated from yogurt and arish cheese specimens. Enterobacter cloacae and Citrobacter freundii isolates were obtained from ice cream, yogurt and arish cheese. Isolates of Providencia rettgeri and Proteus mirabilis were obtained from ice cream and yogurt only.
Table 3. Distribution of Enterobacteriaceae species among different food sources.
The prevalence of different Enterobacteriaceae spp. among food specimens is shown in . Ice cream, yogurt and arish cheese comprised 9 out of 16 identified Enterobacteriaceae spp. Domiati cheese and luncheon contained 7 and 4 spp., respectively. Among ice cream and yogurt isolates, Klebsiella pneumoniae was the highest detected spp. (27% and 19%, respectively) followed by Enterobacter aerogenes (16% per each source). For arish cheese, E. coli was the predominant spp. (42%) followed by Proteus vulgaris (12%). Klebsiella pneumoniae was the highest detected spp. among Domiati cheese (39%) and luncheon isolates (50%).
Antimicrobial susceptibility test of Enterobacteriaceae members
Enterobacteriaceae members showed different susceptibility to 12 tested antimicrobials as shown in . Except for 2.8% of Klebsiella pneumoniae isolates, all the isolates were susceptible to IPM. Most of the tested isolates showed high resistance percentages to FEP and AMP. CIP, AK, CN and CTX were the most effective antimicrobials as low resistance percentages were revealed by most of the isolates. For the other antimicrobials, the identified species showed variable resistance levels.
Table 4. Antimicrobial resistance percentages recorded by different species of Enterobacteriaceae.
Enterobacteriaceae isolates showed 9.2%, 8.4%, 8.4%, 11.5%, 3.05%, 8.4%, 5.3%, 2.3%, 0.7%, 5.3%, 8.4%, 9.9% intermediate resistance to AM, AMC, CZ, FOX, CTX, FEP, CN, AK, IPM, CIP, TE and SXT, respectively.
Various resistance patterns were detected for each species as the following
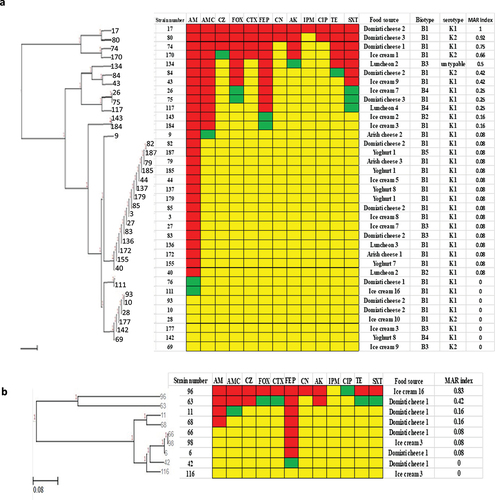
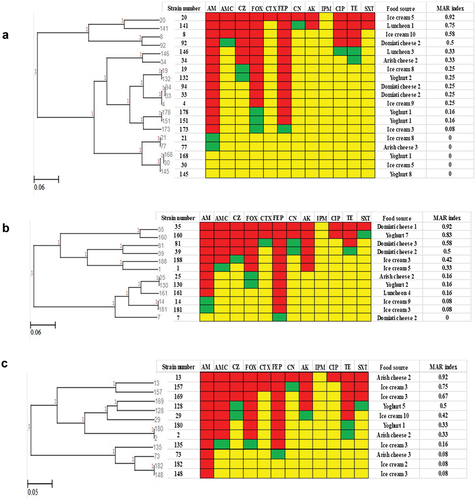
For Klebsiella species, Klebsiella Pneumoniae (36 isolates) showed 14 resistance patterns (). The highest detected resistance pattern included 15 isolates that showed resistance to AMP only. It was noticed that strains 82 and 85 were similar as they were isolated from the same food source (Domiati cheese 2) and have the same biotype and serotype. Also, strains 179 and 185 were identical. Regarding Klebsiella oxytoca (9 isolates), seven resistance patterns were detected (). Three isolates (6, 66, 98) showed the same resistance pattern, they showed resistance to FEP only. Isolates (6, 66) were obtained from the same food source (Domiati cheese 1). The most common multiple antibiotic resistant (MAR) index among Klebsiella spp. was 0.08, however isolate 17 showed the highest MAR index (1).
Figure 2. Antibiogram of Klebsiella species isolates is shown by a Heat map. a: Klebsiella pneumoniae isolates, b: Klebsiella oxytoca isolates, red color: resistant, green color: intermediate, yellow color: sensitive, AM: ampicillin, AMC: amoxicillin-Clavulanic, CZ: cefazolin, FOX: cefoxitin, CTX: cefotaxime, FEP: cefepime, CN: gentamicin, AK: amikacin, IPM: imipenem, CIP: ciprofloxacin, TE: tetracycline, and SXT: sulfamethoxazole-trimethoprim, MAR: multiple antibiotic resistant.
