Abstract
Residual cognitive symptoms are associated with reduced daily life functioning, quality of life and represent a risk factor for relapse of major depressive disorder (MDD). There are few studies targeting self-perceived residual cognitive symptoms after MDD. The current open pilot study examines clinical outcomes and feasibility of a novel internet-delivered cognitive enhancement treatment for mood disorders specifically tailored to target self-perceived residual cognitive symptoms after MDD. A total of 43 adults with self-perceived residual cognitive symptoms after MDD were included. Participants were assessed pre- and post-treatment and at 6-month follow-up. The intervention consists of 10 modules that includes psychoeducation, cognitive strategies, and attention training, coupled with weekly therapist guidance. Results showed a significant reduction from pre- to post-treatment in self-perceived residual cognitive symptoms (d = 0.98) and rumination (d = 0.63). Results remained significant at the 6-month follow-up (d = 1.06; d = 0.86). Reliable change in self-perceived residual cognitive symptoms were obtained in 60% of the participants from pre- to post-treatment. Completion rates (86%) and treatment satisfaction (97%) were high. This open pilot study supports that targeting self-perceived residual cognitive symptoms after MDD through internet-delivered cognitive enhancement therapy for mood disorders may be feasible and provide stable reductions in self-perceived residual cognitive symptoms and rumination.
Major depressive disorder (MDD) is globally the most common mental health disorder and one of the leading causes of disease burden (James et al., Citation2018), associated with substantial personal consequences and societal costs (Whiteford et al., Citation2015). Despite effective psychological and medical treatments about 50% of those with a first episode of depression experience relapse within two years, and the probability of relapse increases with each new episode (Eaton et al., Citation2008; Mueller et al., Citation1999; Solomon et al., Citation2000). Developing effective and accessible interventions preventing depressive relapse are therefore urgent (Beshai et al., Citation2011; Bockting et al., Citation2015; Cuijpers, Citation2015). One promising approach in preventing relapse is to target residual cognitive symptoms after MDD, such as reduced performance on objective neuropsychological tests and self-reported difficulties with attention-, memory-, and executive functions (Conradi et al., Citation2011; Hammar & Årdal, Citation2009; Rock et al., Citation2014; Semkovska et al., Citation2019). More specifically, the literature show that risk of depressive relapse is associated with reduced performance on both objective neuropsychological tests measuring inhibition switching and divided attention (Majer et al., Citation2004; Schmid & Hammar, Citation2013) and subjective self-perceived cognitive difficulties measured by self-report (Saragoussi et al., Citation2017). The latter involves difficulties with daily life activities such as problems concentrating on reading, remembering content of conversations, and getting things organized (Saragoussi et al., Citation2017). Taken together, several intervention studies have targeted objectively measured cognitive impairment (Motter et al., Citation2016), however accessible, engaging, and scalable interventions that target self-perceived residual cognitive symptoms are still scarce.
The precise mechanisms underlying the reduced performance on objective neuropsychological tests in individuals with a history of MDD are not well understood. Several hypotheses exist such as a preexisting cognitive vulnerability, cognitive impairments being acquired during depression that persist into remission, or state-related cognitive impairment changing according to depression symptoms (Allott et al., Citation2016). On the other hand, a possible mechanism of self-perceived cognitive symptoms after MDD is not meeting premorbid levels and expectations of functioning at work or school, and consequently resulting in a negative self-representation. This may explain the fact that self-perceived cognitive symptoms after MDD are associated with reduced daily functioning and quality of life (Dhillon et al., Citation2020; Saragoussi et al., Citation2013; Tatay-Manteiga et al., Citation2019).
Interestingly, depressed adults’ performance on objective neuropsychological tests have not been found to correlate with self-perceived cognitive difficulties (Løvstad et al., Citation2016; Moritz et al., Citation2004; Petersen et al., Citation2019). The lack of correlation has been proposed to be a result of suboptimal effort during testing due to symptoms related with depression such as inattention, lack of engagement, and mood changes (Benitez et al., Citation2011). This discrepancy may also be explained by a cognitive impairment bias, that is an inadequate perception of one’s cognitive resources (Dhillon et al., Citation2020). Therefore, it is important to specifically address self-perceived residual cognitive symptoms in scalable interventions.
Cognitive Enhancement Therapy for mood disorders (CET-MD) is one approach to treatment of objective cognitive impairment and also self-perceived cognitive difficulties in mood disorders (Douglas et al., Citation2019). A strength of CET-MD is the acknowledgment of providing treatment for both the cognitive impairments measured by objective neuropsychological tests and self-perceived cognitive difficulties. Key elements in CET-MD are firstly, psychoeducation including a clear rationale of symptoms and why targeted treatment of objectively measured cognitive impairment or self-perceived cognitive difficulties could be helpful. Secondly, cognitive practise on tasks that activate brain regions proposed to be underactive in depression and strategy training with the support of a therapist. Thirdly, transferring learned strategies and skills by discussion and planning how newly gained skills can be used in daily life. In addition, interventions may target several cognitive domains given that the neuropsychological profile in MDD is heterogenous (Reppermund et al., Citation2009).
