ABSTRACT
Previous publications have reached different conclusions about the balance between the socioeconomic benefits of tilapia introduction for aquaculture and capture fisheries, and the potential negative impacts of these species on ecosystem services such as the provisioning of food, habitat, and water quality. This review (1) provides a new estimate of the global scale of tilapia introduction and the reported occurrence of impacts to ecosystem services; (2) assesses whether reported changes to ecosystem services differ among species, regions and type of ecological effect reported; and (3) determine how perceptions of tilapia introduction are related to the reported occurrence of ecological effects and/or the contribution of tilapia to countries’ economies. Feral tilapia populations now exist in at least 114 countries. The majority of research is consistent with the occurrence of ecological change as a result of tilapia introduction, regardless of species or region of study, but there are important regional influences including the economic contributions of tilapia. There is an increasing recognition that introductions provide both significant benefits and considerable but not fully documented harm to ecosystem services. Stakeholders should consider both ecological effects and socioeconomic context, such as reviewed here, in making decisions about the introduction of tilapia.
Introduction
The dissemination of aquatic species as a result of globalization has often had unexpected and harmful impacts on receiving ecosystems, human communities and economies (Lodge et al., Citation2006; Keller et al., Citation2009), though introduced species in some cases may provide significant benefits (Davis et al., Citation2011; Gleditsch and Carlo, Citation2011; Schlaepfer et al., Citation2011; Barnes et al., Citation2014). Global databases of introductions indicate relatively few negative impacts for many freshwater fish species particularly relative to the economic value of their production (Gozlan, Citation2008). Different authors have, however, reached different conclusions about the extent of undesirable impacts on ecosystems relative to the benefits of aquaculture and fisheries, particularly those based tilapia (Leveque, Citation2002; Canonico et al., Citation2005; Gozlan, Citation2008; Brummett and Ponzoni, Citation2009; De Silva et al., Citation2009; Vitule et al., Citation2009). Since 2002 tilapia (Cichlidae: primarily of genera Oreochromis, Tilapia, and Serranochromis. In the remainder of this paper, “tilapia” refers to these species collectively) have been one of the fastest growing global aquaculture sectors (Bostock et al., Citation2010), expanding twice as fast as fisheries based on salmonids or carps (FAO, Citation2011). Given the rapid expansion of tilapia aquaculture, many decisions are being made to introduce fishes into new watersheds which may have far-reaching ecological, social, and economic effects through changes to ecosystem services.
Ecosystem services include the goods and other resources that contribute to human welfare directly through markets or other non-market values (Millennium Ecosystem Assessment, Citation2005). Changes in the biotic components of an ecosystem, including changes caused by species introduction, may increase some services while simultaneously decreasing others (Hooper et al., Citation2005; Lodge et al., 2012). Specifically, increasing tilapia production may cause a decrease in populations of harvestable native species, a decrease in habitat, or recreational and cultural ecosystem services linked to the native species harmed by tilapia (Canonico et al., Citation2005). Differing opinions on the type of ecosystem services provided or affected by tilapia prevent a comprehensive policy framework for the management of this species (Redpath et al., Citation2013). The extensive review provided here may reduce areas of disagreement and allow more informed future decisions regarding tilapia species.
Global tilapia production in 2012 exceeded 9.6 million tons, 4.5 million of which came from aquaculture and was valued over $7.6 billion USD. From capture fisheries, at least 30% of global tilapia production comes from feral tilapia populations from six main countries: Mexico (62935t), Philippines (44896t), Thailand (38300t), Indonesia (31960t), Sri Lanka (28250t), and Brazil (25246t). In some countries the majority of all reported freshwater fish capture production derives from feral tilapia: Cuba and Panama (100%), Nicaragua (84%), Mexico (58%), and Sri Lanka (54%; FAO Fisheries and Aquaculture Department, 2014). While the production of tilapia from these ecosystems provides easily quantified monetary benefits, the possible tradeoff in terms of losses of other ecosystem goods or services which are likely to have accompanied the introduction and harvest of tilapia are less well quantified.
The ecosystem service tradeoffs potentially caused by tilapia production have been the subject of previous reviews that documented undesirable ecological effects (Pullin et al., Citation1997; Canonico et al., Citation2005). For example, tilapia are considered to be the proximate cause of declines in many native fishes: desert pupfish (Cyprinodon macularius) in the South West United States (Black, Citation1980; Varela-Romero et al., Citation2000), Sinarapan (Mistichthys luzonensis) from Lake Buhi in the Philippines (Gindelberger, Citation1981), and various native cichlids that once comprised important components of fisheries harvests in Lake Nicaragua (McKaye et al., Citation1995) and Lake Victoria (Mkumbo and Ligtvoet, Citation1992; Goudswaard et al., Citation2002). Tilapia have also been implicated in harm to other fisheries such as milkfish aquaculture in Nauru (Ranoemihardjo, Citation1981), and cyprinid harvests in India (Sugunan, Citation2000; Sugunan, Citation1995). Reductions in other ecosystem services caused by tilapia are associated with the loss of aquatic plants and the habitats they provide to native species (Crutchfield, Citation1995), as well as undesirable biotic and abiotic changes associated with eutrophication (Figueredo and Giani, Citation2005). In contrast, some tilapia fisheries may act to protect native species by removing fishing pressure from natives (Arthur et al., Citation2010).
