 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This research demonstrates the impact of maternal high fat diet (HFD) consumption on the structure and function of the rat offspring tibialis muscle and investigates the protective potential of germinating fenugreek (Trigonella arabica) and barley (Hordeum vulgare) grains. Immunohistochemical analysis was performed on four distinct groups (HFD, HFD with fenugreek, HFD with barley and control group). Results revealed adverse effects on tibialis muscle and beneficial microbia in the intestine of offspring of HFD group. There were fluctuations in the levels of biochemical markers indicating potential implications for physiological processes within tibialis muscle. Lipid peroxidation and apoptotic factor caspase3 were increased in HFD group, while antioxidants in tibialis muscle were decreased along with increased levels of cholesterol, triglycerides and insulin resistance in serum. HFD induced inflammatory cells and degenerated muscle fibers with accumulated fat droplets and atrophied mitochondria. Dietary supplementation of either germinating barley and fenugreek grains showed modulatory improvement in antioxidant capacity, lipid peroxidation and apoptotic activity in both muscle fibers. These findings highlight the significance of dietary interventions, especially the inclusion of natural grains, in mitigating negative health outcomes in offspring due to maternal HFD consumption.
Introduction
The ever-evolving landscape of nutritional research emphasizes the profound effects of maternal diet on offspring health, spanning not only during gestation but also during adulthood [Citation1]. Among the wide range of dietary patterns examined, high-fat diets, which are ubiquitously consumed in many parts of the world, have been associated with a range of adverse outcomes for both mothers and their offspring. The consequential health effects of such diets, particularly on tibialis muscle structure and function, remain a significant area of concern [Citation2].
Skeletal muscle, notably the tibialis muscle, plays a pivotal role in locomotion and metabolic health. The early developmental stages of these muscles in neonates are crucial, setting the stage for their future functionality [Citation3]. Any perturbation during this critical window, potentially driven by maternal dietary habits, may have longstanding implications [Citation4].
High-fat diets have been linked to increased inflammation and oxidative stress, both of which can adversely affect skeletal muscle health. Chronic inflammation is associated with the activation of inflammatory pathways and the release of pro-inflammatory cytokines. This inflammatory environment can disrupt normal cellular functions and contribute to insulin resistance in skeletal muscle [Citation5].
Germination has been shown to break down phytic acid, a natural grain component that binds minerals like zinc, iron, and calcium, making them unavailable for absorption. By reducing phytic acid content, germinated grains enhance the bioavailability of these minerals [Citation6].
Fenugreek (Trigonella arabica) and barley (Hordeum vulgare) grains have historically been lauded for their health benefits. Both of them boast a rich profile of bioactive compounds, ranging from antioxidants to potent anti-inflammatory agents [Citation7]. They proved their potential role in modulating the structure and function of neonatal tibialis, particularly when mothers are on a high-fat diet [Citation8].
Methodology
Ethical approval
This study got approval from the Ethical Committee for Animal Experimentation at the Faculty of Science, Mansoura University, Egypt. Approval number is (Sci-Z-M-2020-13).
The following methodology provides a systematic overview of the experimental design, dietary interventions, specimen collection, and analytical procedures adopted in this study. It serves as the foundational framework for the ensuing results and discussions.
Experimental work
Animal model and diet regime
Animal Procurement: 52 albino rats, 12 fertile males and 40 virgin female rats, each weighing around 100 g, were sourced from the Helwan breeding farm under the purview of the Ministry of Health for experimental purposes. They were put in four special cages (three females: one male) [Citation9] means about 10 females and 3 males per one cage. Male rats were used for mating purpose only.
Dietary Groups: The virgin female rats were organized into four distinct dietary groups: Control, High Fat Diet (HFD), HFD with germinating barley grains, and HFD with germinating fenugreek grains.
Housing and mating conditions
Rats were housed under conditions ensuring optimal ventilation and subjected to a 12-hour light–dark cycle. Mating occurred in specialized cages, where one male was paired with three females overnight. Successful conception was ascertained the subsequent morning via the identification of sperm in vaginal smears. Day 1 of conception was earmarked as the initiation of the pregnancy timeline.
Diet preparation and feeding
Control group: Fed on standard diet.
High fat diet (HFD) group: Diet comprised of 20% lamb tail fat amalgamated with a standard nutritional diet [Citation10].
HFD with germinating grains: This diet consisted of 20% of a combination of HFD (lamb tail fat) with either germinating barley or fenugreek grains. Each was mixed with the standard dietary components.
Virgin females in all groups were subjected to their respective diets for 6 weeks before pregnancy [Citation11] and this regime continued throughout their gestation and lactation periods.
Body weight (g)
Body weights of offspring were determined at 1st, 7th and 21st day and recorded.
Sample collection
Postpartum, on the 21st day, offspring from each dietary group were humanely sacrificed by cervical dislocation. Tibialis muscle specimens were meticulously dissected and small intestine specimens were taken for microbiological investigation.
Microbiological investigations
From 3-week-old offspring, eight GI tract samples (2 GI tracts from each group) were promptly procured, preserved in sterilized tubes containing 9 ml of NaCl solution, and subsequently subjected to four serial dilutions to facilitate microbiological examinations [Citation12,Citation13].
Biochemical investigations
A subset of tibialis specimens of 21-day-old offspring were homogenized in 10% ice-cold 2.5 mM-tris buffer (pH 7.5) and centrifuged at 14,000 × g for 15 minutes at 4°C..