There is support for the effect of computerized cognitive training targeting objectively measured cognitive impairment in mood disorders (Motter et al., Citation2016). A systematic review and meta-analysis by Motter et al. (Citation2016) showed that computerized cognitive interventions during depression had moderate to large effects on objective neuropsychological tests measuring global cognitive status, attention, and working memory, while only a small effect on subjective depressive symptoms was obtained. The latter suggests that interventions for formally depressed adults with low depression load should mainly aim to prevent an increase in depressive symptoms. Only a few previous studies have tested the clinical effects of treatments for residual cognitive symptoms after MDD measured by objective neuropsychological tests and self-reports. These studies have included adults with a history of MDD and mainly applied computerized cognitive training or combined this with compensatory transfer sessions (Hammar et al., Citation2020; Listunova et al., Citation2020; Semkovska & Ahern, Citation2017). The studies show promising results such as improved performance on objective neuropsychological tests measuring working memory, attention, planning, long-term verbal memory, switching abilities, and self-reported psychosocial functioning. However, no significant reduction in self-perceived executive functioning or rumination was found (Hammar et al., Citation2020). Further effort to integrate tasks to decrease rumination should however be considered given the association with reduced performance on objective cognitive tests measuring cognitive flexibility and inhibitory systems (Davis & Nolen-Hoeksema, Citation2000; Joormann, Citation2010). Consequently, rumination could thereby occupy essential cognitive resources away from ongoing tasks. In addition, may high levels of rumination increase awareness and worries about self-perceived cognitive symptoms and potentially elevate depressive symptoms (Malivoire et al., Citation2018). Targeting self-perceived residual cognitive symptoms in addition to rumination could therefore be a useful approach to prevent depression relapse. Another limitation to these studies is the lack of reported follow-up data that is of importance when evaluating the durability of cognitive interventions. Previous studies have also applied interventions that are not developed by systematic user-involvement based on adults experiences of self-perceived residual cognitive symptoms after MDD.
Assuming that tailored and effective interventions do exist, accessibility and scalability is a major concern, as there are few neuropsychologists and cognitive trainers who can implement cognitive interventions due to resources required (Rossell, Citation2020). To overcome this issue, digital technology may be a way to distribute the interventions. Several advantages are associated with delivering treatment over the internet. It saves therapist time and has the potential to reach populations that would otherwise not receive treatment (Cuijpers et al., Citation2008). To date, substantial evidence exists for the effects of internet-delivered interventions for common mental health problems such as anxiety and depression (Barak et al., Citation2008; Karyotaki et al., Citation2017; Spek et al., Citation2007). Applying the digital format when delivering CET-MD for self-perceived residual cognitive symptoms after MDD is a promising tool for the provision of low-threshold access to treatment. However, we know little about the feasibility of delivering CET-MD over the internet.
No previous studies have reported pre-, post-, and follow-up results from internet-delivered interventions targeting self-perceived residual cognitive symptoms after MDD. The current study aims to fill this gap of knowledge by examining the clinical effects and feasibility of a novel internet-delivered CET-MD in adults with self-perceived residual cognitive symptoms after MDD.
We hypothesized that:
Self-perceived residual cognitive symptoms would be significantly decreased from pre- to post-treatment and remain stable at the 6-month follow-up.
There would be no significant increase in self-reported depressive symptoms from pre- to post-treatment and at the 6-month follow-up.
Self-reported rumination would be significantly decreased from pre- to post-treatment and remain stable at the 6-month follow-up.
There would be a correlation between rumination and self-perceived cognitive symptoms.
It would be feasible to deliver CET-MD over the internet.
Methods
Design and procedures
The design was an open pilot pre-, post- and 6-month follow-up study. The study protocol was approved by the Regional Committee for Medical Research Ethics of Western Norway (2018/2384/REK vest).
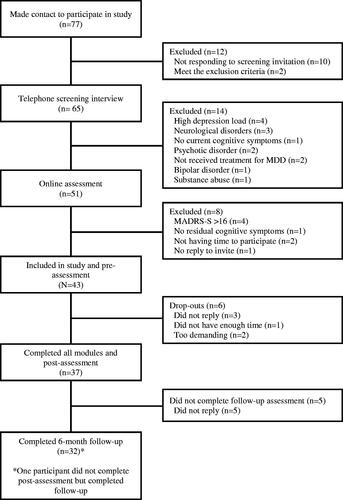
Participants were recruited through national advertisements in social media, public posters, and newspapers. Interested individuals made contact for a brief telephone interview with a clinical psychologist or psychology student under supervision. The Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, Citation1979) was used to assess depression load. The M.I.N.I. Norwegian version, a structural clinical interview for psychiatric diagnoses in the DSM-IV (Leiknes et al., Citation1999) was used to confirm previous depression diagnosis and absence of current depression. Further evaluation of eligibility was conducted online and included The Montgomery-Åsberg Depression Rating Scale Self-Report (MADRS-S; Svanborg & Ekselius, Citation2003; Svanborg & Åsberg, Citation2001). In addition, participants were asked to answer open ended questions such as “Describe your experience with cognitive difficulties” and “Do cognitive difficulties affect your daily life activities?” Responses were evaluated by a clinical psychologist. Inclusion criteria were as follows: (a) previously received treatment for MDD in primary or secondary healthcare services, (b) few or minor depression symptoms with no cardinal symptoms (reported sadness, loss of interest, and inability to feel, <16 MADRS), (c) self-perceived residual cognitive symptoms that affected daily functioning, (d) no changes in psychopharmaca status under the study period, (e) age between 18 and 65 years, and (f) internet access. The following exclusion criteria were used: (a) self-reported substance abuse, (b) neurological conditions or damage (e.g. autism, cerebral hemorrhage, and brain tumour), (c) bipolar disorder, and (d) psychosis.
