ABSTRACT
Nocturnal enuresis is an uncontrollable urination that only happens at night, after most children have stopped. At least three episodes of bedwetting in a patient who has never remained dry for more than six months are necessary for the diagnosis of primary monosymptomatic enuresis (PMNE). Till now, there is no clear connection between the genetic alteration and PMNE. Therefore, there is a crucial need to continue the investigation for a better understanding of the pathophysiology of PMNE. The oxidative stress has been linked to numerous pathological diseases such as cancer, and neurological disease. The current study aimed to investigate the possible association between superoxide dismutase (SOD 1 and 2) single nucleotide polymorphism (SNP), level of metalloproteases and the risk of PMNE persisting into adolescence (10–18 years old). Fifty-three patients (10–18 years old) and 47 healthy children (age- and sex-matched) were included in the study. DNA was extracted for the detection of SNP in SOD1 and SOD2 by using allele-specific primers. Serum malondialdehyde (MDA), nitric oxide (NO), protein carbonyl (PC), SOD, catalase (CAT), and IL-8 were measured in control and patient groups. This study revealed a significant difference between studied groups as regard SOD1 rs17880135 and SOD2 rs4880. There was a significant higher median IL-8 among GT than GG & TT rs17880135 genotype. On the other hand, significant lower median IL-8 was found among CT than CC & TT SOD2 rs4880 genotype. A significantly higher median MDA was found among CT than CC & TT SOD2 rs4880 genotypes. This is the first study to demonstrate that there is an association between the genotype and allele frequencies of SOD1 rs17880135 and SOD2 rs4880 genotypes and the increased risk of nocturnal enuresis. These findings require large scale study among diverse ethnic and race children to better understand the development of the disease and its prevention.
Introduction
Nocturnal enuresis, also known as bedwetting, is the involuntary wetting of the bed at least twice per week for a period of 3 months or more after the age of five [Citation1]. Monosymptomatic enuresis (MSE) and non-monosymptomatic enuresis (NMSE) are the two categories of enuresis. In children, without any additional lower urinary tract symptoms (LUTS) or a history of bladder problems, MSE is classified as enuresis. Enuresis with any daylight LUTS is considered NMSE [Citation2].
MSE is further divided into primary (PMNE) and secondary (SMNE) categories. Children who have never developed a sufficient duration of nocturnal dryness (around 80% of enuretic children meet this requirement) are affected by PMNE. When a child develops enuresis after going dry for at least six months, it is known as SMNE. The prevalence of enuresis rate at five years of age is 15 to 25%, 4% percent at 12-year-old girls, 8% at 12-year-old boys and only 1 to 3% adolescents are still wetting their bed [Citation3,Citation4]. Depending on the etiology and bedwetting alarms, anticholinergics or occasionally tricyclic antidepressants are used in the treatment of PMNE in children [Citation5].
Even though genetic factors can affect all nocturnal enuresis subtypes, prior molecular genetic research was unable to identify a unifying main gene for PMNE. Identification of genes, gene products, and their interactions are therefore crucial [Citation6]. Numerous chronic diseases, including cancers, cardiovascular disease, and neurological diseases, are greatly influenced by oxidative stress [Citation7]. Additionally, oxidative stress is directly linked to pathological symptoms and mechanisms of urinary bladder dysfunction [Citation8]. In the context of this study, some oxidative stress biomarkers and endogenous antioxidants have great clinical relevance in diseases including protein carbonyl (PC), nitric oxide (NO), superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA).
Protein carbonyl groups (ketones and aldehydes) are produced on protein side chains (particularly of Pro, Arg, Thr, and Lys) when they are oxidized. Protein carbonyl derivatives can also be generated through oxidative cleavage of proteins by either the α-amidation pathway or by oxidation of glutamyl side chains [Citation9]. Due to their early production and stability, PC is the most often utilized biomarker of protein oxidative damage and is regarded as a marker of oxidative stress [Citation10]. Nitric Oxide (NO) is used by the kidney, which is produced from L-arginine by the enzyme nitric oxide synthase (NOS), for a variety of biological functions. It takes part in mediating changes in urine flow and Na+ excretion [Citation11]. NO is involved in vasodilation, inflammation, and glomerular ultrafiltration. In pathophysiological situations like hypertension or diabetes, changes in NO bioavailability can cause podocyte destruction, proteinuria, and the early onset of chronic kidney disease (CKD) [Citation12].