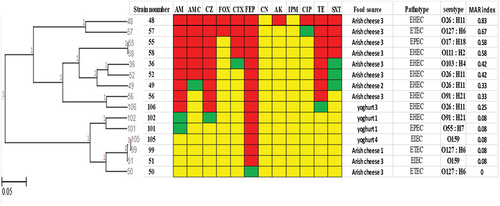
E. coli (15 isolates) revealed 12 resistance patterns (). Isolates (55, 58) showed the same resistance pattern, these isolates were obtained from the same food source but had different pathotypes and serotypes. Another resistance pattern for isolates (51, 99, 105) showed resistance to only one antimicrobial (FEP). They were isolated from different food sources, isolates (51, 105) had the same pathotype and serotype. MAR index for E. coli isolates ranges from 0.08 to 0.83, isolate 48 showed the highest MAR index among E. coli isolates.
Figure 3. Antibiogram of E. coli isolates is shown by a Heat map. Red color: resistant, green color: intermediate, yellow color: sensitive, AM: Ampicillin, AMC: Amoxicillin-Clavulanic, CZ: Cefazolin, FOX: Cefoxitin, CTX: Cefotaxime, FEP: Cefepime, CN: Gentamicin, AK: Amikacin, IPM: Imipenem, CIP: Ciprofloxacin, TE: Tetracycline, SXT: Sulfamethoxazole-Trimethoprim. EHEC: Enterohemorrhagic E. coli, ETEC: Enterotoxigenic E. coli, EPEC: Enteropathogenic E. coli, EIEC for Enteroinvasive E. coli, MAR: multiple antibiotic resistant.
shows the resistance patterns for isolates of different Enterobacter spp. Enterobacter aerogenes (19 isolates) showed 12 resistance patterns (). Three isolates (4, 33, 94) showed the same pattern (resistant to FEP, AMP, FOX), isolates (33, 94) were isolated from the same food source (Domiati cheese 2). Additionally, isolates (151, 178) were obtained from the same yogurt specimen and had the same resistance pattern. Ten resistance patterns of Enterobacter agglomerans isolates are shown in . Two isolates (25, 130) had the same resistance pattern but different sources of isolation. Also, isolates (14, 81) had the same resistance pattern but were isolated from different ice cream specimens. Enterobacter cloacae (11 isolates) showed 9 resistance patterns (). Two pairs of isolates (2, 180 and 148, 182) showed the same resistance patterns but were isolated from different food sources.
Figure 4. Antibiogram of Enterobacter species is shown by a Heat map. a: Enterobacter aerogenes, b: Enterobacter agglomerans, c: Enterobacter cloacae, Red color: resistant, green color: intermediate, yellow color: sensitive, AM: ampicillin, AMC: amoxicillin-Clavulanic, CZ: cefazolin, FOX: cefoxitin, CTX: cefotaxime, FEP: cefepime, CN: gentamicin, AK: amikacin, IPM: imipenem, CIP: ciprofloxacin, TE: tetracycline, and SXT: sulfamethoxazole-trimethoprim, MAR: multiple antibiotic resistant.
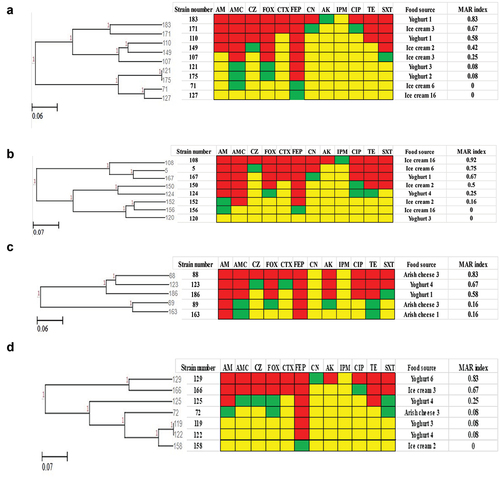
The resistance patterns for Providencia rettgeri, Proteus spp. (Proteus mirabilis, Proteus vulgaris), Citrobacter freundii are shown in . Eight resistance patterns of Providencia rettgeri (9 isolates) were detected (). Only the isolates (121 and 175) showed the same resistance pattern and were isolated from different yogurt specimens. Regarding Proteus spp., eight resistance patterns were detected for Proteus mirabilis isolates () while five resistance patterns were detected for Proteus vulgaris isolates (). It was noticed that no similar patterns were found in either species. Six resistance patterns of citrobacter freundii isolates were recorded (). Only one similar resistance pattern was detected for two isolates (119, 120), they were resistant to FEP and were isolated from different yogurt specimens.