Intervention
The intervention was internet-delivered and developed for adults experiencing self-perceived residual cognitive symptoms after depression. Development of the intervention was made by systematic user-based approaches. This included exploratory qualitative interviews with 16 former depressed adults during intervention planning and development to assess users’ experiences, needs, and feedback on intervention prototypes. Core features of the intervention included: (1) general psychoeducation regarding cognitive domains, such as attention, memory, and executive functions. Information regarding how self-perceived residual cognitive symptoms could affect daily functioning and treatment could be tailored, (2) attention training applying a modified version of Wells Attention Training Technique (Wells, Citation1990), and (3) compensatory strategies. In addition, common-factor elements were used in order to increase knowledge about symptoms, hope, management of personal goals, self-efficacy, and generalization. Ten modules () were provided to participants during the intervention period. Participants were asked to complete two modules each week and encouraged to finish the program within 5–7 weeks. The intervention applied a tunneled design where participants progressed sequentially through modules. To ensure that participants would find the intervention relevant, they could tailor the intervention by choosing which strategies and training tasks they would implement and use in their everyday life. Selected strategies and training tasks were stored in a personal workbook “my plan” where participants also evaluated the usefulness of each task. After completion, participants were asked to continue cognitive training and use the strategies they found helpful. Participants engaged with the program using their smartphones, tablets, or personal computers.
Table 1. Overview of intervention.
Brief telephone guidance was given weekly by a clinical psychologist or an advanced psychology student in clinical psychology with the aim to increase participant motivation and to provide feedback on assignments. All therapists had at least 4 hours of introduction to the intervention and received weekly supervision by a senior clinical psychologist.
Clinical outcomes
Primary outcomes
The Behavior Rating Inventory of Executive Function-Adult (BRIEF-A; Roth et al., Citation2005) is a 75-item self-report questionnaire designed to measure adults’ behaviors associated with executive problems. Participants are asked to rate the frequency of experienced executive problems as “never,” “sometimes,” or “often.” Responses are summed for each score, with higher scores indicating lower executive functioning. Results on the BRIEF-A yield three composite index scores. The Behavioral Regulation Index (BRI) includes the subscales inhibit, shift, self-monitor, and emotional control. The Metacognition Index (MI) consists of initiate, plan/organize, working memory, organization of materials, and task monitor. The BRI and MI can be combined to a Global Executive Composite (GEC), which gives an overall view of the respondents perceptions of their cognitive functioning. BRIEF-A T-scores can be derived from the raw scores; however in this study we analyzed raw scores due to lack of Norwegian norms and similar intervention studies reporting raw scores (Hagen et al., Citation2020; Hammar et al., Citation2020).The BRIEF-A have been validated in adults and have shown high internal consistency (Ciszewski et al., Citation2014; Roth et al., Citation2005). The pretreatment Cronbach’s alpha for the present study was excellent (a = .92). BRIEF-A assessments were distributed pretreatment, post-treatment, and at the 6-month follow-up.
The Perceived Deficits Questionnaire-Depression 5-item (PDQ-5; Sullivan et al., Citation1990) is a five-item questionnaire used for the brief assessment of subjective cognitive difficulties. It covers problems with concentration (e.g. “trouble concentrating on things like watching a television program or reading a book?”), memory (e.g. “forget what you talked about after a telephone conversation?”), and executive functioning (e.g. “have trouble getting things organized?”). Every item is rated on a scale from 0 (Never) to 4 (Almost always) to yield a sum score of 0 to 20, with higher scores indicating greater severity of perceived cognitive difficulties. In the present study the internal consistency of the PDQ-5 was adequate (a = .75). The PDQ-5 assessments were performed at pretreatment, after every other module during treatment (four measure points), post-treatment, and at the 6-month follow-up.
Secondary outcomes
The Rumination Response Scale (RRS; Treynor et al., Citation2003) is a questionnaire frequently used to assess rumination in depression. The 22-item RRS measures the current degree of ruminative responses to depressed mood. Examples of items are “think about how passive and unmotivated you feel”; “think about all your shortcomings, failings, faults, mistakes”; “think ‘What am I doing to deserve this?’” Each item is rated on a scale from 1 (almost never) to 4 (almost always) resulting in a total score ranging from 22 to 88, in which a higher score indicates higher levels of rumination (Roelofs et al., Citation2006). The RRS consists of three subscales: depression-related thoughts (12 items), brooding (5 items), and reflection (5 items). In the present study, the RRS demonstrated excellent internal consistency (a = .92). The RRS shows good psychometric properties in other studies as well (Johnson et al., Citation2008; Nolen-Hoeksema & Morrow, Citation1991). Participants were assessed with the RRS a total of 7 times: at pretreatment, four times throughout the treatment at the end of every other module, at post-treatment, and at the 6-month follow-up.
The Montgomery Åsberg Depression Rating Scale Self-report (MADRS-S; Svanborg & Åsberg, Citation2001) is a self-report questionnaire measuring depressive symptoms during the past three days. The MADRS-S consists of 9 items where the following symptoms are rated on a seven-point scale ranging from 0 to 6: reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts. The total score ranges from 0 to 54 where higher scores reflect higher levels of depressive symptoms. Previous studies have shown that online versions of the MADRS-S have acceptable internal consistency with alpha values between .71 and .83 (Holländare et al., Citation2010). Participants completed the MADRS-S at pretreatment, post-treatment, and the 6-month follow-up.