Catalase is one among the foremost important antioxidant enzymes. CAT neutralizes H2O2 by its conversion to water and oxygen [Citation13]. If there is an excess of H2O2, it causes injuries to DNA, lipids and RNA [Citation14]. Malondialdehyde (MDA) is a natural product of per-oxidation of unsaturated fatty acids with three or more double bonds. According to animal models, oxidative stress (OS) and bladder dysfunction (BD) may be related to ischemia – reperfusion process and bladder outlet obstruction (BOO). A recent article by Sezginer et al. showed that obstruction of bladder function was due to an increase in MDA level [Citation14].
SOD is the major anti-oxidant enzyme [Citation15] that stimulates the conversion of superoxide (O2•), byproducts of cellular respiration, to O2 and H2O2, providing primary route protection from reactive oxygen species (ROS)-mediated cell damage [Citation16,Citation17]. Since SOD is a metalloenzyme, it needs a metal cofactor to function. Many different types of SOD enzyme exist based on the form of metal ion needed as a cofactor [Citation18]. All mammalian cells have three isoforms of SOD: the cytosolic Cu/Zn dimeric form (SOD1), the mitochondrial tetrameric manganese SOD2 and the extracellular tetrameric Cu, Zn SOD3 [Citation19,Citation20].
The human SOD1 gene, which has five exons and four introns, localized at chromosome 21q22.1 [Citation19]. The encoded Cu/Zn SOD1 protein a 16 kDa homodimer of a 153-amino acid polypeptide. Each SOD1 subunit consists of a β-barrel core and seven loops at the edge which are held together by an intramolecular disulfide bond, a binuclear metal binding site, and a global hydrogen bond network [Citation16,Citation18,Citation19]. The second most frequent cause of genetic amyotrophic lateral sclerosis (ALS) is SOD1 mutations, which account for roughly 18.9% of fALS (Familial) and 1.2% of sALS (Sporadic) [Citation21]. Mutant SOD1 is postulated to undergo oligomerization through the disulfide-crosslinking associated with exposure of the SOD1 structural interior, triggering the onset and progression of ALS [Citation22–24].
The human SOD2 gene, located on the long arm of chromosome 6 (6q25.3), consists of 5 exons and 4 introns encoding 222 amino acids (29, 30). SOD2 expression increases cellular oxidant/antioxidant ratios, which are directly associated with tumor progression, angiogenesis and migration, and invasion [Citation20,Citation25]. Mohammedi et al. showed that V-allele of rs4880 (V16A) was associated with the incidence and the progression of diabetic nephropathy, with a faster decline in estimated Glomerular Filtration Rate (eGFR) [Citation26] suggesting a role for SOD2 in the protection against oxidative stress and kidney disease in type 1 diabetes.
Interleukin 8 (IL 8) also known as C-X-C motif chemokine ligand 8 (CXCL8), is a pro-inflammatory chemokine, produced under inflammatory conditions by immune and other cell types. The most prominent role of IL-8 is the attraction of neutrophils to the sites of inflammation [Citation27]. IL-8 also enhances the oxidative metabolism and generation of reactive oxygen species, possibly leading to oxidative stress. Determination of urine IL-8 is more sensitive and specific than that of other inflammatory cytokines and is useful in the diagnosis of Urinary Tract Infection (UTI), providing additional evidence of the role of IL-8 in inflammation [Citation28].
The current study aimed to investigate the possible association between SOD1 and SOD2 single nucleotide polymorphism (SNP) and the risk of PMNE in adolescence (10–18 years old). Additionally, the association of this polymorphism with oxidative stress was evaluated.
Method
Inclusion and exclusion criteria
According to the ethical guidelines of the Institutional Research Board, Faculty of Medicine, Mansoura University the local ethical committee provided approval to the research (approval No. MD.21.03.64), this cross-sectional comparative study assessed adolescence with Nocturnal enuresis. The study involved 100 adolescences included 53 patients diagnosed with nocturnal enuresis (PMNE) and 47 healthy age- and sex-matched adolescences. Inclusion criteria were as follows: primary mono-symptomatic nocturnal enuresis in adolescence (age between 10 and 18 years), have wet nights more than 3 times per week, never received medical pharmacological treatment. Exclusion criteria were as follows: history of neurological disorders, recurrent urinary tract infection and concomitant bladder pathology, vesical stones, vesicoureteral reflux, pelvic surgeries, history of diabetes mellitus or diabetes insipidus, hypertension, cardiac disease or renal disorders and refusing to sign the informed consent by his/her parents to take samples and participate in the study. Same exclusion criteria were applied to the control group.