The following biochemical assays were measured
Assessments of serum biochemical markers
The serum concentrations of insulin, adiponectin, LDH [Citation10], cholesterol, TG, HDL and LDL [Citation14] were assessed utilizing the ELISA kit provided by Cusabio Biotech Company. For this procedure, 25 µl of serum samples were introduced to wells that had been pre-coated with a biotin-conjugated polyclonal antibody specific to the analyte in question. Subsequently, an avidin-conjugated horseradish peroxidase was added. The reaction was then enhanced with the addition of HRP-labeled E2 compound. To instigate the development of a visual output, a 3,3’,5,5’tetramethylbenzidine reagent was introduced and allowed to incubate at ambient temperature for a span of 20 minutes. The resultant blue coloration was then quantified spectrophotometrically at a wavelength of 450 nm.
Assessments of tibialis muscle biochemical markers
Determination of glucose transporter 1 (GLUT1) activity
Glucose transporter 1 (GLUT1) serves as a critical facilitator of glucose transport across the cell membrane. Determining its activity in muscle tissues provides insights into glucose metabolism and cellular energy dynamics. In this experiment, tibialis muscle tissue samples were isolated and homogenized. Post homogenization, the samples underwent a specific biochemical assay designed to quantify GLUT1 activity. This involves monitoring the uptake rate of radiolabeled glucose or using fluorescence-labeled glucose analogs. After the incubation period, samples were washed to remove any unincorporated glucose. The amount of glucose transported into the tissues indicated the activity level of GLUT1 [Citation15].
Determination of ATPase activity
ATPase is an enzyme that plays a pivotal role in energy conversion by hydrolyzing ATP to ADP. To determine its activity in muscle tissues, a specific biochemical assay was employed. This assay measured the inorganic phosphate released during the hydrolysis of ATP. Muscle tissue samples were first isolated and prepared. They were then incubated with a suitable buffer containing ATP [Citation16]. The reaction was stopped after a set period, and the amount of liberated inorganic phosphate was measured, usually using a colorimetric method.
Determination of carbonic anhydrase activity
Carbonic anhydrase facilitates the rapid conversion of carbon dioxide and water into bicarbonate and protons. Its activity can provide valuable insights into the acid–base balance in tissues. In this experiment, the rate at which the enzyme catalyzes the hydration of carbon dioxide was measured. Muscle tissue samples were isolated and treated with a buffer solution containing carbon dioxide. The change in pH, due to the formation of bicarbonate, was then measured over time, often using a pH meter or a colorimetric indicator [Citation17].
Determination of myoglobin activity
Myoglobin, a protein primarily found in muscle tissues, aids in oxygen transport and storage. To determine its activity, a spectrophotometric assay was often employed, leveraging the difference in absorbance of oxygen-bound and oxygen-free myoglobin. Muscle tissue samples were prepared and exposed to varying oxygen concentrations. The changes in absorbance were then measured using a spectrophotometer [Citation18].
Determination of SOD activity
The method is based on the reduction of nitroblue tetrazolium (NBT) by superoxide radicals to blue-colored formazan and assayed at 560 nm [Citation9].
Calculation:
E blank = O.D. of blank at last time – O.D. of blank at zero time
E sample = O.D. of sample at last time – O.D. of sample at zero time = E sample/min
Determination of catalase activity
Catalase catalyzes the conversion of H2O2 to molecular oxygen and water (Catalytic activity).
The absorbance of the sample was read against the distilled water immediately at zero time (A1) and was read again after 30 seconds (A2) at 240 nm. The values were calculated according to the equation of:
10 = dilution factor, T = time, K = constant = 2.3, A1: The absorbance of the sample at zero time, A2: The absorbance of the sample after 30 seconds [Citation9].
Determination of lipid peroxidation end product MDA (thiobarbituric acid reactive substances, TBARS) level
The reddish-pink color was estimated at 532 nm which indicates the extent of peroxidation and expressed as η mol/mg protein. Standardization was carried out by using 1, 1, 3, 3-tetraethoxypropane [Citation9].
Calculation: Malondialdehyde level was expressed as n mol/g tissue.
Determination of caspase-3
It is determined colorimetrically by using a Stressgen kit (catalog No. 907–013). Supernatant of homogenated tibialis muscle tissues were added to a caspase-3 specific peptide that is conjugated to the colored p-nitroaniline molecule (pNA). The level of caspase enzymatic activity is directly proportional to the color reaction at a wavelength of 405 nm [Citation9].
Histological investigations
Light microscopy
As mentioned above, tibialis specimens of 21-day-old offspring were incised and immediately fixed in 10% phosphate-buffered formalin (pH 7.4) and processed for histological investigations. Serial 5-µm-thick sections were cut and stained with hematoxylin and eosin and periodic acid-Schiff stain [Citation19].
Immunohistochemichemisstry of desmin and P53
Five μm histological sections of formalin-fixed, paraffin-embedded tibialis tissues sections taken from 21-day-old offspring were placed on polylysine-coated glass slides and kept at room temperature. Tibialis muscle specimens were incubated with monoclonal mouse anti-human desmin (Sigma, USA, AB907) and monoclonal mouse anti-human p53 antibody (Thermo fisher scientific, fremont, CA, USA). The tissue sections were counter stained with hematoxylin for background of examined tissues. The specimens were investigated with a light microscope and photographed. Data were obtained by using bright-field light Olympus microscope and image analyzer computer system Ltd. (Cambridge, UK). The measurements of the mean area percentage of desmin and P53 were done in 5 non-overlapping fields at a magnification of x400.
Transmission electron microscopy
Tibialis muscle specimens of 21-day-old offspring were fixed in phosphate buffered 2.5% glutaraldhyde (pH 7.4) followed by post-fixed in phosphate buffered 1% osmium tetraoxide at 4°C for 1.5 h. They were dehydrated in ascending grades of ethyl alcohol, cleared in acetone and embedded in epoxy resin. Ultrathin sections were cut with a LKB Ultratome IV and mounted on grids, stained with uranyl acetate and lead citrate, and visualized on a Joel 100CXl transmission electron microscope (Mansoura University, Egypt) [Citation9].