Figure 5. Antibiogram of Providencia rettgeri, Proteus mirabilis, Proteus vulgaris, Citrobacter freundii isolates is shown by a Heat map. a: Providencia rettgeri, b: Proteus mirabilis, c: Proteus vulgaris, d: Citrobacter freundii, Red color: resistant, green color: intermediate, yellow color: sensitive, AM: ampicillin, AMC: amoxicillin-Clavulanic, CZ: cefazolin, FOX: cefoxitin, CTX: cefotaxime, FEP: cefepime, CN: gentamicin, AK: amikacin, IPM: imipenem, CIP: ciprofloxacin, TE: tetracycline, and SXT: sulfamethoxazole-trimethoprim, MAR: multiple antibiotic resistant.
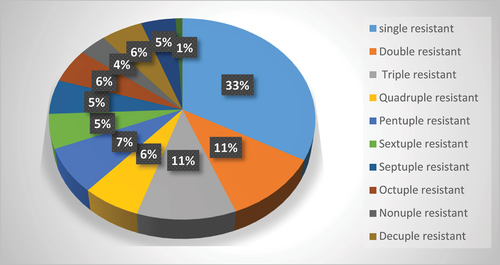
It was noticed that 109 out of 131 isolates showed resistance to one or more antimicrobials, 11 isolates were intermediate to some antimicrobials and the remaining 11 isolates were sensitive to all tested antimicrobials. The percentage of resistance to multiple antimicrobials among the tested isolates is shown in where the highest percentage of the isolates (33%) were resistant to one antimicrobial followed by 11% of isolates showed resistance to each of 2 and 3 antimicrobials. Equal percentages (6%) were noticed for the resistance to 4,8 and 10 antimicrobials. A resistance of 5% was recorded to 6, 7 and 11 antimicrobials. However, only 1% showed resistance to all the tested antimicrobials.
Figure 6. Resistance percentage to multiple antimicrobials among Enterobacteriaceae members isolated from ready-to-eat foods.
The distribution of resistant isolates to multiple antimicrobials among different species is shown in . Only one Klebsiella pneumoniae isolate showed resistance to the 12 antimicrobials (duodecuple). Variable resistance was recorded among different species.
Table 5. Distribution of resistant isolates to multiple antimicrobials and MDR among different Enterobacteriaceae species.
Multiple-drug resistance is defined as non-susceptibility to at least one agent in three or more antimicrobial categories [Citation30]. It was noticed that 38 (29%) of the isolates were MDR. The highest percentage (62.5%) was recorded among Proteus mirabilis isolates where 5 out of 8 isolates were MDR, followed by 60% of Proteus vulgaris isolates. Also, 45.5% of Enterobacter cloacae and 42.9% of Citrobacter freundii isolates were MDR.
Regarding the distribution of MDR isolates among different ready-to-eat food sources, 20 out of 34 (58.8%) different food samples comprised 38 MDR isolates. The highest number of MDR isolates (12 isolates) were found in ice cream samples where they were isolated from 7 out of 14 (50%) samples. From 5 out of 8 (62.5%) of the examined yogurt samples, 10 MDR isolates were obtained. Luncheon samples had recorded the lowest number of MDR isolates (3 isolates), but they were found in 3 out of 4 (75%) of the examined samples.
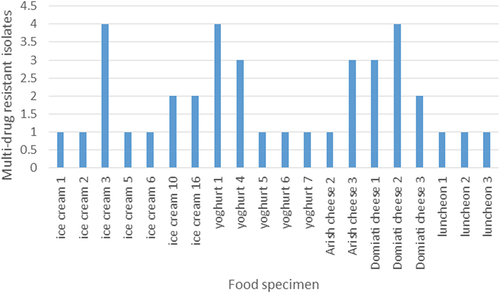
shows the number of MDR isolates per food specimen that harbored MDR isolates. Noticeably, it was found that some food specimens had more than one MDR isolates. The highest number of MDR isolates (4 isolates) were obtained from ice cream 3, yogurt 1, Domiati cheese 4 specimens. Also, three isolates were found in each of yogurt 4, Arish cheese 3 and Domiati cheese 1.
Discussion
Enterobacteriaceae is a group of bacteria that can be used as indicators of the general hygiene status of a food product. Different microbes can be introduced into food products during slicing, packaging, portioning, or other handling techniques. However, this contamination must be controlled by good hygiene practices for both personnel and equipment [Citation31].
In the present study, Enterobacteriaceae isolates were detected among 34 out of 40 (85%) examined ready-to-eat food samples . They were detected in 14 out of 16 (87.5%) examined ice cream samples with a mean count of 3.14 × 102 ± 6.4 x 10 and 8 out of 10 (80%) examined yogurt samples with a mean count of (3.12 x 103 ± 5.32 x 102). In a previous study by Sobeih et al. 2020 [Citation32], a lower percent 64% and 40% of the examined small-scale ice cream and yogurt samples were recorded, respectively but a higher mean counts were recorded, (1.02 x 104 ± 4.13 x 103), (6.95 x 103 ± 4 x103), respectively. Another study by Mohammed et al. 2013 [Citation33] reported nearly close results where 80% of ice cream samples were contaminated with Enterobacteriaceae organisms while the study by El-Diasty et al. 2009 [Citation34] revealed that Enterobacteriaceae were found in yogurt samples with a higher mean count (1.5 x 104 ± 1.2 x 104).