Feasibility measures
The recruitment process was measured by the number of individuals who showed interest in the study and were finally included in the study. Adherence was measured by drop-out rates and completion of modules. Acceptability was assessed by measuring participants’ satisfaction with the program and perceived negative effects by using a feedback questionnaire constructed by the research team. In this paper we report participants’ responses on general satisfaction (“Overall, how satisfied are you with the treatment you have received?”) and perceived negative effects of treatment (“Do you believe the treatment had any unfortunate effects on you and your life?”). Participants could rate their satisfaction on a scale ranging from 1 (not happy at all) to 5 (pleased) and dissatisfaction from 1 (very unfortunate effects) to 6 (only positive effects).
Statistics
SPSS Statistics version 25 was used to analyze the data. Linear mixed models for repeated measures analysis were performed to test outcome data on the group level at pretreatment, after every other module, at post-treatment, and at the 6-month follow-up. The intention-to-treat principle was used, such that all participants providing pre-assessments were included in the analysis. The model was fitted with full information maximum likelihood estimation to handle missing data by estimating the missing data from the observed data based on the assumption of data missing at random (Gueorguieva & Krystal, Citation2004).
Calculation of effect sizes (Cohen’s d) were derived from the estimated changes in means from pre- to post-treatment divided by the standard deviation before treatment.
A correlation analysis was conducted to identify the relationship between change scores on the BRIEF-A GEC and RRS.
Correction analysis was conducted by dividing alpha (.05) with number of tests (9).
Individual change in self-perceived residual cognitive symptoms was determined using the reliable change index (RCI) (Jacobson & Truax, Citation1991) for the primary outcome measure BRIEF-A GEC. The RCI was calculated using the formula 1.96*(SD1*√2*√(1-rel)), where SD1 is the observed standard deviation at pretreatment (17.38), and rel is the pretreatment reliability (.92).
Reliable change in the BRIEF-A GEC was categorized into three: (1) reliable change, (2) no change, (3) deterioration.
Results
Participants and recruitment
A total of 43 participants were included in the study. All participants completed the pre-intervention measures and at least one treatment module. Participant demographics are listed in .
Table 2. Participant characteristics.
The recruitment period lasted from March 2019 to September 2019, and a total of 77 individuals from different regional parts of Norway made contact to participate in the study; 43 were found eligible. See for participant flow through the study.
Primary outcomes
presents a summary of the results from linear mixed models. Results from the primary outcome measures BRIEF-A GEC and PDQ-5 showed large effect sizes and significant reduction in the scores from pre- to post-treatment. Significant changes were observed in both the BRIEF-A MI composite and BRI composite. The effect size observed in the MI was large and moderate in the BRI. The main effects over time in BRIEF-A and PDQ-5 were also significant at the 6-month follow-up.
Table 3. Primary and secondary outcomes.
Secondary outcomes
Participants reported a significantly lower level of total rumination in the RRS from pre- to post-treatment. Analysis also showed a significant reduction in the depression-, brooding-, and reflection subscales. The within-group effect sizes were moderate in both the total RRS, depression-, brooding-, and reflection subscales. Follow-up assessments at 6-months also showed a significant reduction in the total RRS. Correlation of change scores including BRIEF-A GEC and RRS showed a positive significant result (r = 0.40, p = .015).
Depression scores measured by the MADRS-S showed no significant change from pre-to post- and follow-up assessments.
Reliable change
A reliable change in the BRIEF-A GEC from pre- to post-treatment was obtained in 60% of the completers (). No reliable change from pre to post-treatment was observed in 35%, and deterioration in the BRIEF-A GEC was shown in 5% of the completers. Among the 31 participants that completed the intervention and the 6-month follow-up assessment, a reliable change in the BRIEF-A GEC was observed in 55%, with no reliable change in 42% and deterioration in 3%.
Table 4. Reliable changes from pretreatment to post-treatment and at 6-month follow-up, as measured by the BRIEF-A GEC.
Feasibility outcomes
Adherence to the provided modules were good with thirty-seven participants (86%) completing all modules and post-treatment assessments. The average number of completed modules were 9 (range 3–10). Reasons for not completing all modules were the following: participants did not provide any reason (n = 3); did not have enough time (n = 1); and the intervention was too demanding (n = 2). Attrition rates (74%) at the 6-month follow-up assessment were somewhat lower. Acceptability was high with 97% of those participants who completed the post-treatment assessment reported to be “pleased” or “mostly pleased” with the treatment they had received. Nobody replied “not being happy at all” with the treatment. With regard to perceived negative effects of treatment, 70% reported only positive effects, 22% no negative effects of matter, and 8% a few negative effects.
Discussion
The present study is the first to report pre-, post- and follow-up effects from a novel internet-delivered cognitive enhancement therapy for mood disorders (CET-MD) targeting self-perceived residual cognitive symptoms after MDD measured using self-reports. A total of 43 adults with self-perceived residual cognitive symptoms after MDD participated in the study.