Statistical analysis
Quantitative data derived from the research were statistically analyzed using appropriate tools (one way ANOVA, SPSS (version 2023)) between the studied groups. Data are presented as means ± standard error (SE) with significant differences determined at p < 0.05. Qualitative observations from histological and electron microscopy studies were systematically cataloged and interpreted in the context of the dietary regimes.
Results
Results of this work revealed that maternal HFD had damaging effects on the tibialis muscle of the offspring. HFD induced oxidative stress, inflammation, fibrosis in the muscle tissue and even metabolic disorders. Barley and fenugreek showed a noticeable improvement in tibialis muscle health and the metabolism in body. This was shown by improved muscle fibers with enhanced performance, reduced oxidative stress, and other HFD-related disorders as shown in the following results.
Effect on body weight
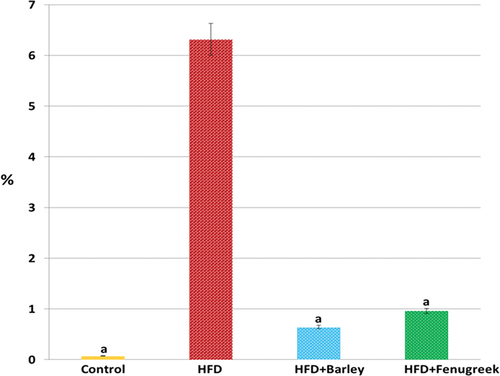
Offspring from maternal HFD groups exhibited greater weight gain than control groups. Rats treated with germinating barley and fenugreek grains showed moderate body weight gain with a significant difference (p ≤ 0.05) compared to the HFD and control groups, indicating their potential role in weight management (; ).
Figure 1. Mean body weight (g) of different experimental groups at 1-, 7-, and 21-day-old offspring rats of hyperlipidemic mothers treated with HFD (20%) and HFD with either fenugreek or barley treatment (20%). N = 5/group. Letters shown in pair of groups that is significantly different from each other (p ≤ 0.05).
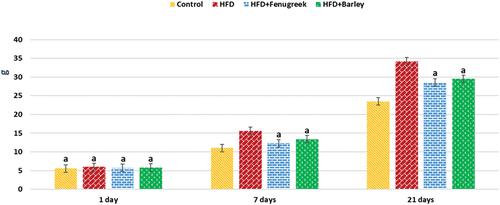
Table 1. Mean body weight (g) of different experimental groups at 1-, 7-, and 21-day-old offspring rats of hyperlipidemic mothers treated with HFD (20%) and HFD with either fenugreek or barley treatment (20%).
Effect on beneficial and pathological bacteria
showcases the impact of different treatments on the abundance of four bacterial strains (Lactobacillus brevis, Lactobacillus plantarum, Escherichia coli DH5alpha, and Escherichia coli DSM 1576) in the context of varying experimental conditions.
Table 2. Identification of microbiota in the intestine of 21-day-old rats treated with HFD with or without fenugreek or barley.
As shown in (), maternal HFD altered the composition of beneficial and pathogenic bacteria in the intestine of 21-day-old rats. Barley and Fenugreek supplementation showed the potential to restore a more balanced microbial profile, reducing pathogenic bacteria and increasing beneficial strains, indicating their role in promoting gut health.
Table 3. Mean count of bacterial strains in intestine of 21-day-old offspring rats of hyperlipidemic mothers treated with HFD (20%) with or without germinating fenugreek or barley grains treatment (20%).
In terms of beneficial bacterial strains, Lactobacillus brevis and Lactobacillus plantarum, it can be observed that the highest counts associated with fenugreek treatment, and barley treatments. The count of Lactobacillus brevis and Lactobacillus plantarum significantly increased in HFD+Fenugreek group and decreased in HFD group compared to control group. In HFD+Barley group, Lactobacillus plantarum significantly increased and Lactobacillus brevis non-significantly increased compared to control group.
Moving to the pathogenic bacterial strains, Escherichia coli DH5alpha and Escherichia coli DSM 1576, the lowest counts are consistently seen in barley and fenugreek treatments. Both of which non-significantly increased in HFD group and non-significantly decreased in HFD+Fenugreek and HFD+Barley groups compared to control group, indicating a potential inhibitory effect of both barley and fenugreek on these pathogenic strains.
Effect on biochemical parameters in serum samples
Insulin, cholesterol, triglycerides and LDH significantly increased in serum samples of all treatments compared to control group, while they significantly decreased in HFD+Fenugreek and HFD+Barley compared to HFD group as shown in ().
Table 4. Biochemical parameters in serum samples of 21-day-old offspring from hyperlipidemic mothers treated with HFD (20%) with or without germinating barley or fenugreek grains supplementation.
Adiponectin significantly decreased in serum samples of all treatments compared to control group, while significantly increased in HFD+Fenugreek and HFD+Barley groups compared to HFD group.
LDL significantly increased in serum samples of HFD compared to control group and significantly decreased in HFD+Fenugreek and HFD+Barley compared to both control and HFD groups.
HDL significantly decreased in serum samples of HFD compared to control group and significantly increased in HFD+Fenugreek and HFD+Barley compared to both control and HFD groups
Effect on biochemical parameters in tibialis muscle samples
Antioxidant enzymes: SOD significantly decreased and catalase non-significantly decreased in all treatments compared to control group. However, SOD significantly increased and catalase non-significantly increased in HFD+Barley and HFD+Fenugreek groups compared to HFD group as shown in ().
Table 5. Biochemical parameters in tibialis muscle samples of 21-day-old offspring from hyperlipidemic mothers with or without germinating barley or fenugreek grains supplementation.