Sample collection and DNA extraction
Blood samples were taken from each patient in the morning and separated into portions. The first portion was used to collect serum by centrifugation at > 300 × g for 10 min to be used for the investigation of biochemical analysis. The second portion was collected in an EDTA – containing tube for DNA extraction (ABIOpureTM Genomic DNA, Cat. No.:M501DP100). All samples showed bands, which represent the genomic DNA when gel electrophoresis was applied. The DNA quantity and quality were measured by reading the absorbance at λ260 nm and λ280 nm by Thermo Scientific™ NanoDrop.
PCR amplification for SOD 1 (C/T rs1041740 and G/T rs17880135) and SOD2 gene (T/C rs4880):
Primers in this study were designed by using https://www.ncbi.nlm.nih.gov/tools/primer-blast/ and are provided in . Fifty nanograms of genomic DNA and 1 µL of each allele-specific primers (10 µM stock) were mixed with 10 µL 2× My Taq Red mix master mix (Meridian BIOSCIENCE cat. No: MTRX-020105A). PCR conditions were 95°C for 4 min, then 40 cycles at 95°C for 30 sec, annealing at the corresponding temperature () for 30 sec, extension at 72°C for 30 sec and a final extension at 72°C for 10 min. The agarose gel (2%) electrophoresis (Intron biotechnology, Cat. No.: 32033) was performed at 90 V and a 50 bp ladder RTU (GeneDireX, Cat. No: DM012-R500) to visualize the specific band for each allele.
Table 1. primers used in this study for the detection of SNP in SOD1 and SOD2 genes.
Biochemical measurements
Serum was used to measure IL-8 (Cat. No: E-EL-H6008) by ELISA from Elabscience. Nitric oxide was determined according using Griess reagent [Citation29]. Protein carbonyl was detected using a modified method of the traditional one proposed by Levine et al., and Mesquita et al., based on the reaction between 2,4-dinitrophenylhydrazine (DNPH) and carbonyl groups [Citation30,Citation31]. The serum malondialdehyde (MDA) level was measured by a method described previously by Ohkawa et al., [Citation32]. Thiobarbituric acid (TBA) reacts with MDA in an acidic medium at 94°C for 30 min to yield thiobarbituric acid reactive product which is pink and measured at 532 nm.
Catalase activity was assessed by incubating the enzyme sample in 1.0 ml substrate (65 mmol/ml hydrogen peroxide in 60 mmol/l sodium – potassium phosphate buffer, pH 7.4) at 37°C for 3 min. The method is based on the reaction of ammonium molybdate with the remaining of hydrogen peroxide in the presence of catalase. The product is measured spectrophotometrically 405 nm [Citation33]. Superoxide dismutase activity was measured with a kit provided by Biodiagnostic company (Cairo, Egypt) which is based on the method established by Nishikimi et al. [Citation34].
Statistical analysis and data interpretation:
Data analysis was performed by SPSS software, version 18 (SPSS Inc., PASW statistics for windows version 18. Chicago: SPSS Inc.). Qualitative data were described using number and percent. Quantitative data were described using median (minimum and maximum) (interquartile range) for non-normally distributed data and after testing normality using Kolmogrov-Smirnov test. Significance of the obtained results was judged at the (0.05) level. Chi-Square was used to compare qualitative data between groups as appropriate. Mann Whitney U test was used to compare between 2 studied groups for non-normally distributed data. The Spearman’s rank-order correlation is used to determine the strength and direction of a linear relationship between two non-normally distributed continuous variables and/or ordinal variables.
Results
The current study represents characteristics of children patients with primary monosymptomatic nocturnal enuresis and healthy controls. From , the study participants included 53 patients (Cases) suffering from primary monosymptomatic nocturnal enuresis and control group contained 47 apparently healthy individuals. Their ages ranged from 10 to 18 years. There were no significant differences in age and gender of patients with primary monosymptomatic nocturnal enuresis in comparison to control group (p = 0.567 and 0.305, respectively).
Table 2. Comparison of age and gender among the studied groups. χ2: chi-square test.
showed the comparison of SOD, PC, MDA, NO, CAT and IL8 levels. The results revealed that SOD activity was lower among cases than control group (42.83 vs 60.37, respectively) and significant higher PC among cases than control group (5.15 vs 8.39, respectively). A non-significant difference was detected between the cases and control groups as regard; MDA, NO, CAT and IL8 (p > 0.05).
Table 3. Comparison of biochemical parameters within the studied groups. Z: Mann Whitney U test. Parameters are described as median (min-max) (interquartile range). P is significant when < 0.05. *Statistically significant.