All five hypotheses were supported. First, significant large effect sizes of change of self-perceived residual cognitive symptoms were reported from pre-to post-treatment. The effects remained stable at follow-up. On an individual level, 60% of the participants who completed the intervention showed a reliable significant decrease in self-perceived residual cognitive symptoms at post-treatment, and 54% obtained reliable change at 6-month follow-up. Second, no significant change in self-reported depressive symptoms was identified from pre- to post-treatment and follow-up. Third, significant moderate effects for rumination were observed from pre- to post-treatment, and a further reduction in rumination was shown at follow-up. Fourth, a significant correlation between rumination and self-perceived residual cognitive symptoms was observed. Fifth, completion rates (86%) and overall treatment satisfaction among participants completing the intervention were high (97%), supporting the feasibility of the intervention.
Individual improvement rates (60%) observed in the current study are similar to those previously reported in internet-delivered treatment studies in general (Berger et al., Citation2009; Karyotaki et al., Citation2018; Warmerdam et al., Citation2010). Deterioration rates (5%) in the present study are also comparable with a meta-analysis reporting of internet-delivered treatment deterioration rates, which have an average of 5.8% (Rozental et al., Citation2017). However, there are few cognitive intervention studies for individuals experiencing cognitive problems after MDD that have reported reliable change. One exception is a study by Listunova et al. (Citation2020) that found reliable improvement on objective cognitive tests measuring attention in 34% of the participants at post-treatment assessment. This was somewhat lower than identified in the current study. This discrepancy may be explained by the fact that the current intervention was tailored to the user group, including systematic user involvement. Also, the results could be attributed to the use of different measurements as the study by Listunova et al. did report individual change in an objective cognitive test measuring attention and not self-perceived cognitive difficulties. The current study complements and expands on the existing literature by reporting individual change in self-perceived residual cognitive symptoms.
The moderate effects in rumination and significant correlation with changes in self-perceived residual cognitive symptoms found in the present study is in line with a previous study showing that strengthening of cognitive abilities is linked to less rumination (Demeyer et al., Citation2012). This finding has clinical implications that support the view that providing tasks that target cognition could be a tool that helps patients gain control over excessive rumination, which could expand patients’ capacity for attentiveness toward everyday demands or tasks.
Lastly, regarding the feasibility, the intervention had high completion and satisfaction rates, thus supporting the fifth hypothesis. Other cognitive interventions delivered to the same target group have shown lower or slightly higher compliance rates between 62% and 95% (Hammar et al., Citation2020; Semkovska & Ahern, Citation2017). The high number of completers and high level of satisfaction with treatment are promising results for a future trial and implementation in routine care.
The present study has clinical implications such as supporting the use of the internet to deliver CET-MD and potentially increasing access to treatment. Furthermore, the observed reduction in rumination and long-term effects are of importance. The findings may also have implications for patients with neurological disorders, such as multiple sclerosis, where depression symptoms have been found to be associated with self-perceived cognitive difficulties rather than objective cognitive impairment measured using neuropsychological tests (Middleton et al., Citation2006). Another contribution is the successful use of psychology students as therapists, suggesting that experienced therapists could take a role as supervisors and allow less experienced therapists to have patient contact, which could increase the cost-effectiveness of a large scale trial.
The current study has limitations. The first limitation is the low sample size and no control or comparison group that could have inflated effect-sizes. Conclusions are therefore preliminary, and results should be interpreted in the light of this. The second concerns the results, which should be generalized with caution as the majority of the participants were females and had higher education. Still, this was a group with a history of severe mental health problems, where 74% had experienced more than 2 episodes of MDD and 57% had been depressed for more than 2 years of their lives. Altogether, this indicates that the sample was highly clinical and in need of treatment. A third limitation is the fact that objective neuropsychological tests were not applied in this study. Complementing objective neuropsychological assessment with self-perceived residual cognitive symptoms measured using self-reports could provide a further understanding about the association between the two and the effects of the current intervention.
Conclusions
Despite the abovementioned limitations, the current study supports that internet-delivered CET-MD may reduce self-perceived residual cognitive symptoms and rumination. High completion and satisfaction rates support the feasibility of the intervention. Results are promising given that there is a great need for scalable interventions targeting self-perceived residual cognitive symptoms after MDD. Further, longitudinal assessments within a controlled trial will be crucial to identify the effects of the intervention in preventing an increase of depressive relapse rates.