In this study, Arish cheese showed the lowest percentage of positive Enterobacteriaceae samples (3/5, 60%) with a mean count of (3.16 x 102 ± 1.09 x 102). Enterobacteriaceae were detected in all Domiati cheese and luncheon samples with a mean count of (2.5 x 103 ± 1.96 x 103 and 2.3 × 103 ± 3.39 x 102, respectively). Results of Mohammed et al. 2013 [Citation33] revealed a higher percentage of Enterobacteriaceae in Kareish (Arish cheese) (93.3%). Also, the study by Ahmed and Abdelrahman 1988 [Citation35] recorded a higher Enterobacteriaceae percentages (80%) regarding Arish cheese while a lower percentage (35%) was recorded regarding Domiati cheese. Akwieten et al. 2022 [Citation36] study showed that Enterobacteriaceae obtained from luncheon were recorded in a lower percentage (40%) with a higher mean count (2.1 × 104 ± 0.81 × 103). Additionally, Etanani et al. 2021 [Citation37] recorded a lower mean count (5 x 102 ± 3 x 102) of Enterobacteriaceae form their luncheon samples.
In our study, a total of 139 Enterobacteriaceae isolates were obtained from different ready-to-eat food samples as identified by different biochemical tests. Seven genera comprising 16 species were detected. The most frequent genus was Klebsiella with total number of 48 isolates including 36 Klebsiella pneumoniae, 9 Klebsiella oxytoca, 2 Klebsiella ozaenae, 1 Klebsiella terrigena, followed by Enterobacter genus with total number of 45 isolates including 19 Enterobacter aerogenes, 12 Enterobacter agglomerans, 11 Enterobacter cloacae, 2 Enterobacter hafniae, and 1 Enterobacter sakazakii. A number of 15 isolates of Escherichia coli were obtained. Proteus genus (13 isolates) included 8 Proteus mirabilis, 5 Proteus vulgaris. Providencia rettgeri (9 isolates), citrobacter genus (8 isolates including 7 citrobacter freundii and one citrobacter diversus) and Serratia liquefaciens (1 isolate) were the least detected Enterobacteriaceae members. In a study by Fakruddin et al. 2014 [Citation38], a lower number of genera and isolates were reported. They detected five genera of Enterobacteriaceae (Shigella, Escherichia, Klebsiella, Enterobacter and Citrobacter) among 40 food samples. They identified a lower number of isolates (12 isolates: 2 Klebsiella, 3 Enterobacter, 3 Citrobacter, 3 Shigella and one E. coli). In contrast, different genera of family Enterobacteriaceae were detected by Baylis et al. 2011 [Citation10], these genera included Salmonella, Shigella, Cronobacter, Yersinia. Similar spp. were identified in a study by Tsai et al. 2022 [Citation39], in addition to some Salmonella and Shigella were also detected. A study by Gaffer et al. 2019 [Citation40], similar species were detected among the collected food samples.
The difference in the number and type of detected genera and species and the number of isolates among various studies may be attributed to the difference in geographical location, difference in food specimens, and the extent of adherence to hygiene practices.
The prevalence of different Enterobacteriaceae spp. among food specimens is shown in . Ice cream, yogurt and arish cheese comprised 9 out of 16 identified Enterobacteriaceae spp. Domiati cheese and luncheon contained 7 and 4 spp., respectively. Among ice cream and yogurt isolates, Klebsiella pneumoniae was the highest detected spp. (27% and 19%, respectively) followed by Enterobacter aerogenes (16% per each source). For arish cheese, E. coli was the predominant spp. (42%) followed by Proteus vulgaris (12%). Klebsiella pneumoniae was the highest detected spp. among Domiati cheese (39%) and luncheon isolates (50%). In the study conducted by Sobeih et al. 2020 [Citation32], a larger number of spp. were detected regarding yogurt and ice cream samples where 13 and 14 different spp. were detected, respectively. For yogurt, in contrary to our results, the most predominant spp. was E. coli followed by Serratia liquefaciens, while for ice cream samples, the highest percentages were recorded for Serratia marcescens followed by E. coli spp. In a study by El-Gendy et al. 2014 [Citation41], also a larger number of spp. were detected for luncheon samples where different species rather than ours were observed (Enterobacter, Serratia and Edwardsiella). Unlike our study, the most predominant spp. was E. coli but similarly the second predominant spp. was Enterobacter. The larger number of spp. recorded in the previous studies may be due to a larger number of food specimens being examined.
Concerning both Arish cheese and Domiati cheese, in the study by Kamal et al. 2017 [Citation42], a lower number of spp. (only 5 spp.) were detected for both. For Kareish (Arish) cheese similar results were obtained in which the most predominant species was E. coli followed by Enterobacter aerogenes while different results were recorded for Damietta (Domiati) cheese where the predominant species was E. coli followed by Citrobacter freundii.