Due to the diversity of reported ecological effects and the lack of a quantitative review that considers the strength of inferences from different kinds of study designs conflicting perceptions of the ecological impacts of tilapia have emerged. Assessments of tilapia impacts range from no negative impacts to extremely harmful (Canonico et al., Citation2005; Gozlan, Citation2008). This range of perceptions is perpetuated because areas where tilapia have been introduced for culture and capture fisheries are concentrated where resources to support ecological research are limited (Canonico et al., Citation2005; UNESCO, Citation2007; De Silva et al., Citation2009; Josupeit, Citation2010; Skelton & Swartz, Citation2011; Amano and Sutherland, Citation2013). It is likely that the absence of evidence for ecological effects is often substituted in management and policy decisions for evidence of the absence of ecological effects, in which case the monetized value of tilapia production may drive perceptions and decisions about tilapia introduction (Lövei et al., 2012). The goal of this review is to comprehensively assess the reported effects of tilapia introductions on ecosystem services, motivated by a desire to inform those making decisions about the introduction and management of tilapia.
To accomplish this general goal, three specific questions are addressed: (1) What proportion of tilapia introductions are associated with ecological effects? (2) Does the presence or absence of ecological effects vary by the type of ecological effect considered, the particular tilapia species, the time and place where the introduction has occurred, or the study design used to assess effects? (3) Do perceptions of the relative harm or benefit differ by species, region, or the presence of ecological and socioeconomic effects?
The first question addresses the number of countries in which tilapia introductions have caused ecological effects. A review of global freshwater fish introductions (Gozlan, Citation2008) reported that 5–20% of cichlid introductions (of which tilapia are the major constituent (Welcomme, Citation2011)) have resulted in negative ecological impacts, in contrast with the experience of tilapia researchers in particular cases (Vitule et al., Citation2009). A new count of the number of countries that have received tilapia introductions is provided, and an improved estimate for the proportion of countries for which tilapia introductions have been associated with ecological effects is derived. Ecological effects in this review are defined as demonstrable changes in ecosystem function and/or the provisioning of ecosystem goods and services.
The second question asks if the presence or absence of ecological effects vary by the tilapia species, type of effect, the year, and place of the study, or the study design. The life-history traits of tilapia that have made them an aquacultural success (e.g., rapid somatic and population growth, wide environmental tolerances) are the same traits often associated with invasive species (Bruton and van As, Citation1986; Lorenzen, Citation2000; Norrgren et al., Citation2000; Canonico et al., Citation2005). These and other traits differ between common species of tilapia, such that different species might be expected to have different ecological effects. For example, while most tilapia are opportunistic omnivores, species of the genus Tilapia are typically macrophyte feeders while Oreochromis are typically microphagous (Beveridge and Baird, Citation2000). As a result, Tilapia have previously been used to control aquatic weeds (Hauser et al., Citation1977), suggesting these species may impact plant habitats relatively frequently. Similarly, O. mossambicus is the only cichlid listed as among the world's worst 100 hundred invasive species (Lowe et al., Citation2000), raising the expectation that O. mossambicus should be associated with more undesirable ecosystem impacts than other tilapia.
The occurrence and magnitude of the ecological effects from a species introduction is often dependent on the characteristics of the community and ecosystem that receives them (Kolar and Lodge, Citation2001; Bruno et al., Citation2005; Zenni and Nuñez, Citation2013). Tilapia are native to Africa and a small portion of the Middle East, but are now found throughout the world, particularly in tropical regions. Thus, it was predicted that hybridization and competition between introduced and native species of tilapia would be greatest in Africa because basic ecological theory expects these interactions to be strongest among similar species though recent work provides some evidence to the contrary in fish (Abrams, Citation1983; Perry et al., Citation2002; Mouchet et al., Citation2013). To answer this second question, mixed-model logistic regression was used to estimate the importance of study design, species, and regions to the reported presence or absence of ecological impacts.
Finally, the third question examines whether perceptions of the relative harm or benefit of tilapia introduction differ by species, region, or the presence of ecological and socioeconomic effects. For each of the research papers reviewed the authors’ conclusion, if any, about whether the effect on ecosystem services of the introduced tilapia studied was positive (overall increase in ecosystem services), negative (decrease in ecosystem services), or both (positive and negative changes) were recorded. Mixed-model ordinal regression was used to model the contribution of ecological effects, species of tilapia, region, study design, and the relative monetary value of introduced tilapia fisheries to a country's economy in explaining the observed perception of the impact on ecosystem services. It was predicted that reports of ecological effects would be negatively correlated with the perception of tilapia introduction, but that the contribution of tilapia production to a country's economy would be positively correlated with perception as well as the amount of research put towards tilapia ecology.