The apoptotic marker (caspase-3) significantly increased in all treatments compared to control group and significantly decreased in HFD+Barley and HFD+Fenugreek groups compared to HFD group.
ATPase, carbonic anhydrase and GLUT-1 significantly decreased in all treatments compared to control group while they significantly decreased in HFD+Fenugreek and HFD+Barley groups compared to HFD group, indicating oxidative stress and muscular dystrophy.
Myoglobin significantly decreased in HFD+Barley and non-significantly decreased in HFD+Fenugreek compared to control group, while it significantly increased in HFD+Fenugreek and HFD+Barley compared to HFD group.
Also, lipid peroxidation (MDA) significantly increased in all treatments compared to control group while it significantly decreased in HFD+Fenugreek and HFD+Barley compared to HFD group, indicating inflammatory conditions .
Histological changes and effects on tibialis muscle
Light microscopy
Histological examination revealed that maternal HFD induced degenerative changes in tibialis muscle.
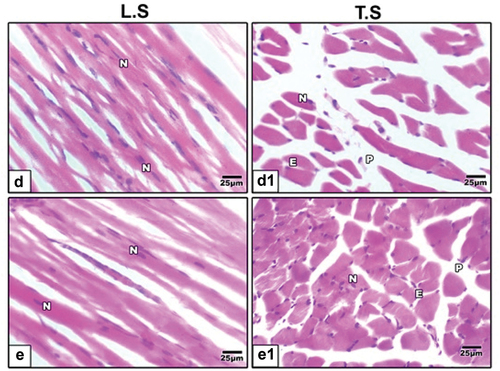
Hematoxylin and eosin (H&E) staining showed regular muscle fibers in control group (), increased inflammatory cells, separation between muscle fibers and degenerated muscle fibers in muscles of maternal HFD offspring (). Barley () and fenugreek () supplementation showed improvements in muscle fibers, attenuating the degenerative effects of HFD and suggesting their potential protective role in tibialis muscle.
Figure 2. Photomicrographs of histological tibialis muscle fibers sections stained by hematoxylin and eosin (H&E) in 21-day-old rat. (a & a1) control rats showing regular muscle fibers. (b, b1, c & c1) neonates of hyperlipidemic mother showing increased inflammatory cells, separation between muscle fibers and degenerated muscle fibers.
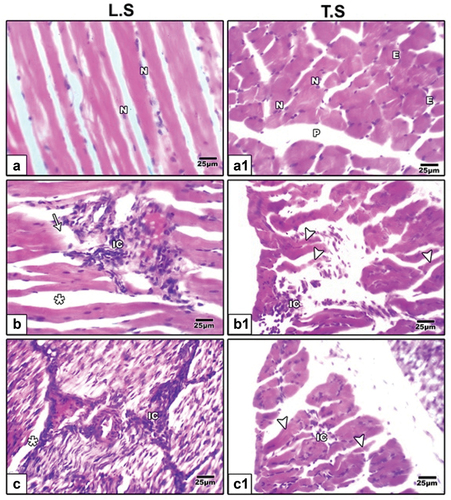
Figure 3. Photomicrographs of histological sections of tibialis muscle stained by hematoxylin and eosin (H&E) in 21-day-old rat. High fat diet plus germinating (d & d1) barley and (e & e1) fenugreek grains supplementation showed improvement in muscle fibers.
Periodic acid Schiff (PAS) staining of tibialis muscle showed negative expression in control group (), strong expression in HFD offspring () indicating inflammatory cells. However, it was weak in barley () and fenugreek () supplementation.
Figure 4. Periodic acid schiff (PAS) staining of tibialis muscle fibers biopsies in 21-day-old rats. a & a1 control showing negative expression. b & b1 neonates of hyperlipidemic mother showing strong expression. c & c1 high fat diet plus germinating barley grains supplementation showing weak expression. d & d1 high fat diet plus germinating fenugreek grains supplementation showing weak expression. Arrow head indicating histochemical reaction of PAS.
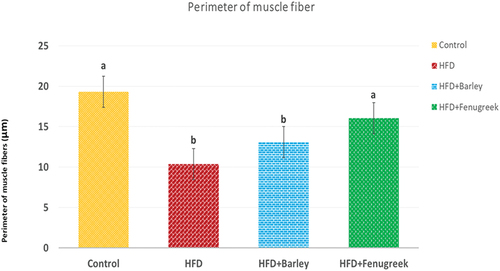
Muscle fiber perimeter was measured and found to be decreased in HFD offspring. However, it was improved in both of HFD+barley and HFD+fenugreek groups ().
Figure 5. Average perimeter of tibialis muscle fibers in 21-day-old rats treated with HFD, HFD+Barley and HFD+Fenugreek compared to control group. Statistical analysis shows that HFD and HFD+Barley groups are significant (p ≤ 0.05) while HFD+Fenugreek group is non-significant compared to control group.
Immunohistochemistry of desmin and P53
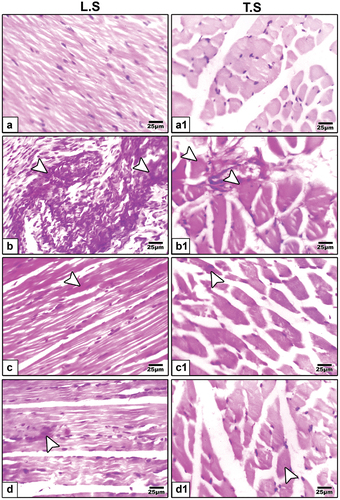
immunohistochemical staining using antibodies specific for desmin ( and ) and p53 ( and ) revealed that maternal HFD induced intense expression of these cell deathrelated proteins compared to control group that revealed weak immunohistochemical reaction of these proteins. Barley and fenugreek grains supplementation showing decrease reaction of these proteins.