Genetic polymorphism and genotype frequencies
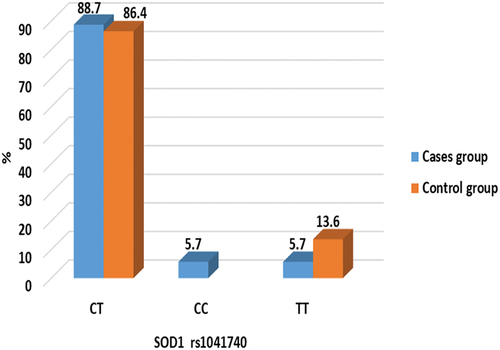
Genotype analysis of SOD1 rs1041740
The genetic polymorphisms in the SOD1 rs1041740 were investigated, and the genotypes are shown in . The frequencies of the genotype of the SOD1 rs1041740 between nocturnal enuresis and healthy controls are listed in and . Neither the genotype nor allele frequencies of the SOD1 rs1041740 C/T polymorphism differed significantly between nocturnal enuresis cases and controls. In , there were no significant differences in SOD1 rs1041740 CT, CC and TT genotype frequencies between children with nocturnal enuresis and control subjects (p > 0.05).
Figure 1. Gel electrophoresis (2%) for PCR product of SOD1 rs1041740. Lane M indicates DNA marker (50 bp). Specific 421 bp band illustrates C or T alleles; Lanes (C1, C2 and C3) represent CC homozygous genotyping, where T allele absents in control group. Lanes (P1, P2, P3, P4 and P5) represent CT heterozygous genotyping; where both C and T alleles were detected at 421 bp in cases group; lane P6 represents TT homozygous genotyping where T allele appears in cases group.
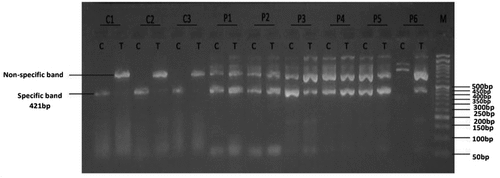
Table 4. Comparison of SOD1 rs1041740 genotypes between the studied groups. Data was expressed as frequency and percentage. χ2: Chi-Square test, Z: Mann Whitney U test, FET: Fisher’s exact test. Parameters are described as numbers (Percentage). P is significant when < 0.05.
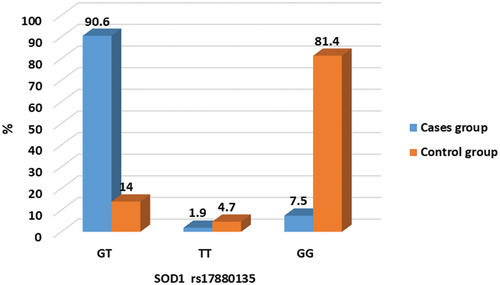
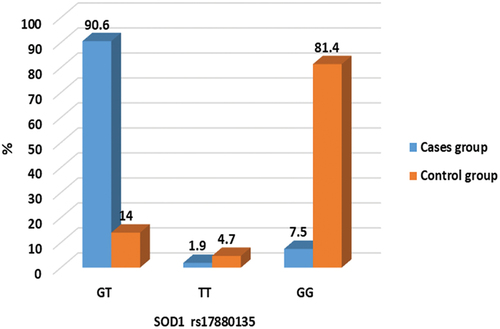
Genotype analysis of SOD1 rs17880135
The genetic polymorphisms in the SOD1 rs17880135 were investigated, and the genotypes are shown in . The frequencies of the genotype of the SOD1 rs17880135 between nocturnal enuresis and healthy controls are listed in and . shows that there was a significant difference in SOD1 rs17880135 genotyping between the studied groups where 90.6% vs 14% were GT genotype with odds ratio 59.2. This finding indicates that GT genotype increases the risk of noctural enuresis by 59.2 times more than other genotypes where 81.4% vs 7.5% were GG genotype.
Figure 3. Gel electrophoresis (2%) for the PCR product of SOD1 rs17880135; Lane M indicates DNA marker (50 bp). Specific 256 bp bands illustrate G or T alleles; Lanes (C1, C2 and C3) represent GG homozygous genotyping where T allele absents in control group. Lane (P1, P2, P4, P5 and P6) represent GT heterozygous genotyping, where G and T alleles appear at 256 bp in cases group. Lane P3 represents TT homozygous genotyping where T allele appears in cases group.
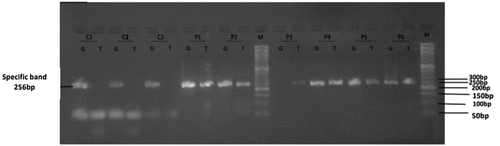
Table 5. Comparison of SOD1 rs17880135 genotypes between the studied groups. χ2: Chi-Square test, Z: Mann Whitney U test. Parameters are described as numbers (Percentage). P is significant when < 0.05. * Statistically significant.