Methods
Literature review
Original published reports of the ecological impacts of tilapia introduction were sought from all sources, dates and locations, including peer reviewed publications, books, government and non-governmental organization reports, conference proceedings, and theses. A comprehensive keyword search using the following Boolean search string, “TS = (tilapi* OR oreochromis OR sarotherodon) AND TS = (impact OR invasiv* OR effect OR introduc* OR compet* OR pred* OR hybrid* OR exotic OR biodiversity OR interact* OR nonnative OR non-native OR nonindigenous OR non-indigenous OR genetic* OR establish* OR habitat OR feral OR consequence* OR naturaliz* OR threat)” was used in the following online databases: Academic Search Premier, Applied Science and Technology Index, Biological Abstracts, General Science Abstracts, and Web of Science. All relevant search results were retrieved. Only original work in each report was reviewed; cited works were retrieved and reviewed separately whenever possible. All reports containing original records of introduction and/or ecological effects were subsequently forward-searched using Web of Science. Identified reports were deemed unavailable if they could not be located in the WorldCat database (http://www.worldcat.org/) or retrieved through Inter-Library Loan at the University of Notre Dame Libraries. Reports in languages other than English (7% of total identified) were not included.
For each paper the focal tilapia species and the country or geographic region of introduction was recorded. Relying on the original authors’ interpretations, reported ecological effects attributed to tilapia introductions were placed into four categories: effects on resident fish; effects on non-fish biota; effects on abiotic factors; and no effects considered. Effects on resident fish and non-fish biota were defined as changes in at least one demographic parameter (e.g., population size or somatic growth rates) of the resident species population(s) attributed to the introduced tilapia species. Changes in these parameters were recorded as positive, negative, or complex, the later representing multiple parameter interactions. The resident fish category included native fish species and naturalized introduced species. Effects on non-biotic habitat components consisted of common water quality measurements (e.g., dissolved oxygen, pH, and chlorophyll) typically as an indicator of eutrophication. The “no effects considered” category consisted of studies on introduced tilapia populations for which none of the first three categories of ecological effects were considered. These studies were typically focused narrowly on tilapia ecology, for example, on a comparison of somatic growth of tilapia under different conditions with no explicit consideration of the reciprocal effects of tilapia on the ecosystem.
Ecological mechanisms by which any reported effects were attributed by the original authors were also categorized: competition, disease, and interspecific hybridization. The competition category consisted of any type of competition for food or space, and included interference and exploitative competition. Disease consisted of instances when tilapia served as a vector for nonindigenous pathogens or parasites, and/or provided a reservoir which maintained or propagated the incidence of an existing pathogen or parasite. The hybridization category consisted of interbreeding with native or previously introduced tilapia species.
Different study designs allow different strengths of inference about cause and effect (Diamond, Citation1986), e.g., about whether a given impact on ecosystem services is caused by tilapia introduction or some other (potentially unmeasured) cause. Thus, conclusions about impacts were examined contingent on the type of study design employed. Four components of study designs used by the original authors to make inferences about ecological effects and mechanisms were categorized: quantitative measurement; time-series; observations or experiments with replicates; and observations or experiments with control or reference treatments (i.e., lacking introduced tilapia). Quantitative measurement was defined as the numerical measurement of the parameters implicated in the effect or mechanism categories; purely anecdotal reports, presence-absence reports, and species lists were not included as quantitative measures. Time series was defined as the use of data collected either before and after tilapia introduction, or collected at least three data points across 10 years post tilapia introduction (Walpole et al., Citation2009). Replication was defined as the presence of at least two replicates (i.e., n ≥ 2) for at least one tilapia introduction treatment or where the populations of introduced tilapia can be reasonably considered to be distinct from other populations. In most cases, this meant multiple isolated water bodies where tilapia had been introduced. Distant populations in a large lake or river, or experimental ponds completely drained between replicates were also included. Finally, a control or reference treatment was defined as a similar waterbody where tilapia were not introduced. For the hybridization mechanism, quantitative and control data were defined more specifically. Quantitative data was defined as the determination of hybridization using morphometric or meristic methods, genetic techniques, or experimental crosses. A hybridization study was recorded as having control or reference treatments when the study reported high-order genetic mixing (e.g., via the detection of backcrosses and/or the fertility of hybrids).
Time-series, replication, and controls were not necessarily quantitative, e.g., anecdotes of tilapia impacts may still have a time-series component. Thus, study design components were independent, and for each paper, we extracted a fully crossed matrix of impacts and mechanisms times study design (i.e., a 7 × 4 matrix), in which was recorded the presence, absence, or not considered of each study design feature for each impact and each mechanism. The full review instrument and citations are provided in the online supplement.