Enterobacteriaceae are important causes of serious infections, and a large number of the most important members of this family is becoming increasingly resistant to the current available antimicrobials. Antimicrobial resistance has been reported in bacteria isolated from different dairy products [Citation43].
In this study, antimicrobial resistance percentages of different species of Enterobacteriaceae were investigated (). Enterobacteriaceae members showed different susceptibility to 12 tested antimicrobials. All the isolates were susceptible to IPM except a small number of Klebsiella pneumoniae isolates. Most of the tested isolates showed high resistance percentages to FEP and AM. CIP, AK, CN and CTX showed high activity against most of the isolates. Variable resistance levels were found for the other antimicrobials.
By the analysis of the present results for each species, the resistance percent of Klebsiella pneumoniae isolates were nearly similar to the results by Gundogan et al. 2007 [Citation44] concerning some antimicrobials (AM, CN, CTX) but lower percent was recorded to FEP. The study by Ammar et al. 2020 [Citation45] recorded higher resistance percent, Klebsiella pneumoniae isolates (isolated from milk products and meat products) showed absolute resistance to AM and AMC (100%) followed by FEP (72.72%). For Klebsiella oxytoca, also nearly similar resistance percent was recorded by Gundogan et al. 2007 [Citation44] to AM, SXT, CTX, IPM but all the isolates were sensitive to FEP, AK, CN in contrast to our results that showed resistance. In the study by Youssif et al. 2023 [Citation46], Klebsiella oxytoca showed a higher resistance percent (100%) to AM, TE, SXT, AMC, FOX, CN but no resistance was recorded to AK. Our results showed 22% resistance to AK.
Regarding E. coli, our results were similar to Virpari et al. 2013 [Citation47] for CN as all the isolates were sensitive. Also, nearly similar resistance percent was recorded by Sultana et al. 2021 [Citation48] for SXT and CIP. In addition, the present study showed agreement with the study by Madani et al. 2022 [Citation49] as the isolates were highly susceptible to CN, AK, FOX and more than half of the strains showed resistance to TE. A study performed by Ibrahim et al. 2022 [Citation50] recorded a similar high resistance to FEP followed by AM, CZ, then TE and SXT. The studies by Parussolo et al. 2019 and Ombarak et al. 2018 [Citation51,Citation52] revealed that all isolates were highly sensitive to IPM in agreement with our study. Lower resistance percent was recorded against AM and CTX in the study by Virpari et al. 2013 [Citation47]. Also, the isolates were more susceptible to AMC in the study by Madani et al. 2022 [Citation49]. However, E. coli isolates showed high percent resistance (60%) to CN in the study by Sultana et al. 2021 [Citation48].
In agreement with a study by Paterson 2006 [Citation43], Enterobacter spp. showed significant resistance to aminopenicillins, CZ, and FOX due to production of constitutive chromosomal AmpC b-lactamases. Resistance to third-generation cephalosporins appeared in approximately 20% of patients during treatment for Enterobacter bacteremia. Different results were obtained by Fakruddin et al. 2014 [Citation53] in which all Enterobacter isolates (4 isolates) showed resistance to AM and IPM, all the isolates were sensitive to TE and CN, all except one isolate were sensitive to CIP and AK. Higher resistance percent was obtained by Youssif et al. 2023 [Citation46], Enterobacter spp. showed 100% resistance to FOX, TE, SXT, AMC, the isolates were 100% sensitive to CN and higher sensitivity to AK was also recorded.
Concerning Providencia rettgeri isolates, a study by Al-Gburi and Mohammed 2020 [Citation54] showed similar results regarding resistance to AMC and no isolates were resistant to IPM but higher percent resistance to TE (100%) was recorded.
For Proteus spp., Ronanki et al. 2022 [Citation55] revealed that Proteus mirabilis isolates showed nearly the same resistance percent to CN but higher resistance were recorded toward AM, CIP and TE (100%). While Proteus vulgaris isolates recorded higher resistant percentages to AM, CN, TE and a lower resistance was recorded to CIP. Lower resistance percentages were recorded in a study by Owoseni et al. 2021 [Citation56]. Proteus mirabilis isolates showed 9%, 6%, 3%, 2% resistance to TE, CTX, AM, CN. All of the isolates were sensitive to both AK and CIP, Proteus vulgaris isolates showed 10%, 4%, 3%, 2%, resistance to TE, AM, CTX, CIP, respectively. All the isolates were sensitive to AK. Proteus vulgaris isolates recorded 1% resistance to CN while our results showed no resistance.
Regarding Citrobacter freundii isolates, Liu et al. 2020 [Citation57] reported similar resistance percentages to our study for CIP and TE, higher resistance percentages were recorded to AM, CTX, FOX, SXT, CN and IPM, while lower percentages were recorded for both FEP and AK. In a study by Nam et al. 2010 [Citation58], Citrobacter freundii had exceptionally higher level of susceptibility to AM, CZ,TE and CN . However, all the isolates were susceptible to AK like our results in which no resistance was recorded.