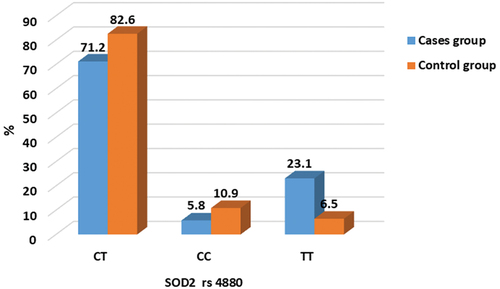
Genotype analysis of SOD2 rs4880
The most reported polymorphism in SOD2 (rs4880) was investigated, and the genotypes are shown in . The frequencies of the genotype of the SOD2 rs4880 between nocturnal enuresis and healthy controls are listed in and . revealed that there were significant higher TT genotyping in SOD2 rs4880 in cases group compared to the control group, implying that SOD2 rs4880 could be a risk factor for the development of nocturnal enuresis in adolescence.
Figure 5. Gel electrophoresis (2%) for PCR product of SOD2 rs4880. Lane M indicates DNA marker (50 bp). Specific 356 bp band illustrates T or C allele. Lanes (C1, C2 and C3) represent TT homozygous genotyping where C allele absents in control group. Lanes (P1, P2) represent CC homozygous genotyping where C allele appears at lanes P1 and P2 at 356 bp in cases group while lanes (P3, P4, P5 and P6) represent TC heterozygous genotyping where T and C alleles appear at 356 bp in cases group.
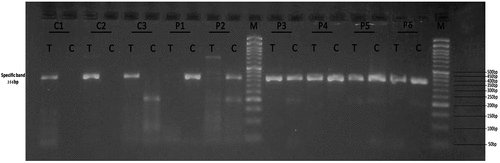
Table 6. Comparison of SOD2 rs4880 genotypes between the studied groups. χ2: Chi-Square test, parameters are described as number (percentage).
SNP genotypes and laboratory findings
The statistical analysis was performed to evaluate the significant difference between the laboratory markers within each group. We found that there were no significant relation between SOD1 rs1041740 genotype and SOD, MDA, PC, NO, CAT and IL8 (p > 0.05) within control group or cases group (Supplementary Files: Table S1 and S2). Regarding SOD1 rs17880135, the analysis revealed no significance within control cases (Supplementary Files: Table S3). On the other hand,
demonstrates that there is statistically significant higher median IL-8 among GT than GG&TT genotype (33.58 vs 12.46, respectively) while no significant relation between SOD1 rs17880135 and the measured laboratory markers SOD, MDA, NO, PC or CAT.
Table 7. Relation between SOD1 rs17880135 SNP genotypes and laboratory findings among cases. Z: Mann Whitney U test, *statistically significant. Parameters are described as median (min-max) (interquartile range).
The genotyping of SOD2 rs4880 among control group showed no statistically difference in the level of SOD, MDA, PC, NO, CAT or IL8 (p > 0.05) (Supplementary Files: Table S4). The analysis revealed that there is a statistically significantly higher median MDA among cases with CT than CC & TT SOD2 rs4880 genotypes (5.38 vs 3.11, respectively) (). Additionally, a significant lower median IL-8 among CT than CC&TT genotype (31.46 vs 71.76, respectively) was found among cases group while no significant relation between SOD2 rs4880 genotyping and the SOD, PC, NO or CAT.
Table 8. Relation between SOD2 rs4880 SNP genotypes and laboratory findings among cases. Z: Mann Whitney U test, *statistically significant. Parameters are described as median (min-max) (interquartile range).
Discussion
Nocturnal enuresis (NE) is a prevalent clinical issue in children, influenced by genetic factors, endocrine and metabolic diseases, inflammation, bladder instability, developmental delays in urinary control, and disturbances in sleep arousal. The etiology of NE is complex [Citation35], and understanding the interaction between genetics and its pathophysiology remains unclear. This study investigated single nucleotide polymorphisms (SNPs) in SOD1 (rs1041740, rs17880135) and SOD2 (rs4880) among Egyptian children to explore their association with NE, aiming to contribute toward reducing its incidence and advancing understanding of its causes and treatments.