The original authors’ description of the socioeconomic impact of tilapia was categorized as positive, negative, both, or not considered. Determinations of “positive” or “negative” perceptions were made only when the original paper included clear and explicit statements. Examples of phrases that led to a score of “negative” included: “We therefore consider that O. niloticus poses an unacceptable risk to its congenerics in the Limpopo River system” (Zengeya et al., Citation2012), and “The species have become widely established, with a range of negative consequences for the rich natural fish fauna of this Central American country” (McCrary et al., Citation2007). Statements scored as “positive” included: “The general importance of tilapias to the Asian region is evident from the fact that the first ever, multination, selective breeding programme on a cultured finfish species in Asia was on O. niloticus” (De Silva et al., Citation2006) and “Use of these non-native tilapia and carp species in fisheries enhancement in mainland SE Asia supported substantial increases in harvestable biomass while having only mild impacts on native fish communities” (Arthur et al., Citation2010). In contrast, where authors acknowledged both significant benefits and harms, “both” was assigned. The default category was “not-considered.”
Analyses
Proportion of introductions associated with ecological impacts
The number of countries from which ecological impacts have been attributed to tilapia was divided by the total number of countries to which tilapia had been introduced and in which a specified level of research effort had been directed toward detecting ecological impacts. Research effort was categorized into six bins based on study designs (see below). The bins represent a gradient of increasing strength of inference as a result of increasing sophistication of the research effort. A range of answers to the first research question is provided contingent on which bin of effort was used as a denominator. This approach recognized that detecting impacts of tilapia is analogous to ecological sampling for the presence of cryptic, rare, or endangered species where non-detection may be a result of insufficient sampling effort. This metric does not indicate the overall scientific quality of the papers reviewed, because for many papers, the information relevant to the current purpose was not the main focus of the original paper.
Study design bins contained the number of countries which meet the following criteria. Bin 1 is the total number of countries in which tilapia species have been introduced. Bin 2 comprises the number of countries in which established, feral populations of tilapia exist. Bins 1 and 2 were based on fishbase.org (Froese and Pauly, Citation2012) and the FAO Database on Introduced Aquatic Organisms (FAO, 2012). The literature review described above also provided data for bins 1 and 2, and was the sole source of data for bins 3–6, as follows. Bin 3 contained all reports without quantitative data, while bin 4 reports were based on only quantitative data no other data types. Bin 5 reports contained quantitative and control data, and bin 6 contained quantitative data, control data, and replicate or time-series data. Replicate and time-series data were considered equivalent here because of the common logistical tradeoff made in ecological studies between replication in time and space. The conservative assumption was used that “no effect considered” papers represented absence of ecological effects, acknowledging that in many cases the original author may not have intended to address the ecological effects of tilapia. The justification for this assumption is that it is possible that the reason ecological effects were not considered was because effects did not exist. This assumption makes it more difficult to reject the null hypothesis that ecological effects are uncommon as reported in Gozlan (Citation2008).
Moving from bin 1 to bin 6 implies increasing strength of inference, that is, increasing assurance that the reported presence or absence of an ecological effect represents the true state of the system. For example, an effect reported from a particular paper making use of quantitative data, replication, and controls (bin 6) is considered more likely to represent a real ecological effect than a paper reporting that an impact occurs but does not use controls (bin 4). Thus, it was expected that the global probability of impacts calculated from higher bins are better representations of the true probability of impact. A plateau in the detection of ecological effects with increasing bin number would suggest that the estimate is approaching the true value of the prevalence of ecological impacts.
Presence or absence of ecological effects as a function of effect type, species, region, and study design
Mixed-model logistic regression was used to test whether the presence or absence of ecological effects was influenced by year of publication, tilapia species, region, effect type, and study design. Only reports from the literature review (bins 3–6) were included in this analysis, on the basis of results for Question 1 (see Results) study design was coded simply as the inclusion (or not) of controls or reference treatments. The data set was also limited to reports that explicitly considered the ecological impacts of tilapia, excluding “no effect considered” papers because doing so assured that relevant controls and references were applicable specifically to the ecological effects of tilapia. Study design and publication year were used as fixed effect covariates, and these were nested according to species or regions as random effects. That is, the effects of particular species and years are assumed to be drawn from a normal distribution of effects with a single global mean. Logistic regression models were fit for all possible combinations of including or excluding each fixed or random effect, yielding 16 basic models. These models were run for all ecological effects combined, and separately for effects on resident fish, the biotic community, and habitat. The best models were selected using Akaike's Information Criterion (AIC). Analyses were carried out in R (R Core Team, Citation2012), using the lmer function in the lme4 package for the logistic regression with mixed effects.
Perceptions of tradeoffs among ecosystem services resulting from tilapia
Three approaches were used to test how the perception of tilapia is related to the relative magnitude of the socioeconomic benefit of tilapia. First, an ordinal regression employing a cumulative link mixed model tested for a relationship between perception of tilapia introduction outcomes (i.e. positive, negative, and both) and the presence or absence of ecological effects. This model, as in question 2, also included fixed effects for publication year, and study design, random effects for tilapia species, and region, while also including the presence or absence of ecological effects as an additional fixed effect. These ordinal models were estimated using the clmm function in the ordinal package in R. Model selection was performed in the same manner as described for question 2.