Acknowledgements
We want to thank all participants. We want to thank the students in clinical psychology Silje Stevens, Ellen Marie Løkslid, Iselin Furset, Martine Hansen, and clinical psychologist Marthe Myklebost for providing therapist guidance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allott, K., Fisher, C. A., Amminger, G. P., Goodall, J., & Hetrick, S. (2016). Characterizing neurocognitive impairment in young people with major depression: State, trait, or scar? Brain and Behavior, 6(10), e00527. https://doi.org/10.1002/brb3.527
- Barak, A., Hen, L., Boniel-Nissim, M., & Shapira, N. A. (2008). A comprehensive review and a meta-analysis of the effectiveness of internet-based psychotherapeutic interventions. Journal of Technology in Human Services, 26(2–4), 109–160. https://doi.org/10.1080/15228830802094429
- Benitez, A., Horner, M. D., & Bachman, D. (2011). Intact cognition in depressed elderly veterans providing adequate effort. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 26(3), 184–193. https://doi.org/10.1093/arclin/acr001
- Berger, T., Hohl, E., & Caspar, F. (2009). Internet-based treatment for social phobia: A randomized controlled trial. Journal of Clinical Psychology, 65(10), 1021–1035. https://doi.org/10.1002/jclp.20603
- Beshai, S., Dobson, K. S., Bockting, C. L., & Quigley, L. (2011). Relapse and recurrence prevention in depression: Current research and future prospects. Clinical Psychology Review, 31(8), 1349–1360. https://doi.org/10.1016/j.cpr.2011.09.003
- Bockting, C. L., Hollon, S. D., Jarrett, R. B., Kuyken, W., & Dobson, K. (2015). A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review, 41, 16–26. https://doi.org/10.1016/j.cpr.2015.02.003
- Ciszewski, S., Francis, K., Mendella, P., Bissada, H., & Tasca, G. A. (2014). Validity and reliability of the Behavior Rating Inventory of Executive function – adult version in a clinical sample with eating disorders. Eating Behaviors, 15(2), 175–181. https://doi.org/10.1016/j.eatbeh.2014.01.004
- Conradi, H., Ormel, J., & De Jonge, P. (2011). Presence of individual (residual) symptoms during depressive episodes and periods of remission: A 3-year prospective study. Psychological Medicine, 41(6), 1165–1174. https://doi.org/10.1017/S0033291710001911
- Cuijpers, P. (2015). Psychotherapies for adult depression: Recent developments. Current Opinion in Psychiatry, 28(1), 24–29. https://doi.org/10.1097/YCO.0000000000000121
- Cuijpers, P., Van Straten, A., & Andersson, G. (2008). Internet-administered cognitive behavior therapy for health problems: A systematic review. Journal of Behavioral Medicine, 31(2), 169–177. https://doi.org/10.1007/s10865-007-9144-1
- Davis, R. N., & Nolen-Hoeksema, S. (2000). Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research, 24(6), 699–711. https://doi.org/10.1023/A:1005591412406
- Demeyer, I., De Lissnyder, E., Koster, E. H., & De Raedt, R. (2012). Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: A prospective study in remitted depressed adults. Behaviour Research and Therapy, 50(5), 292–297. https://doi.org/10.1016/j.brat.2012.02.012
- Dhillon, S., Videla-Nash, G., Foussias, G., Segal, Z. V., & Zakzanis, K. K. (2020). On the nature of objective and perceived cognitive impairments in depressive symptoms and real-world functioning in young adults. Psychiatry Research, 287, 112932. https://doi.org/10.1016/j.psychres.2020.112932
- Douglas, K. M., Peckham, A., Porter, R., & Hammar, A. (2019). Cognitive enhancement therapy for mood disorders: A new paradigm? The Australian and New Zealand Journal of Psychiatry, 53(12), 1148–1150. https://doi.org/10.1177/0004867419873711
- Eaton, W. W., Shao, H., Nestadt, G., Lee, H. B., Lee, B. H., Bienvenu, O. J., & Zandi, P. (2008). Population-based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry, 65(5), 513–520. https://doi.org/10.1001/archpsyc.65.5.513
- Gueorguieva, R., & Krystal, J. H. (2004). Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the archives of general psychiatry. Archives of General Psychiatry, 61(3), 310–317. https://doi.org/10.1001/archpsyc.61.3.310
- Hagen, B. I., Lau, B., Joormann, J., Småstuen, M. C., Landrø, N. I., & Stubberud, J. (2020). Goal management training as a cognitive remediation intervention in depression: A randomized controlled trial. Journal of Affective Disorders, 275, 268–277. https://doi.org/10.1016/j.jad.2020.07.015
- Hammar, Å., & Årdal, G. (2009). Cognitive functioning in major depression-a summary. Frontiers in Human Neuroscience, 3, 26–27. https://doi.org/10.3389/neuro.09.026.2009
- Hammar, Å., Semkovska, M., Borgen, I. M., Myklebost, S., Ronold, E. H., Sveen, T., Ueland, T., Porter, R., & Johnson, S. L. (2020). A pilot study of cognitive remediation in remitted major depressive disorder patients. Applied Neuropsychology: Adult. https://doi.org/10.1080/23279095.2020.1726919
- Holländare, F., Andersson, G., & Engström, I. (2010). A comparison of psychometric properties between internet and paper versions of two depression instruments (BDI-II and MADRS-S) administered to clinic patients. Journal of Medical Internet Research, 12(5), e49. https://doi.org/10.2196/jmir.1392
- Jacobson, N. S., & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. https://doi.org/10.1037/10109-042
- James, S. L., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., Abbastabar, H., Abd-Allah, F., Abdela, J., Abdelalim, A., Abdollahpour, I., Abdulkader, R. S., Abebe, Z., Abera, S. F., Abil, O. Z., Abraha, H. N., Abu-Raddad, L. J., Abu-Rmeileh, N. M. E., Accrombessi, M. M. K., … Murray, C. J. L. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
- Johnson, S. L., McKenzie, G., & McMurrich, S. (2008). Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognitive Therapy and Research, 32(5), 702–713. https://doi.org/10.1007/s10608-007-9158-6
- Joormann, J. (2010). Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science, 19(3), 161–166. https://doi.org/10.1177/0963721410370293
- Karyotaki, E., Ebert, D. D., Donkin, L., Riper, H., Twisk, J., Burger, S., Rozental, A., Lange, A., Williams, A. D., Zarski, A. C., Geraedts, A., van Straten, A., Kleiboer, A., Meyer, B., Ünlü Ince, B. B., Buntrock, C., Lehr, D., Snoek, F. J., Andrews, G., … Cuijpers, P. (2018). Do guided internet-based interventions result in clinically relevant changes for patients with depression? An individual participant data meta-analysis. Clinical Psychology Review, 63, 80–92. https://doi.org/10.1016/j.cpr.2018.06.007
- Karyotaki, E., Riper, H., Twisk, J., Hoogendoorn, A., Kleiboer, A., Mira, A., Mackinnon, A., Meyer, B., Botella, C., Littlewood, E., Andersson, G., Christensen, H., Klein, J. P., Schröder, J., Bretón-López, J., Scheider, J., Griffiths, K., Farrer, L., Huibers, M. J. H., … Cuijpers, P. (2017). Efficacy of self-guided internet-based cognitive behavioral therapy in the treatment of depressive symptoms: A meta-analysis of individual participant data. JAMA Psychiatry, 74(4), 351–359. https://doi.org/10.1001/jamapsychiatry.2017.0044
- Leiknes, K. A., Legange, R. S., Malt, E. A., & Malt, U. (1999). Mini internasjonalt neuropsykiatrisk intervju. In D. Sheehan, J. Janavs, J. Baker, K. Harnett-Sheenan, E. Knapp & M. Sheehan (Eds.), Mini International Neuropsychiatric Interview. University of South Florida.