In our study, children from study and control groups did not differ between sex and age in case group and control group. Our findings are in agreement with the finding of Szymanik-Grzelak et al., in terms of sex or age [Citation5]. SOD is an endogenous antioxidant enzyme, protects the human body from reactive oxygen species [Citation16–18]. Based on the findings from the current study, the serum MDA level did not differ, but SOD activity was decreased in the case group compared with the control group. MDA is recognized as an oxidative stress marker. MDA, a lipid peroxide product, reflects the severity of the damage caused by free radicals [Citation36]. Miao-Shang Su et al., found that the MDA content in serum is significantly high in the rat model of chronic intermittent hypoxia (CIH) – induced enuresis while less SOD activity was recorded in the model [Citation37].
Previous studies have suggested that enuresis may be associated with the genes on chromosomes 8, 6, 21 and 22 [Citation38]. SODs are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide [Citation17]. In blood vessels, SOD1 accounts for ~ 85% of the total cellular SOD activity of most mammalian cells where it preserves NO release from the endothelium [Citation39]. The human SOD1 gene (Entrez Gene ID 6647) is located on chromosome 21q22.11, and codes for the monomeric SOD1 polypeptide. Our results showed no significant differences in SOD1 rs1041740 CT, CC and TT genotype frequencies between children with PMNE and control subjects.
SOD1 and SOD2 genes are associated with a higher risk of cancer and are also associated with the progression of the kidney pathologies [Citation40]. In the current study, it was revealed that significant difference between cases groups as regard SOD1 rs17880135 were associated with an increased risk of disease by 59.2 times more than other genotypes. This means that this polymorphism plays an important role in PMNE. Regarding the role of this polymorphism in the levels of genomic damage, no previous studies were found linking both parameters. Also, the groups did not differ in nitric oxide NO, CAT and PC cases group compared to control group.
We also did not find any differences in terms of interleukin-8 level between children with PMNE and healthy children. However, we found significant higher median IL-8 among GT than GG & TT in SOD1 rs17880135 genotype among cases. IL-8 has been detected in the urine of superficial bladder cancer patients to whom Mycobacterium bovis bacillus Calmette-Guerin (BCG) cells were administered intravesical after transurethral resection of tumors [Citation28]. Olivier Oregioni et al., found that IL-8 is increased in urines during UTIs especially with Escherichia coli infection [Citation41]. IL-8 can be produced by epithelial cells of the renal tract in response to a variety of inflammatory stimuli. The cytokine has been shown in inflamed renal tissue in patients with acute allograft rejection, and high serum concentrations have been reported in patients with renal failure [Citation42]. A recent finding by Rashwan et al. showed that IL-8 level is higher in the children suffering from obstructive sleep apnea [Citation43]. All those data support our findings that IL-8 plays a role in PMNE.
A common polymorphism in SOD2, a T/C transition (Val16Ala), was shown to alter the active site [Citation44,Citation45]. Numerous studies linked the reduction of SOD2 to the increased DNA damage and higher incidence of cancer either in animal model or in vitro [Citation46,Citation47]. In our study, no statistically significant relation between SOD2 rs4880 genotype and the level of SOD, MDA, PC, NO, CAT or IL8 among control group which is in accordance with the finding by Paul Terry et al., [Citation48]. However, we found a significant higher median MDA among cases with CT than CC & TT SOD2 rs4880 genotypes (5.38 vs 3.11, respectively). Also, we found that there was a significant lower median IL-8 with CT than CC & TT g SOD2 rs4880 genotypes (31.46 vs 71.76, respectively) among cases group. Our findings agree with Zhao et al., who showed that the inflammation plays a role in the induction of cyclophosphamide-induced cystitis in rat model [Citation49].
Conclusions
This study focused on investigating single nucleotide polymorphisms (SNPs) in SOD1 (rs1041740, rs17880135) and SOD2 (rs4880) among Egyptian children to explore their relationship with PMNE. Our findings indicate that SOD1 rs17880135 genotype GT was significantly associated with an increased risk of PMNE, highlighting its potential role in the condition. Additionally, while no significant differences were found in SOD1 rs1041740 genotype frequencies between PMNE cases and controls, differences in SOD2 rs4880 genotypes were associated with varying levels of oxidative stress markers (MDA) and inflammatory cytokines (IL-8) in PMNE cases compared to controls. These results suggest that oxidative stress and inflammation may contribute to the pathogenesis of PMNE, underscoring the need for further research into genetic predispositions and targeted therapies for this condition.