Figure 6. Photomicrographs of formalin fixed histological sections of tibialis muscle fibers immunohistochemical stained with desmin of 21-day-old rat. (a) Control showing weak reaction of desmin. (b) High fat diet showing increased dark brown reaction of desmin in muscle fibers indicating cell death. High fat diet treated with (c) barley and (d) fenugreek supplementation showing decreased immunohistochemical reaction of desmin. Arrow head indicating desmin immunohistochemical reaction.
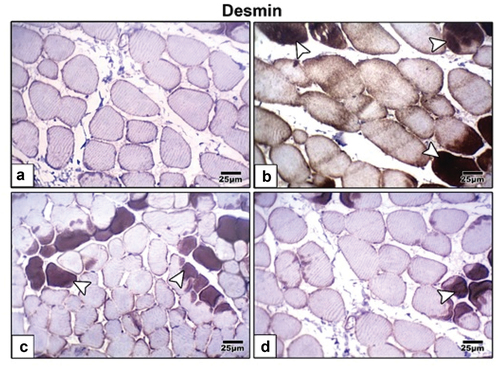
Figure 7. Histogram illustrating the percentages of immunohistochemical reaction of desmin in tibialis muscle of 21-day-old rats fed on diet rich in fat with or without fenugreek or barley grains supplementation. Note over expression of desmin in tibialis muscle of 21-day-old rats fed on diet rich in fat and improved in that received either fenugreek or barley grains plus a high fat diet. Statistical analysis showing that HFD+Barley group is significant with control group (p ≤ 0.05) while HFD+Fenugreek group is non-significant with control and HFD+Barley groups and HFD group is significant with all other groups (p ≤ 0.05).
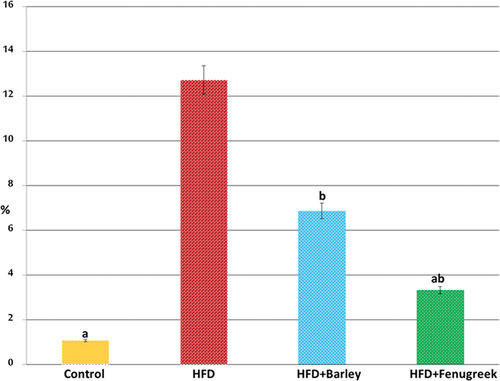
Figure 8. Photomicrographs of formalin fixed histological sections of skeletal muscle fibers immunohistochemical stained with P53 of 21-day-old rat. (a) Control revealed weak immunohistochemical reaction of P53. (b) High fat diet revealed increased dark brown P53 immunohistochemical reaction in muscle fibers manifesting cell death. High fat diet with (c) barley and (d) fenugreek supplementation revealed decreased immunohistochemical reaction of P53. Arrow head indicating immunohistochemical reaction of P53.
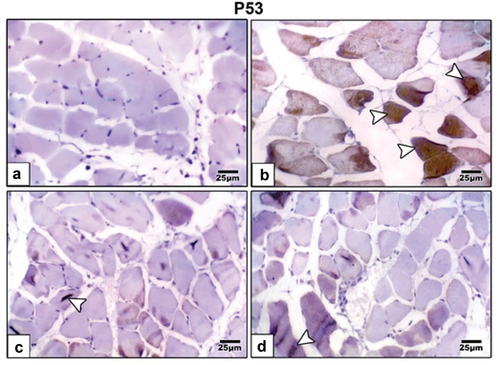
Figure 9. Histogram illustrating the percentages of immunohistochemical reaction of P53 in tibia muscle of 21-day-old rats fed on diet rich in fat with or without fenugreek or barley grains supplementation. Note over expression of P53 in tibia muscle of 21-day-old rats fed on diet rich in fat and improved in that received either fenugreek or barley grains plus a high fat diet. Statistical analysis showing that HFD+Barley and HFD+Fenugreek groups are non-significant with control and HFD group is significant to all other groups(p ≤ 0.05).
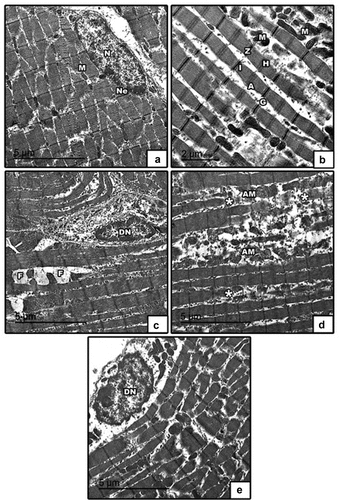
Transmission microscopy
The ultrastructural investigation to detect possible cellular and intracellular alterations in tibialis muscles was performed using transmission electron microscopy (TEM). Control showed regular arranged muscle fibers, normal nucleus with peripheral arrangement of heterochromatin, abundant mitochondria in between muscle fibers and glycogen granules (). Maternal HFD undergo profound degenerative changes including degenerated nucleus with clamped chromatin, fat droplets, degenerated muscle fibers, atrophied mitochondria ().
Figure 10. Transmission electron micrographs of skeletal muscle in 21-day-old rat. (a & b) control showed regularly arranged muscle fibers with normal distribution of A-band, I-band, Z-line & H-zone, normal nucleus with peripheral arrangement of heterochromatin, abundant mitochondria in between muscle fibers and glycogen granules. (c, d & e) high fat diet showing degenerated nucleus with clamped chromatin, fat droplets, degenerated muscle fibers, atrophied mitochondria. Abbreviation: A, dark band; G, glycogen granules; H, H-zone; I, light band; M, mitochondria; N, nucleus; Ne, nuclear envelope; Z, Z-line; asterisk*, degenerated muscle fibers; AM, atrophied mitochondria; F, fat droplets; DN, degenerated nucleus.