Second, the ordinal regression modeling described above was repeated, but included a socioeconomic term, which was the percentage of country-specific gross domestic product (GDP) contributed by tilapia fisheries. Model selection was carried as described above, and these ordinal regression models including GDP were then compared to those described above without GDP. Percentage GDP was estimated for each country for which a literature-derived result for impact of tilapia was available. Countries’ GDP was retrieved from the World Bank DataBank (http://databank.worldbank.org; accessed Dec. 13, 2012). The total value of tilapia harvest in each country was calculated from a country's value per ton of tilapia aquaculture production and linearly extrapolated to the countries’ total tilapia production from aquaculture and wild harvest (FAO Fisheries and Aquaculture Department Citation2014). This estimation was performed for every year data was available from 1950 to 2010 using 2010 dollars ($USD). The percent of GDP from tilapia production was then matched to the year closest to the publication year within +/− 5 years.
Finally, whether research effort or the perception of tilapia was related to the proportion of GDP contributed by tilapia was tested. Two one-way ANOVAs were used to test whether percentage GDP differed across countries with different levels of research effort (indexed by study design bins described above) or for perception of tilapia ecosystem service impact (i.e., negative, positive, and both).
Results
The initial literature search conducted on May 25, 2010 identified 290 relevant publications for review. References within these publications and from forward literature searches identified a further 532 relevant publications for a total of 822 articles screened. Of these, 144 were unavailable. Of the 678 publications reviewed in detail, 416 included information on ecological interactions or fisheries involving tilapia, and 352 specifically addressed ecological effects of tilapia.
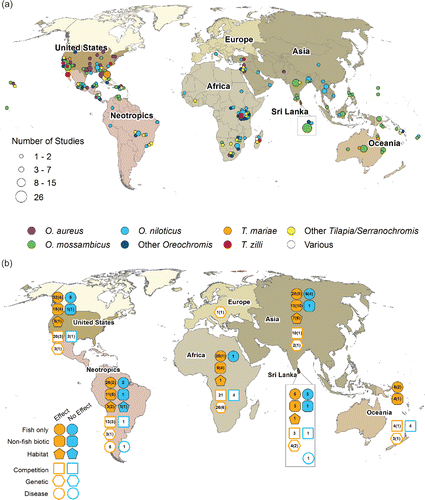
The rate of tilapia publications increased from the 1950s through the 1970s and plateaued in the early 1980s at about 25 publications annually (data not shown). There were 140 countries into which at least one tilapia species had been introduced. maps the global distribution of tilapia publications by focal species and summarizes the presence or absence of ecological effects across major regions for both all reporting publications and for only studies which use controls. The United States and Sri Lanka were the two largest contributors to the tilapia literature with 93 and 30 reviewed publications, respectively, followed by India (n = 21), Mexico (20), Australia (17), Brazil (13), Kenya (11), Bangladesh (8), Israel (7), Philippines (6), South Africa (5), and Madagascar (4; ). Due to the large discrepancy in sample size between the United States and Sri Lanka, and all other countries, countries other than the United States and Sri Lanka were pooled into five regions for analysis (): Africa (31 countries), Asia (25), Neo-tropics (21), Lake Victoria (18), and Oceania (14). Publications from 37 countries reported ecological effects attributed to tilapia (). The Neotropics was the only region to report the presence of the full suite of ecological effects considered. In publications that explicitly considered the presence or absence of ecological effects, 152 reported effects while 22 reported that effects did not occur. An additional 53 publications reported both the presence and absence of different ecological effects or mechanisms.
Figure 1. (A) Map of the global distribution of reports on the ecological effects of tilapia introduction by focal species in each publication. Locations were mapped to specific waterbodies when possible, or placed in the geographic center of the country or state/province where the study took place and offset to limit overlap. (B) Map of reported ecological effects (solid symbols) and mechanisms (hollow symbols) by region. Numbers within symbols indicate the total number of studies reporting that effect or mechanism, while the number in parenthesis indicates the number of studies which report that effect and use quantitative data, and controls or reference treatments.