In this study, various resistance patterns were detected in all the identified species, this revealed that the isolates had different behavior toward the used antimicrobials so that variable resistance percentages were determined. Some isolates showed the same resistance patterns, the largest number of similar resistance patterns were detected among Klebsiella pneumoniae isolates. Some of these patterns showed similar isolates in which they were isolated from the same food specimen, they had the same biotypes and serotypes. For example, isolates 82 and 85 were isolated from Domiati cheese 2 with biotype B1 and serotype K1, isolates 179 and 185 obtained from yogurt 1 with biotype and serotype K1 and B1, respectively. So, further investigation by molecular typing methods is recommended to detect whether these isolates are identical or not.
Multiple antibiotic resistance index (MAR) (number of antibiotics to which test isolate displayed resistance divided by total number of the tested antibiotics) for each test isolate was calculated as recommended by Krumperman [Citation59]. According to Krumperman, values of MAR higher than 0.25 pose a high-risk source of contamination. In our study 49 isolates (37.4%) showed MAR exceeding the high-risk level (0.25). So, the area of the study is considered as an area of contamination.
Multi-drug resistant (MDR) is defined as resistance to at least one antimicrobial of three or more distinct classes: beta-lactams, quinolones, aminoglycosides, tetracyclines, phenolics, sulfonamides or nitrofuran. Enterobacteriaceae showing resistance to penicillins and cephalosporins due to extended-spectrum β-lactamases (ESBLs) or plasmid-mediated AmpC enzymes have spread worldwide during the last decades [Citation60]. Our results revealed that 29% of isolates were MDR. These isolates were distributed by variable percentages within the different species (). Regarding their distribution among the different food sources, MDR isolates were found in 58.8% of the collected food samples, 12 MDR isolates were found in ice cream samples, and 3 luncheon samples harbored 3 MDR isolates. Some food specimens contained MDR isolates while others did not, the highest number of MDR isolates (4 isolates) were obtained from ice cream 3, yogurt 1, Domiati cheese 4 samples. Also, three isolates were found in each of yogurt 4, Arish cheese 3 and Domiati cheese 1. The increasing frequency and the rapid spread of multidrug resistance among the Enterobacteriaceae is a true and complex public health problem.
Conclusion
The high prevalence of members of Enterobacteriaceae in different food sources is a reliable index of food contamination. So, it is recommended to reconsider restrictions on hygiene and sanitary control in the current food acts and regulations. The isolates showed different resistance patterns, some of them showed high MAR index and some were MDR. This indicates that the phenomenon of multiple antibiotic resistant bacteria in the environment is of global concern since it is an international rather than national problem. A larger scale study is recommended with a larger number of food specimens to be collected from different areas all over Egypt. Furthermore, the study of the phenotypic and genotypic virulence of the identified isolates is suggested and further investigation by molecular typing methods is recommended to detect whether these isolates are identical or not.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Tsang. Microbiological guidelines for ready to eat food. J Road Environmental Hygiene Department, Hongkong. 2002;2(1):115–116.
- Musa O, Akande T. Effect of health education intervention on food safety practice among food vendors in Ilorin. J Sahel Medical Journal. 2002;5(3):120.
- Odu N, Akano U. The microbiological assessment of ready-to-eat-food (Shawarma) in Port Harcourt City, Nigeria. J Nat Sci. 2012;10(8):1–8.
- Jacques Y, Pierre NJ, Antoine M, et al. Toxicological risk assessment of dairy products marketed in the markets of the city of Yaoundé, Cameroon. Issues Bio Sci Pharma Res. 2022;10(1):7.
- Kelly A, Osburn B, Salman M. Veterinary medicine’s increasing role in global health. Lancet Glob Health. 2014;2(7):e379–e80.
- Organization WH. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. World Health Org. 2015;11(15–17).
- Amira OF, Nagah MH, Halawa M, et al. Occurrence of Enterobacteriaceae in skimmed milk soft cheese. Assiut Vet Med J. 2017;63(154):20–27.
- Janda JM, Abbott SL. The changing face of the family Enterobacteriaceae (Order:“Enterobacterales”): new members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. J Clin Microbiol Rev. 2021;34(2).
- Zilberberg MD, Nathanson BH, Sulham K, et al. 30-day readmission, antibiotics costs and costs of delay to adequate treatment of Enterobacteriaceae UTI, pneumonia, and sepsis: a retrospective cohort study. J Antimicrobial Resistance Infection Control. 2017;6(1):1–7.
- Baylis C, Uyttendaele M, Joosten H, et al. The Enterobacteriaceae and their significance to the food industry. ILSI Europe. 2011;30(2):17–28.
- Orallo GO, Pangan AH, Cabrera EC. Microbial analysis of ice cream produced by big-scale and small-scale. manufacturers in metro Manila. J Phil J Microbiol Infect Dis. 1999;28(3):99–101.