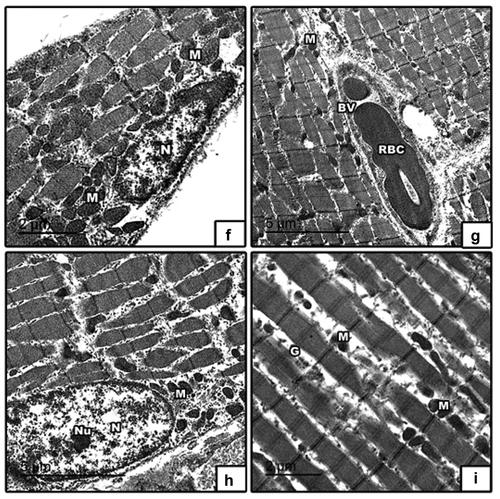
High fat diet with barley () and fenugreek () germinating grains supplementation showed improvement in muscle fibers and nucleus with peripheral arrangement of heterochromatin and showed abundant mitochondria in between muscle fibers, glycogen granules, blood vessel and red blood cell.
Figure 11. Transmission electron micrographs of skeletal muscle in 21-day-old rat. High fat diet with (f & g) barley and (h & i) fenugreek grains supplementation showed improvement in muscle fibers and nucleus with peripheral arrangement of heterochromatin and showed abundant mitochondria in between muscle fibers, glycogen granules, blood vessel and red blood cell. Abbreviation: M, mitochondria; N, nucleus; nu, nucleolus; BV, blood vessel; RBC, red blood cell; G, glycogen granules.
Discussion
The current study delved into the consequences of a maternal high-fat diet on the beneficial microbia in the intestine and the structure and function of the tibialis in the offspring and the potential mitigative effects of germinating fenugreek and barley grains on these outcomes. The relevance of this research lies in the increasing global trend of high-fat dietary intake and its implications on maternal and neonatal health.
One of the most significant findings was the evident increase in body weight among offspring from the high-fat diet (HFD) group. This mirrors findings from several studies that have reported an association between maternal high-fat diets and increased body weight and adiposity in the offspring [Citation20]. Such an effect could be due to the alterations in the intrauterine environment, subsequently influencing fetal programming and predisposing the neonate to obesity and related metabolic disorders in later life [Citation21].
A small dimension added by our study was the microbiological investigations of the GI tract samples. The balance of gut microbiota is crucial for overall health, including muscle function [Citation22,Citation23]. Our results highlighted the possible dysbiosis in the HFD group, which was notably rebalanced in the barley and fenugreek groups. Dietary fibers and bioactive compounds in these grains could be fostering a favorable gut environment, promoting the growth of beneficial bacteria [Citation24,Citation25].
Our findings pertaining to insulin resistance, adiponectin levels, and lipid dysregulation align closely with previous investigations [Citation26] that have reported the beneficial effects of fenugreek and barley in the management of metabolic syndrome and the enhancement of insulin sensitivity. Offspring from mothers on an HFD showed elevated levels of cholesterol [Citation27], triglycerides, LDL and lactate dehydrogenase (LDH), a marker of tissue damage, along with reduced adiponectin and HDL levels-characteristic features of dyslipidemia. Fenugreek could be used for anti-hyperlipidemic and anti-obesity activity as it decreased the levels of blood glucose, insulin, total cholesterol and triglycerides [Citation28] and increased antioxidant enzymes and decreased the oxidative stress marker MDA and the apoptotic marker caspase3 [Citation29]. Barley showed similar effects on cholesterol levels [Citation30].
The dysregulation of GLUT1, ATPase, and carbonic anhydrase in the maternal HFD group hints at metabolic disturbances, likely associated with insulin resistance and perturbed muscle functionality, as corroborated by studies conducted by Badale et al. [Citation31]. Obese rats showed higher content of GLUT1 in plasma membranes of their tibialis muscles [Citation32]. Muscular dystrophy was expressed by decreased carbonic anhydrase in tibialis muscle [Citation33]. Obesity led to less muscle and less ATPase enzyme units in skeletal muscle [Citation34]. HFD was found to induce fat deposition, damage in muscle structure, inflammatory cellular infiltration and decreased distribution of myoglobin inside lingual myofibers which may affect its contractile function [Citation35]. The inflamed myocardial fibers and lung tissues in obese rats showed over expression of caspase3 [Citation36]. MDA and caspase3 were increased in rats maternally fed on HFD [Citation37].
Histological observations further elucidated the adverse structural changes in the tibialis muscles of the HFD group. The occasional disrupted muscle fiber architecture and mild inflammatory infiltration echo previous research that has demonstrated muscle tissue impairment due to high-fat diets [Citation23]. The disrupted muscle histology might be attributed to oxidative stress and inflammation instigated by the fatty acid overload [Citation38] and muscle fiber perimeter was found to be decreased in HFD offspring [Citation39].
However, a salient feature in our findings was the ameliorative impact of germinating barley and fenugreek grains. Both grains are renowned for their rich array of bioactive compounds, including antioxidants and anti-inflammatory agents [Citation40]. The moderated muscle fiber disruptions and the improved serum lipid profile in the barley and fenugreek groups underscore the grains’ protective potential against the detriments of an HFD. These grains likely exert their beneficial effects by modulating lipid metabolism, reducing oxidative stress, and promoting an anti-inflammatory environment [Citation41].