- Listunova, L., Kienzle, J., Bartolovic, M., Jaehn, A., Grützner, T. M., Wolf, R. C., Aschenbrenner, S., Weisbrod, M., & Roesch-Ely, D. (2020). Cognitive remediation therapy for partially remitted unipolar depression: A single-blind randomized controlled trial. Journal of Affective Disorders, 276, 316–326. https://doi.org/10.1016/j.jad.2020.07.008
- Løvstad, M., Sigurdardottir, S., Andersson, S., Grane, V., Moberget, T., Stubberud, J., & Solbakk, A. (2016). Behavior Rating Inventory of Executive Function Adult Version in patients with neurological and neuropsychiatric conditions: Symptom levels and relationship to emotional distress. Journal of the International Neuropsychological Society: JINS, 22(6), 682–694. https://doi.org/10.1017/S135561771600031X
- Majer, M., Ising, M., Künzel, H., Binder, E., Holsboer, F., Modell, S., & Zihl, J. (2004). Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychological Medicine, 34(8), 1453–1463. https://doi.org/10.1017/S0033291704002697
- Malivoire, B. L., Hare, C. J., & Hart, T. L. (2018). Psychological symptoms and perceived cognitive impairment in multiple sclerosis: The role of rumination. Rehabilitation Psychology, 63(2), 286–294. https://doi.org/10.1037/rep0000180
- Middleton, L. S., Denney, D. R., Lynch, S. G., & Parmenter, B. (2006). The relationship between perceived and objective cognitive functioning in multiple sclerosis. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 21(5), 487–494. https://doi.org/10.1016/j.acn.2006.06.008
- Montgomery, S. A., & Åsberg, M. (1979). A new depression scale designed to be sensitive to change. The British Journal of Psychiatry: The Journal of Mental Science, 134(4), 382–389. https://doi.org/10.1192/bjp.134.4.382
- Moritz, S., Ferahli, S., & Naber, D. (2004). Memory and attention performance in psychiatric patients: Lack of correspondence between clinician-rated and patient-rated functioning with neuropsychological test results. Journal of the International Neuropsychological Society: JINS, 10(4), 623–633. https://doi.org/10.1017/S1355617704104153
- Motter, J. N., Pimontel, M. A., Rindskopf, D., Devanand, D. P., Doraiswamy, P. M., & Sneed, J. R. (2016). Computerized cognitive training and functional recovery in major depressive disorder: A meta-analysis. Journal of Affective Disorders, 189, 184–191. https://doi.org/10.1016/j.jad.2015.09.022
- Mueller, T. I., Leon, A. C., Keller, M. B., Solomon, D. A., Endicott, J., Coryell, W., & Maser, J. D. (1999). Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry, 156(7), 1000–1006. https://doi.org/10.1176/ajp.156.7.1000
- Nolen-Hoeksema, S., & Morrow, J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology, 61(1), 115–121. https://doi.org/10.1037/0022-3514.61.1.115
- Petersen, J. Z., Porter, R. J., & Miskowiak, K. W. (2019). Clinical characteristics associated with the discrepancy between subjective and objective cognitive impairment in depression. Journal of Affective Disorders, 246, 763–774. https://doi.org/10.1016/j.jad.2018.12.105
- Reppermund, S., Ising, M., Lucae, S., & Zihl, J. (2009). Cognitive impairment in unipolar depression is persistent and non-specific: Further evidence for the final common pathway disorder hypothesis. Psychological Medicine, 39(4), 603–614. https://doi.org/10.1017/S003329170800411X
- Rock, P., Roiser, J., Riedel, W., & Blackwell, A. (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. https://doi.org/10.1017/S0033291713002535
- Roelofs, J., Muris, P., Huibers, M., Peeters, F., & Arntz, A. (2006). On the measurement of rumination: A psychometric evaluation of the ruminative response scale and the rumination on sadness scale in undergraduates. Journal of Behavior Therapy and Experimental Psychiatry, 37(4), 299–313. https://doi.org/10.1016/j.jbtep.2006.03.002
- Rossell, S. L. (2020). Identifying the barriers to implementing cognitive remediation therapy for mood disorders. The Australian and New Zealand Journal of Psychiatry, 54(2), 203–204. https://doi.org/10.1177/0004867419887233
- Roth, R. M., Isquith, P. K., & Gioia, G. A. (2005). Behavior rating inventory of executive function-adult version (BRIEF-A). Psychological Assessment Resources.