- Abou Dobara M, El-Shihy ES. Simultaneous detection of seven foodborne Enterobacteriaceae pathogens using multiplex PCR. J Egypt Acad Soc. 2019;20(1):61–78.
- Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603.
- Klipstein F, Engert RF. Purification and properties of Klebsiella pneumoniae heat-stable enterotoxin. J Infection and Immunity. 1976;13(2):373–381.
- Euzéby JP. List of bacterial names with standing in nomenclature: a folder available on the Internet. J International Journal of Systematic Evolutionary Microbiology. 1997;47(2):590–592.
- Martín MC, Martínez N, Del Rio B, et al. A novel real-time polymerase chain reaction-based method for the detection and quantification of lactose-fermenting Enterobacteriaceae in the dairy and other food industries. J Dairy Sci. 2010;93(3):860–867.
- Khater DF, Lela RA, El-Diasty M, et al. Detection of harmful foodborne pathogens in food samples at the points of sale by MALDT-TOF MS in Egypt. BMC Res Notes. 2021;14(1):1–6.
- Ahmed AM, Shimamoto T. Isolation and molecular characterization of Salmonella enterica, Escherichia coli O157: H7 and Shigella spp. from meat and dairy products in Egypt. J International Journal of Food Microbiology. 2014;168(2):57–62.
- Roberts D, Greenwood M. Practical food microbiology. USA: John Wiley & Sons; 2008.
- Wehr HM, Frank JF. Standard methods for the examination of dairy products. American Public Health Association: Ignatius Press; 2004.
- EG NM, Hossam AI, AS NA. Enterobacteriaceae in beef products from retail outlets in Alexandria. Alexandria J Veterinary Sci. 2014;41(3):80–86.
- Bergey DH. Bergey’s manual of determinative bacteriology. Philadelphia: Lippincott Williams & Wilkins; 1994.
- Kok T, Worswich D, Gowans E. Some serological techniques for microbial and viral infections. In: Collee J, Fraser A, Marmion B, Simmons A, editors. Practical medical microbiology. 14th Edinburgh Churchill Livingstone UK, 1996: 179–204
- Edmondson A, Cooke EM. The production of antisera to the Klebsiella capsular antigens. J Appl Bacteriol. 1979;46(3):579–584.
- Barry A, Garcia F, Thrupp L. An improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens. Am J Clin Pathol. 1970;53(2):149–158.
- Giriyapur RS, Nandihal NW, Patil AB. Comparison of disc diffusion methods for the detection of extended-spectrum beta lactamase-producing Enterobacteriaceae. J Lab Physicians. 2011;3(1):033–036.
- CLSI. Performance standards for antimicrobial susceptibility testing. Clinical and laboratory standards institute. Wayne PA: Book and Periodical Publishing. 2017;106–112
- Donay JL, Fernandes P, Lagrange PH, et al. Evaluation of the inoculation procedure using a 0.25 McFarland standard for the BD Phoenix automated microbiology system. J Clin Microbiol. 2007;45(12):4088–4089.
- Bauer AW, Kirby WM, Sherris JC, et al. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496.
- Rafailidis PI, Kofteridis D. Proposed amendments regarding the definitions of multidrug-resistant and extensively drug-resistant bacteria. Expert Rev Anti Infect Ther. 2022;20(2):139–146.
- Baumgartner A, Grand M, Liniger M, et al. Detection and frequency of Cronobacter spp.(Enterobacter sakazakii) in different categories of ready-to-eat foods other than infant formula. Intern J Food Microbiolog. 2009;136(2):189–192.
- Sobeih AM, AL-Hawary I, Khalifa E, et al. Prevalence of Enterobacteriaceae in raw milk and some dairy products. Kafrelsheikh Veterin Med J. 2020;18(2):9–13.
- Mohammed G, EL-GHiaty H, Riad E. Prevalence of enteric bacteria producing toxins in ice-cream and kareish cheese in Port-Said City markets. Assiut Veterinary Med J. 2013;59(136):16–21.
- El-Diasty EM, El-Kaseh R. Microbiological monitoring of raw milk and yoghurt samples collected from El-Beida city. Arab J Biotechn. 2009;12(1):57–64.
- Ahmed AA, Abdel-Rahman H, Moustafa M. Incidence of Enterobacteriaceae in some selected food stuffs. Assiut Veterinary Med J. 1988;20(40):103–108.
- Akwieten H, Hamad R, Saleh A, et al. Microbial profile of some ready to eat meat products retailed for sale in Al Beida City, Libya. Damanhour J Veterinary Sci. 2022;7(2):1–5.
- Eltanani GS, Arab WS. Quality assurance of some meat products. Alexandria J Vet Sci. 2021;69(1):70–75.
- Fakruddin M, Rahaman MM, Ahmed MM, et al. Antimicrobial resistance and virulence factors of Enterobacteriaceae isolated from food samples of Bangladesh. J Microbiol Immunol Res. 2014;3(1):12–18.