The transmission electron microscopy observations provided deeper insights into the ultrastructural modifications of muscle cells under different dietary regimens. While HFD appeared to induce mitochondrial swelling, indicative of mitochondrial dysfunction and increased oxidative stress [Citation42], the offspring from the barley and fenugreek integrated diets exhibited relatively healthier mitochondria. The restoration of mitochondrial health is pivotal as these organelles play a central role in muscle energy metabolism [Citation1,Citation4].
In summary, while a maternal HFD showed potential negative repercussions on the offspring’s tibialis and overall health, the dietary inclusion of germinating barley and fenugreek grains emerged as a promising intervention. Future studies could further explore the molecular pathways involved and evaluate the long-term effects of such dietary modifications.
Conclusion
In conclusion, the findings of this study highlight the potential role of germinating barley and fenugreek grains as functional foods with antioxidant and health-protective properties. The study sheds light on the complex interactions between diet, oxidative stress, and physiological outcomes, providing valuable insights for the development of dietary strategies to mitigate oxidative stress-related disorders.
Acknowledgments
Facilities provided by Mansoura University, supervisors and family are greatly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Chyang PJ, and Mawaddah M. The effect of hordeum vulgare L. Extracts on blood cholesterol level and lipid peroxidation activity in a hypercholesterolemia rodent model. Int J Res Pharm Sci. 2020;11(4):6244–6249. doi: 10.26452/ijrps.v11i4.3305
- Zhang J, Luo Y, Feng S, et al. Effects of liposoluble components of highland barley spent grains on physiological indexes, intestinal microorganisms, and the liver transcriptome in mice fed a high-fat diet. Food Sci Nutri. 2023;11(6):3096–3110. doi: 10.1002/fsn3.3291
- Iqbal S, Zebeli Q, Mazzolari A, et al. Feeding barley grain steeped in lactic acid modulates rumen fermentation patterns and increases milk fat content in dairy cows. J Dairy Sci. 2009;92(12):6023–6032. doi: 10.3168/jds.2009-2380
- Zebeli Q, Ametaj BN. Relationships between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. J Dairy Sci. 2009;92(8):3800–3809. doi: 10.3168/jds.2009-2178
- Szabó K, Gesztelyi R, Lampé N, et al. Fenugreek (Trigonella Foenum-Graecum) seed flour and diosgenin preserve endothelium-dependent arterial relaxation in a rat model of early-stage metabolic syndrome. Int J Mol Sci. 2018;19(3):798. doi: 10.3390/ijms19030798
- El-Sayyad HIH, El-Mansi AA, Efekrin SM. Dietary supplements of barley and date-palm fruit improved the growth defects of Ovaries of Rat Offspring Maternally Fed on hypercholesterolemic diet. Biosci Biotech Res Asia. 2019;16(2):359–376. doi: 10.13005/bbra/2752
- Rana N, Dahiya S. Proximate composition, in vitro digestibility and anti nutritional factors of millets and legume grains. Agric Science Digest. 2022;42(1):80–83. doi: 10.18805/ag.D-5248
- Attia AI, Reda FM, Patra AK, et al. Date (Phoenix dactylifera L.) by-products: chemical composition, nutritive value and applications in poultry nutrition, an updating review. Animals. 2021;11(4):1133. doi: 10.3390/ani11041133
- Emara EM, El-Sayyad HIH, Mowafy AM, et al. The therapeutic effect of nano-zinc on the optic nerve of offspring rats and their mothers treated with lipopolysaccharides. Egypt J Basic Appl Sci. 2023;10(1):302–315. doi: 10.1080/2314808X.2023.2192582
- Emara EM, El-Sayyad HI, El-Ghaweet HA. Bovine whey supplementation in a high-fat diet fed rats alleviated offspring’s cardiac injury. Macedonian Vet Rev. 2022;45(1):89–99. doi: 10.2478/macvetrev-2022-0017
- Christians JK, Lennie KI, Wild LK, et al. Effects of high-fat diets on fetal growth in rodents: a systematic review. Reprod Biol Endocrinol. 2019;17(1):39. doi: 10.1186/s12958-019-0482-y
- Ashraf M, Arshad M, Siddique M, et al. In-vitro screening of locally isolated lactobacillus species for probiotic properties. Pakistan Vet J. 2009;29(4):186–190.
- Schierack PRömer A, Jores JKaspar H, Guenther S, et al. Isolation and characterization of intestinal Escherichia coli clones from wild boars in Germany. Appl Environ Microbiol. 2009;75(3):695–702. doi: 10.1128/AEM.01650-08
- El-Sayyad HI, Abou-El-Naga AM, Gadallah AA, et al. Protective effects of allium sativum against defects of hypercholesterolemia on pregnant rats and their offspring. Int J Clin Exp Med. 2010;3(2):152–163.
- Avanzato D, Pupo E, Ducano N, et al. High USP6NL levels in breast cancer sustain chronic AKT phosphorylation and GLUT1 stability fueling Aerobic Glycolysis. Cancer Res. 2018;78(13):3432–3444. doi: 10.1158/0008-5472.CAN-17-3018
- Bartolommei G, Moncelli MR, Tadini-Buoninsegni F, et al. A method to measure hydrolytic activity of adenosinetriphosphatases (ATPases). PLOS One. 2013;8(3):e58615. doi: 10.1371/journal.pone.0058615
- Fuchs W, Steger F, Reich J, et al. A simple and straightforward method for activity measurement of carbonic anhydrases. Catalysts. 2021;11(7):819. doi: 10.3390/catal11070819
- Arakaki LS, Ciesielski WA, Thackray BD, et al. Simultaneous optical spectroscopic measurement of hemoglobin and myoglobin saturations and cytochrome aa3 oxidation in vivo. Appl Spectrosc. 2010;64(9):973–979. doi: 10.1366/000370210792434387
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31–43.