Proportion of introductions associated with ecological impacts
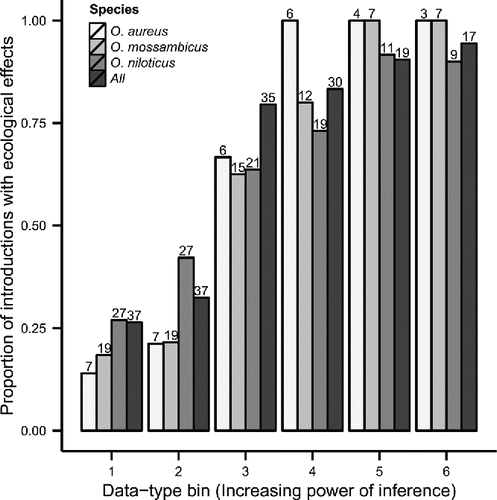
Tilapia species have been introduced into at least 140 countries around the world. Previous studies have estimated this closer to 90 to 100 countries (Pullin et al., Citation1997; Coward and Little, Citation2001). At present, 55% of all countries in the world have reported populations of non-native tilapia species established outside of aquaculture. At the country scale at least 26% of all known tilapia introductions were associated with ecological impacts (, bin 1). From bin 1 to bin 6, the proportion of countries with impacts increased. For bin 4 and above, the proportion of countries which report effects is greater than 80%, and reaches 100% for some species. In almost all countries where even a modest level of ecological research effort has been targeted at tilapia, ecological impacts have been observed. By bin 5, the increase in proportion of countries reporting ecological effects of tilapia has reached near the maximum relative to bin 4 through bin 6 (). Based on this result, subsequent analyses (for the logistic regression addressing question 2, and the ordinal regression addressing question 3) used the presence or absence of controls or references as a threshold of inferential power.
Figure 2. The proportion of countries reporting ecological effects of tilapia introduction across all effect types by all species combined and by important species arranged in order of increasing power of inference in attributing ecological impacts to tilapia (see Methods for complete description of the bins, which represent increasing research effort). The numbers above the bars are the number of countries reporting ecological impacts of tilapia.
Presence or absence of ecological effects as a function of effect type, species, region, and study design
Logistic regression provided no evidence that the year a study was conducted, the particular species, or the regions where the study occurred influenced detection of any ecological effects. The best logistic regression model (lowest AIC) for the presence or absence of all effects combined was the simplest, intercept only model, implying no influence of any of the tested covariates on the overall presence or absence of ecological effects. In all 16 base models, dAIC was less than 7.7, suggesting negligible discrimination between these models and providing no support for effects of study design, year, species, or region on the presence or absence of ecological effects.
Different species, particularly O. mossambicus, were expected to be more strongly associated with ecological effects, but this was not the case. O. mossambicus may simply be considered the “worst” invasive fish (Lowe et al., Citation2000) due to its relatively large global distribution rather than the presence of ecological effects. Both Oreochromis and Tilapia species were implicated in the occurrence of ecological effects despite substantive differences between these genera in life-history, such as parental care and diet (Beveridge and Baird, Citation2000; Klett and Meyer, Citation2002). As expected, hybridization was reported more often in Africa () but was also reported among feral introduced tilapia in all regions considered and regional effects were not significant.
When considering different ecological effects and mechanisms separately by logistic regression, only impacts on resident fish and biotic community had sufficient representation across species and regions for using our logistic regression model. In both cases, the best selected model was the intercept only model. For impacts on resident fish, the top seven models were all within dAIC = 2 of the best model. For impacts on biotic community the top four models were all within dAIC = 2, each with the addition of a second parameter (year, region, or species, respectively) indicating little ability to distinguish between the null model and the influence of these parameters.
Perceptions of tradeoffs among ecosystem services resulting from tilapia
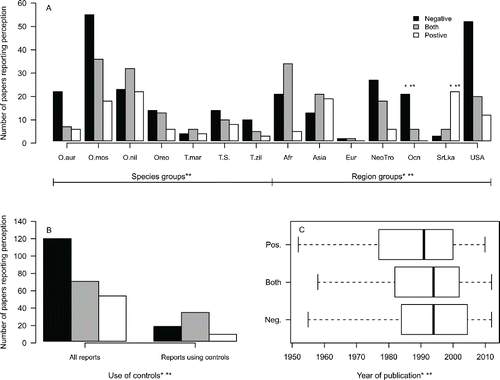
For all ecological effect types combined, the best ordinal regression included terms for the presence of region and species (), use of controls (), and publication year (). The second best model additionally included species groupings, but no species significantly deviated from the mean species effect. The two top models (dAIC <1.6) also supported significant effects of year of publication, with older publications more likely to report positive outcomes (). These two top models also indicated a significant effect of region, with Oceania dominated by negative perceptions and Sri Lanka by positive perceptions ().
Figure 3. The number of papers categorized by perception of tilapia effects on ecosystem services for (a) particular species of tilapia and region; (b) different study designs (all reports compared to reports with quantitative data and controls); (c) and year of publication. Significant effects are indicated by (*) the best or (**) the second best ordinal regression model. O. aur = O. aureus, O. moss = O. mossambicus, O. nil = O. niloticus, Oreo = All other Oreochromis species, T. mar = T. mariae, T.S. = All other Tilapia or Sarotherodon species, T. zil = T. zilli, Afr = Africa, Eur = Europe, NeoTro = Neotropics, Ocn = Oceania, SrLka = Sri Lanka.