Supplementary files.docx
Download MS Word (21.2 KB)Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2314808X.2024.2371747
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Porter JS, Paladino AJ, Russell K et al. Nocturnal enuresis in sickle cell: sociodemographic, medical, and quality of life factors. J Pediatr Psychol. 2022;47(1):75–85. doi: 10.1093/jpepsy/jsab079
- Franco I, von Gontard A, De Gennaro M. Evaluation and treatment of nonmonosymptomatic nocturnal enuresis: a standardization document from the international children’s continence society. J Pediatr Urol. 2013;9(2):234–243. doi: 10.1016/j.jpurol.2012.10.026
- Thiedke CC. Nocturnal enuresis. American Family Physician. 2003;67(7):1499–1506.
- Gaonkar N, Nahon Irmina JM, Bagalkot Praveen S, et al. Prevalence of nocturnal enuresis in 6–15 years school children and its awareness among parents in Dharwad. Indian J Phy and Occu Ther - An Inter Jou. 2018;12(3):11–16. doi: 10.5958/0973-5674.2018.00048.5
- Szymanik-Grzelak H, Daniel MU, Skrzypczyk P, et al. Is copeptin a reliable biomarker of primary monosymptomatic nocturnal enuresis? Cent Eur J Immunol. 2019;44(1):38–44. doi: 10.5114/ceji.2019.84013
- Ece A, Coşkun S, Şahin C, et al. BDNF and NGF gene polymorphisms and urine BDNF-NGF levels in children with primary monosymptomatic nocturnal enuresis. J Pediatr Urol. 2019;15(3):e255.1–.e255.7. doi: 10.1016/j.jpurol.2019.03.010
- Sardaro N, DELLA Vella F, Incalza MA, et al. Oxidative stress and oral mucosal diseases: an overview. In Vivo. 2019;33(2):289–296. doi: 10.21873/invivo.11474
- Miyazaki N, Yamaguchi O, Nomiya M, et al. Preventive effect of hydrogen water on the development of detrusor overactivity in a rat model of bladder outlet obstruction. J Urol. 2016;195(3):780–787. doi: 10.1016/j.juro.2015.10.117
- Dalle-Donne I, Rossi R, Giustarini D, et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/S0009-8981(03)00003-2
- Chiaradia E, Renzone G, Scaloni A, et al. Protein carbonylation in dopaminergic cells exposed to rotenone. Toxicol Lett. 2019;309:20–32. doi: 10.1016/j.toxlet.2019.04.002
- Gecit İ, Eryılmaz R, Kavak S, et al. The prolidase activity, oxidative stress, and nitric oxide levels of bladder tissues with or without tumor in patients with bladder cancer. J Membr Biol. 2017;250(5):455–459. doi: 10.1007/s00232-017-9971-0
- Semenikhina M, Stefanenko, M, Spires, D. R, et al. Nitric-oxide-mediated signaling in podocyte pathophysiology. Biomolecules. 2022;12(6). doi: 10.3390/biom12060745
- Nandi A, Yan LJ, Jana CK, et al. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid Med Cell Longev. 2019 Nov 11;2019:9613090. doi: 10.1155/2019/9613090
- Sezginer EK, Yilmaz‐Oral D, Lokman U, et al. Effects of varying degrees of partial bladder outlet obstruction on urinary bladder function of rats: a novel link to inflammation, oxidative stress and hypoxia. LUTS: Lower Urinary Tract Symptoms. 2019;11(2):O193–O201. doi: 10.1111/luts.12211
- Jamieson D, Chance B, Cadenas E, et al. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48(1):703–719. doi: 10.1146/annurev.ph.48.030186.003415
- Fridovich I. Superoxide radical and superoxide dismutase. Acc Chem Res. 1972;5(10):321–326. doi: 10.1021/ar50058a001
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J of Neurosci Res. 2005;79(1–2):157–165. doi: 10.1002/jnr.20280
- Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase: site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248(13):4793–4796. doi: 10.1016/S0021-9258(19)43735-6
- Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci USA. 1982;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634
- Kamarajugadda S, Cai Q, Chen H, et al. Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death Dis. 2013;4(2):e504. doi: 10.1038/cddis.2013.20
- Mathis S, Goizet C, Soulages A, et al. Genetics of amyotrophic lateral sclerosis: a review. J Neurolog Sci. 2019;399:217–226. doi: 10.1016/j.jns.2019.02.030
- Huai J, Zhang Z. Structural properties and interaction partners of familial ALS-associated SOD1 mutants. Frontiers in neurology. Front Neurol. 2019;10:527. doi: 10.3389/fneur.2019.00527
- Tokuda E, Anzai I, Nomura T, et al. Immunochemical characterization on pathological oligomers of mutant Cu/Zn-superoxide dismutase in amyotrophic lateral sclerosis. Mol Neurodegener. 2017;12(1):2. doi: 10.1186/s13024-016-0145-9
- Momtaz S, Memariani Z, El-Senduny FF, et al. Targeting ubiquitin-proteasome pathway by natural products: novel therapeutic strategy for treatment of neurodegenerative diseases. Front Physiol. 2020;11:361. doi: 10.3389/fphys.2020.00361
- Alateyah N, Gupta, I, Rusyniak, R. S, et al. SOD2, a potential transcriptional target underpinning CD44-Promoted Breast Cancer Progression. Molecules, 2022, 27(3).