- Masuda K, Truscott K, Lin PC, et al. Determination of myoglobin concentration in blood-perfused tissue. Eur J Appl Physiol. 2008;104(1):41–48. doi: 10.1007/s00421-008-0775-x
- He ML, Stanford K, Dugan MER, et al. Association of leptin genotype with growth performance, adipocyte cellularity, meat quality, and fatty acid profile in beef steers fed flaxseed or high-oleate sunflower seed diets with or without triticale dried distiller’s grains. J Anim Sci. 2020;98(4). doi: 10.1093/jas/skaa104
- Gong L, Gong L, Zhang Y. Intake of tibetan hull-less barley is associated with a reduced risk of metabolic related syndrome in rats fed high-fat-sucrose diets. Nutrients. 2014;6(4):1635–1648. doi: 10.3390/nu6041635
- Sievenpiper JL. Low-carbohydrate diets and cardiometabolic health: the importance of carbohydrate quality over quantity. Nutr Rev. 2021;78(Supplement_1):69–77. doi: 10.1093/NUTRIT/NUZ082
- Tanner GJ, Blundell MJ, Colgrave ML, et al. Creation of the first ultra-low gluten barley (hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol J. 2016;14(4):1139–1150. doi: 10.1111/pbi.12482
- Tari LM, Perera WNU, Zaefarian F, et al. Influence of barley inclusion method and protease supplementation on growth performance, nutrient utilisation, and gastrointestinal tract development in broiler starters. Anim Nutr. 2022;8(1):61–70. doi: 10.1016/j.aninu.2021.06.008
- Koteb N. Effect of fenugreek on carbohydrate and lipid metabolisms of rats treated with cypermethr in combined with high-fat-diet and Low Dosestreptozotosin. Ann Agric Sci Moshtohor. 2019;57(2):367–374. doi: 10.21608/assjm.2019.44295
- Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113(12):1640–1661. doi: 10.1016/j.jand.2013.07.010
- Semalty A, Kumar R, Semalty M. Anti-hyperlipidemic and anti-obesity activities of ethanolic extract of trigonella foenum graecum (seeds) of Himalayan region in diet induced obese mice. Adv Biomed Pharm. 2015;2(5):229–234. doi: 10.19046/abp.v02i05.05
- Alu’datt MH, Rababah TA, Ereifej KA, et al. Effects of barley flour and barley protein isolate on chemical, functional, nutritional and biological properties of pita bread. Food Hydrocolloids. 2012;26(1):135–143. doi: 10.1016/j.foodhyd.2011.04.018
- Idehen E, Tang Y, Sang S. Bioactive phytochemicals in barley. J Food Drug Anal. 2017;25(1):148–161. doi: 10.1016/j.jfda.2016.08.002
- Badale A, Pallag A, Kozma M, et al. Fenugreek seed and its active agent diosgenin treatment effects on different metabolic parameters in rats. Farmacia. 2019;67(1):92–98. doi: 10.31925/farmacia.2019.1.12
- Chadt A, H A-H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch - Eur J Physiol. 2020;472(9):1273–1298. doi: 10.1007/s00424-020-02417-x
- Dowling P, Gargan S, Zweyer M, et al. Proteomic profiling of carbonic anhydrase CA3 in skeletal muscle. Expert Rev Proteomics. 2021;18(12):1073–1086. doi: 10.1080/14789450.2021.2017776
- Tran L, Langlais PR, Hoffman N, et al. Mitochondrial ATP synthase β-subunit production rate and ATP synthase specific activity are reduced in skeletal muscle of humans with obesity.Experimental phusiology. Exp Physiol. 2018;104(1):126–135. doi: 10.1113/EP087278
- El-Nefiawy N, Kallini D, Abu-Hussien A, et al. Effect of obesity on the structure and myoglobin distribution within the lingual muscles of the adult male albino rats with reference to obstructive sleep apnea syndrome. Egypt J Anat. 2011;34(2):41–53. doi: 10.21608/ejana.2011.3660
- El-Sayyad HI, El-Ghawet HA, El-Bayomi KS, et al. Bovine whey improved the myocardial and lung damage of mother rats fed on a high fat diet. Stud Stem Cells Res Ther. 2020;6(1):001–008. doi: 10.17352/sscrt.000014
- Abbas OA, El-Sayyad HI, Greash ZA. Dietary malted barley grain improved structure and function of spinal cord of mother rats fed on a high cholesterol diet. Biosci Biotech Res Asia. 2020;17(1):89–101. doi: 10.13005/bbra/2814
- Kheirabadi S, Dehghan-Banadaky M, Ganjkhanlou M. Effects of different dietary fat levels and sources on diet digestibility, fattening performance and meat quality of holstein young bulls when substituted for dietary barley grain. Arch Anim Nutr. 2022;76(1):34–49. doi: 10.1080/1745039X.2021.2013114
- Wang X, Qian Y, Gu T, et al. Dietary tea polyphenols change flesh quality with dose-related manner in the GIFT tilapia fed with a high-fat diet. Aquaculture Nutr. 2020;27(2):519–532. doi: 10.1111/anu.13203
- Yang Y, Ferreira G, Teets CL, et al. Effects of feeding hull-less barley on production performance, milk fatty acid composition, and nutrient digestibility of lactating dairy cows. J Dairy Sci. 2017;100(5):3576–3583. doi: 10.3168/jds.2017-14082
- Mio K, Yamanaka C, Kohyama N, et al. Effect of roasted barley flour on lipid metabolism and gut fermentation in mice fed high-fat diets. J Cereal Sci. 2021;102:103351. doi: 10.1016/j.jcs.2021.103351
- Yang WZ, Beauchemin KA, Rode LM. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. J Dairy Sci. 2001;84(10):2203–2216. doi: 10.3168/jds.S0022-0302(01)74667-X