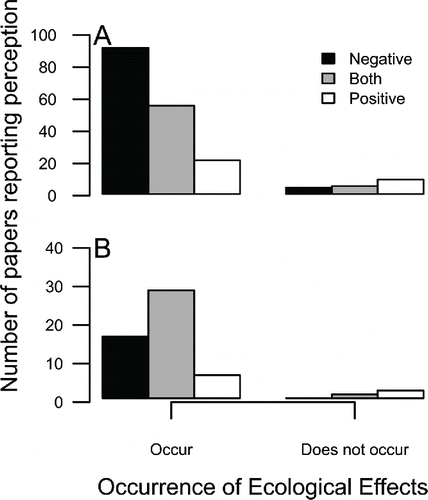
The presence of ecological effects was the most influential determinant of perceptions of tilapia introduction in the ordinal regression model, increasing the probability of negative perceptions of tilapia introduction (). As expected, authors’ perceptions about tilapia impact on ecosystem services were consistent with measures of ecological effects (). For studies in which impacts were documented, over four times as many papers concluded that the impacts of tilapia were negative than concluded that impact was positive, with an intermediate number of papers having both positive and negative components. In contrast, for papers reporting no effects of tilapia, the opposite trend suggests positive perceptions are more associated with lack of ecological effects (). The largest single category of publications reported both ecological impacts and a negative perception of tilapia introduction (). For papers that included a control or reference treatment, the pattern was similar but less pronounced (). The inclusion of increasing research effort, and hence increasing inference ability, decreased the probability of reporting negative perceptions such that the occurrence of both impacts and controls made “both” outcomes more likely.
Table 1. The best ordinal regression models of the perceived benefit of tilapia introduction for all ecological effect categories combined. Summary model selection statistics, fixed, and random effects coefficients for species and regions. Items in parentheses indicate random effects. –LL = negative log likelihood, AIC = Akiake's information criteria, dAIC = difference in Akaike's information criteria to best model, Ctrl = presence or absence of control treatments, Yr = year of publication, Effects = presence or absence of ecological effects, GDP = Percent contribution of tilapia to GDP. and “na” indicates that GDP was not included in the model selection. Bold indicates statistically significant effects p < 0.05.
Figure 4. The number of papers categorized by perception of tilapia effects on ecosystem services and the reported occurrence of ecological effects in the same papers—including all effect types, species, and regions—(a) for all papers and (b) only for papers that consider quantitative data and include a control.
As the proportional contribution of tilapia fisheries to country GDP increased, the expected trend of increased research effort (i.e., bin assignment) is observed, but this trend was not statistically significant (F = 1.16, p = 0.33). As the proportional contribution of tilapia fisheries to country GDP increased, there was a significant trend toward increased positive perception about the ecosystem service impact of tilapia (F = 4.135, p = 0.016). Including GDP in the ordinal regression required a loss in sample size due to missing GDP data, reducing from 191 publications in the overall model to 129. The best model included GDP as well as control treatment, impact, and region effects (). The second and third best models, however, were also highly supported (dAIC < 2) but neither included GDP. The second model was more parsimonious than the first, and the third model was identical to the best model from the previous ordinal regression which excluded GDP. These results suggest that while GDP and perception were related, percent contribution of tilapia to GDP was not a sufficient predictor of perception of the ecosystem tradeoffs.
Discussion
This study represents the most comprehensive review of tilapia literature to date and reveals that previous studies have underestimated the ecological effects of tilapia at the global scale. In this analysis, the lowest proportion of countries that have received introductions and reported ecological effects (26%, ) is much higher than the average 5% and even the most extreme 20% previously reported (Gozlan, Citation2008). The ecological effects of tilapia introduction are reported almost everywhere tilapia introductions are studied with no significant effects of species or region. Indeed, the geographic extent in this analysis includes all continents and most major freshwater habitat types, except polar and montane freshwater (Abell et al., Citation2008) and includes all major tilapia species. The widespread reporting of ecological effects across species and regions suggests that the occurrence of ecological effects may be expected wherever established feral tilapia populations exist in similar habitats.
The perception that tilapia negatively affect ecosystem services was significantly associated with documented ecological effects on ecosystem services, but with some strong regional differences. Trends in tilapia contributions to a county's GDP are consistent with the interpretation that these regional differences may be explained by the socioeconomic benefits of tilapia introduction. However, contribution to GDP is insufficient to predict whether tilapia introduction are viewed as positive or negative forces on ecosystem services because increasing research effort associated with higher contributions to GDP also tends to increase the recognition of both positive and negative perspectives. These results quantitatively demonstrate that studies with higher research effort were more likely to temper claims of negative ecological impacts with appeals to socioeconomic benefits, or equivalently, to temper claims of positive socioeconomic impact with appeals to ecological impacts.