- Rozental, A., Magnusson, K., Boettcher, J., Andersson, G., & Carlbring, P. (2017). For better or worse: An individual patient data meta-analysis of deterioration among participants receiving Internet-based cognitive behavior therapy. Journal of Consulting and Clinical Psychology, 85(2), 160–177. https://doi.org/10.1037/ccp0000158
- Saragoussi, D., Haro, J. M., Boulenger, J. P., Jönsson, B., Knapp, M., Caillou, H., Chalem, Y., Milea, D., & François, C. (2013). Patient-reported cognitive dysfunction negatively impacts functioning in patients with major depressive disorder–preliminary findings from the PERFORM study. Value in Health, 16(7), A543–A544. https://doi.org/10.1016/j.jval.2013.08.1383
- Saragoussi, D., Touya, M., Haro, J. M., Jönsson, B., Knapp, M., Botrel, B., Florea, I., Loft, H., & Rive, B. (2017). Factors associated with failure to achieve remission and with relapse after remission in patients with major depressive disorder in the PERFORM study. Neuropsychiatric Disease and Treatment, 13, 2151–1265. https://doi.org/10.2147/NDT.S136343
- Schmid, M., & Hammar, A. Å. (2013). A follow-up study of first episode major depressive disorder. Impairment in inhibition and semantic fluency-potential predictors for relapse? Frontiers in Psychology, 4, 633–613. https://doi.org/10.3389/fpsyg.2013.00633
- Semkovska, M., & Ahern, E. (2017). Online neurocognitive remediation therapy to improve cognition in community-living individuals with a history of depression: A pilot study. Internet Interventions, 9, 7–14. https://doi.org/10.1016/j.invent.2017.04.003
- Semkovska, M., Quinlivan, L., O’Grady, T., Johnson, R., Collins, A., O’Connor, J., Knittle, H., Ahern, E., & Gload, T. (2019). Cognitive function following a major depressive episode: A systematic review and meta-analysis. The Lancet Psychiatry, 6(10), 851–861. https://doi.org/10.1016/S2215-0366(19)30291-3
- Solomon, D. A., Keller, M. B., Leon, A. C., Mueller, T. I., Lavori, P. W., Shea, M. T., Coryell, W., Warshaw, M., Turvey, C., Maser, J. D., & Endicott, J. (2000). Multiple recurrences of major depressive disorder. The American Journal of Psychiatry, 157(2), 229–233. https://doi.org/10.1176/appi.ajp.157.2.229
- Spek, V., Cuijpers, P., Nyklíček, I., Riper, H., Keyzer, J., & Pop, V. (2007). Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: A meta-analysis. Psychological Medicine, 37(3), 319–328. https://doi.org/10.1017/S0033291706008944
- Sullivan, M. J., Edgley, K., & Dehoux, E. (1990). A survey of multiple sclerosis: I. Perceived cognitive problems and compensatory strategy use. Canadian Journal of Rehabilitation, 4(2), 99–105.
- Svanborg, P., & Åsberg, M. (2001). A comparison between the Beck Depression Inventory (BDI) and the self-rating version of the Montgomery Åsberg Depression Rating Scale (MADRS). Journal of Affective Disorders, 64(2–3), 203–216. https://doi.org/10.1016/S0165-0327(00)00242-1
- Svanborg, P., & Ekselius, L. (2003). Self-assessment of DSM-IV criteria for major depression in psychiatric out- and inpatients. Nordic Journal of Psychiatry, 57(4), 291–296. https://doi.org/10.1080/08039480307281
- Tatay-Manteiga, A., Cauli, O., Tabarés-Seisdedos, R., Michalak, E. E., Kapczinski, F., & Balanzá-Martínez, V. (2019). Subjective neurocognition and quality of life in patients with bipolar disorder and siblings. Journal of Affective Disorders, 245, 283–288. https://doi.org/10.1016/j.jad.2018.11.012
- Treynor, W., Gonzalez, R., & Nolen-Hoeksema, S. (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. https://doi.org/10.1023/A:1023910315561
- Warmerdam, L., Smit, F., van Straten, A., Riper, H., & Cuijpers, P. (2010). Cost-utility and cost-effectiveness of internet-based treatment for adults with depressive symptoms: Randomized trial. Journal of Medical Internet Research, 12(5), e53. https://doi.org/10.2196/jmir.1436
- Wells, A. (1990). Panic disorder in association with relaxation induced anxiety: An attentional training approach to treatment. Behavior Therapy, 21(3), 273–280. https://doi.org/10.1016/S0005-7894(05)80330-2
- Whiteford, H. A., Ferrari, A. J., Degenhardt, L., Feigin, V., & Vos, T. (2015). The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS One, 10(2), e0116820. https://doi.org/10.1371/journal.pone.0116820