- Mohammedi K, Bellili-Muñoz N, Driss F, et al. Manganese superoxide dismutase (SOD2) polymorphisms, plasma advanced oxidation protein products (AOPP) concentration and risk of kidney complications in subjects with type 1 diabetes. PLOS ONE. 2014;9(5):e96916. doi: 10.1371/journal.pone.0096916
- Vilotić A, Nacka-Aleksić M, Pirković A, et al. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int J Mol Sci. 2022;23(23):14574. doi: 10.3390/ijms232314574
- Ko YC, Mukaida N, Ishiyama S, et al. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61(4):1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993
- Giustarini D, Rossi, R, Milzani, A, et al. Nitrite and nitrate measurement by griess reagent in human plasma: evaluation of interferences and Standardization. Methods Enzymol. 2008;440:361–380. doi: 10.1016/S0076-6879(07)00823-3
- Levine RL, Garland, D, Oliver, C. N, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478.
- Mesquita CS, Oliveira R, Bento F, et al. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem. 2014;458:69–71. doi: 10.1016/j.ab.2014.04.034
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2–3):143–151. doi: 10.1016/0009-8981(91)90067-M
- Nishikimi M, Roa N, Yogi K. Measurement of superoxide dismutase. Biochem Biophys Res Commun. 1972;46(2):849–854. doi: 10.1016/S0006-291X(72)80218-3
- Austin PF, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35(4):471–481. doi: 10.1002/nau.22751
- Li W, Yang S, Yu F-Y, et al. Hydrogen ameliorates chronic intermittent hypoxia-induced neurocognitive impairment via inhibiting oxidative stress. Brain Res Bull. 2018;143:225–233. doi: 10.1016/j.brainresbull.2018.09.012
- Su MS, Xu L, Gu S-G, et al. Therapeutic effects and modulatory mechanism of alpiniae oxyphyllae fructus in chronic intermittent hypoxia induced enuresis in rats. Sleep Breath. 2020;24(1):329–337. doi: 10.1007/s11325-019-01983-4
- von Gontard A, Schaumburg H, Hollmann E, et al. The genetics of enuresis: a review. J Urol. 2001;166(6):2438–2443. doi: 10.1016/S0022-5347(05)65611-X
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999
- Kitada M, Xu J, Ogura Y, et al. Manganese superoxide dismutase dysfunction and the pathogenesis of kidney disease. Front Physiol. 2020;11:755. doi: 10.3389/fphys.2020.00755
- Oregioni O, Delaunay P, Bruna P, et al. Urinary interleukin-8 is elevated in urinary tract infections independently of the causative germs. Cytokine. 2005;31(6):415–418. doi: 10.1016/j.cyto.2005.06.009
- Rao WH. The significance of interleukin 8 in urine. Arch Dis Child. 2001;85(3):256–262. doi: 10.1136/adc.85.3.256
- Abdelmonem NI, Tabbakh, M. T. E, Farid, A. M, et al. Interleukin 8 in children with obstructive sleep apnea before and after adenotonsillectomy. Egypt J Otolaryngol. 2023;39(1):104. doi: 10.1186/s43163-023-00467-3
- Taufer M, Peres A, de Andrade VM, et al. Is the Val16Ala manganese superoxide dismutase polymorphism associated with the aging process? J Gerontol A. 2005;60(4):432–438. doi: 10.1093/gerona/60.4.432
- Bag A, Bag N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3298–3305. doi: 10.1158/1055-9965.EPI-08-0235
- Ambrosone CB, Ambrosone, C. B., Freudenheim, J. L., et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59(3):602–606.
- Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37. doi: 10.1152/physiolgenomics.00122.2003
- Terry PD, Umbach DM, Taylor JA. No association between SOD2 or NQO1 genotypes and risk of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(3):753–754. doi: 10.1158/1055-9965.EPI-04-0574
- Zhao J, Song Q, Wang L, et al. Detrusor myocyte autophagy protects the bladder function via inhibiting the inflammation in cyclophosphamide-induced cystitis in rats. PLOS ONE. 2015;10(4):e0122597. doi: 10.1371/journal.pone.0122597