- Tsai K, Hoffmann V, Simiyu S, et al. Bacteroides microbial source tracking markers perform poorly in predicting Enterobacteriaceae and enteric pathogen contamination of cow milk products and milk-containing infant food. Front Microbiol. 2022;12(7):778921.
- Gaffer W, Gwida M, Samra RA, et al. Occurrence and molecular characterization of extended spectrum beta-lactamase producing Enterobacteriaceae in milk and some dairy products. Slovenian Veterinary Res. 2019;56(11):475–485.
- El-Gendy NM, Ibrahim HA, Al-Shabasy NA, et al. Enterobacteriaceae in beef products (Luncheon, Pasterma, Frankfurter and Minced meat) from Alexandria retail outlets. Alexandria J Veterinary Sci. 2014;41(1):80–86.
- Kamal AM, El-Makarem HSA, Amer AA. Safety and public health hazards associated with Egyptian soft cheese consumption. Alexandria J Vet Sci. 2017;54(1):135–141.
- Paterson DL. Resistance in gram-negative bacteria. Enterobacteriaceae Am J Infection Control. 2006;34(5):20–28.
- Gundogan N, Yakar UA. Siderophore production, serum resistance, hemolytic activity and extended‐spectrum β‐lactamase‐producing Klebsiella species isolated from milk and milk products. J Food Safety. 2007;27(3):251–264.
- Ammar AM, El-Aziz A, Norhan K, et al. Biofilm formation and its correlation with antimicrobial resistance in Klebsiella pneumoniae. Zagazig Vet J. 2020;48(4):366–377.
- Youssif NH, Hafiz NM, Halawa MA, et al. Potential risk of antimicrobial resistance related to less common bacteria causing subclinical mastitis in cows. J Adv Vet Res. 2023;13(2):222–229.
- Virpari P, Nayak J, Brahmbhatt M, et al. Study on isolation, molecular detection of virulence gene and antibiotic sensitivity pattern of Escherichia coli isolated from milk and milk products. Vet World. 2013;6(8):541–545.
- Sultana T, Rabbi M, Sarker B, et al. Prevalence and antibiotic resistance patterns of Escherichia coli isolated from milk and milk product. J Bio-Sci. 2021;29(2):81–91.
- Madani A, Esfandiari Z, Shoaei P, et al. Evaluation of virulence factors, antibiotic resistance, and biofilm formation of Escherichia coli isolated from milk and dairy products in Isfahan, Iran. Foods. 2022;11(7):960.
- Ibrahim AH, Ali ME, Ahmed MF, et al. Prevalence and characterization of Escherichia coli in raw milk and some dairy products at Mansoura City. J Adv Vet Res. 2022;12(4):363–370.
- Parussolo L, Sfaciotte RAP, Dalmina KA, et al. Detection of virulence genes and antimicrobial resistance profiles of Escherichia coli isolates from raw milk and artisanal cheese in Southern Brazil. Semina: Ciências Agrárias. 2019;40(1):163–178.
- Ombarak RA, Hinenoya A, Elbagory A-RM, et al. Prevalence and molecular characterization of antimicrobial resistance in Escherichia coli isolated from raw milk and raw milk cheese in Egypt. J Food Prot. 2018;81(2):226–232.
- Fakruddin M, Rahaman MM, Ahmed MM, et al. Antimicrobial resistance and virulence factors of Enterobacteriaceae isolated from food samples of Bangladesh. Intl J Microbiol Immunol Res. 2014;3(1):12–18.
- Al‐Gburi A, Mohammed N, Mestorino N. Isolation and molecular identification and antimicrobial susceptibility of Providencia spp. from raw cow’s milk in Baghdad, Iraq. Vet Med Int. 2020;2020(1):1–6.
- Ronanki SP, Ramya P, Babu AJ, et al. Multidrug resistant-Proteus mirabilis and Proteus vulgaris isolated from milk and meat samples in Tirupati, Andhra Pradesh: an emerging public health threat. The pharma journal. 2022;11(11):1427–1434.
- Owoseni MC, Oyigye O, Sani B, et al. Antimicrobial resistance and virulence genes profiling of proteus species from poultry farms in Lafia, Nigeria. BioRxiv. 2021;2021(7):425673.
- Liu L, Qin L, Hao S, et al. Lineage, antimicrobial resistance and virulence of Citrobacter spp. Pathogens. 2020;9(3):195. doi: 10.3390/pathogens9030195.
- Nam H-M, Lim S-K, Kim J-M, et al. In vitro activities of antimicrobials against six important species of gram-negative bacteria isolated from raw milk samples in Korea. Foodborne pathogens. 2010;7(2):221–224.
- Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. J Applied Environmental Microbiology. 1983;46(1):165–170.
- Okafor JU, Nwodo UU. Molecular characterization of antibiotic resistance determinants in Klebsiella pneumoniae isolates recovered from hospital effluents in the eastern cape Province, South Africa. Antibiotics (Basel, Switzerland). 12(7):2023.