The divergence of conclusions about tilapia impacts apparent in the literature was addressed by separately assessing the ecological effects of tilapia introduction, which are rarely monetized, from the more easily monetized ecosystem service provided by tilapia production and harvest. Geographical and socioeconomic contexts of tilapia introduction, as well as ecological effects, play a significant role in the perspective of benefits or harm. While documented ecological changes were strongly associated with negative perspectives, this relationship was also dependent on the socioeconomic context, particularly the value of tilapia production and latent regional factors. These results are in agreement with previous work emphasizing that concepts of invasion are context specific and mutable (Lodge and Shrader-Frechette, Citation2003; Nuñez and Simberloff, Citation2005), and that complex perceptions are typical for introduced species that provide some ecosystem services while harming others (Davis et al., Citation2011; Gleditsch and Carlo, Citation2011; Schlaepfer et al., Citation2011). Some general considerations and potential analyses to further inform decision and policy making regarding tilapia introductions are discussed below.
One of the main results of this review was the discovery that regional context partly determines the perception of ecosystem services provided by, or lost to, tilapia introduction. Very coarse country and regional groupings were used in this analysis to construct reasonable sample sizes of publications for analysis. Regional patterns may therefore be partly due to the grouping of publications and introductions across regions considered. This effect may be particularly true for the United States and Sri Lanka, the two largest contributors of tilapia research by a considerable margin. Together, these two countries represent 35% of global tilapia research publications, but only 2% of countries to which tilapia have established feral populations. Moreover, reports from these countries had generally opposing perspectives towards the ecosystem service outcome of tilapia introduction. Approximately 80% of US publications emphasized negative impacts on ecosystem services, while in Sri Lanka, 75% of the publications emphasized positive outcomes. While the results from these countries dominate the literature they should, however, also be viewed with caution because neither the United States nor Sri Lanka represents typical biogeography of tilapia introductions. The United States exists at the very edge of the environmental tolerances of tilapia (Zambrano et al., Citation2006) and winter die-offs occur even in southern states (Germany and Noble, Citation1977). Meanwhile Sri Lanka is environmentally well suited for tilapia but there are few if any native freshwater fish species (De Silva, Citation1989) to resist or be affected by introductions (Moyle and Marchetti, Citation2006). Thus the ecological effects and resulting tradeoffs reported in the United States and Sri Lankan habitats may not be comparable to other regions or countries, despite the fact that these countries are the largest contributors to tilapia ecology publications.
There are very few reports of tilapia in Europe, and this presents an opportunity for decision makers. On the one hand, introductions of tilapia in Europe appear relatively rare () but feral populations have recently been reported in Italy (Bianco and Turin, Citation2010) and Turkey (Akin et al., Citation2005). The fate of these populations as well as future introductions may still lie in the hand of decision makers. Meanwhile, much of Oceania has already experienced tilapia introductions and a fairly large number of the publications reviewed herein (30) are from this region. These analyses suggest that Oceania is associated with negative outcomes more so than any other region. Yet, Oceania represents probably the largest and most geographically dispersed region included in the study. There are doubtless undocumented islands to which tilapia have become established, and others to which it has not, with obvious potential for a large scale natural experiment.
Tilapia introductions often occur simultaneously with other major drivers of ecological change in freshwaters such as habitat degradation, heavy fishing pressure, flow modifications, and pollution (Dudgeon et al., Citation2006; De Silva et al., Citation2009). But, almost none of the studies reviewed herein comprehensively address these possibly confounding factors with rigorous study designs. Few of the publications were experimental; most were observational studies of natural experiments and fishery-derived data. Replicates and controls were rare and their association to treatments often tenuous. As with other invasive species, assigning causation for observed changes under these circumstances is difficult (Lodge and Shrader-Frechette, Citation2003).
In this review bins of increasing inferential ability partly overcomes the problems of study designs with individually low inferential ability by increasing confidence that publications are reporting tilapia as the true cause of observed ecological effects. Nonetheless, this review analysis suffers the weaknesses of a “vote-counting” approach (Gurevitch and Hedges, Citation2001) within each bin. One of these weaknesses is the reliance on the statistical power within each study, which is often severely limited by small sample sizes and insufficient control. Another limitation is inability to estimate effect magnitude, i.e., the size (not just the occurrence) of the ecological effects on ecosystem services. An extensive set of tools have been developed for meta-analysis of ecological literature that estimates and compares these magnitudes in terms of effect sizes (Osenberg et al., Citation1999; Cadotte et al., Citation2012; Borenstein et al., Citation2009). Such an analysis should be conducted on the small subset of reports herein that used both controls and replicates and otherwise adequate study designs and reporting for meta-analysis techniques.
Conclusion
This review quantitatively demonstrates that tilapia introductions often represent a tradeoff between ecosystem services provided by tilapia and services which are negatively affected by tilapia. Being able to consider this tradeoff explicitly in the decision making process is important to adapting to ongoing environmental change and resolving the conflicting attitudes pervasive in the global tilapia literature. On a global scale, increasing research effort increases the probability of detecting ecological impacts of tilapia introduction. More than 80% of published ecological research on tilapia reported changes in ecosystem services. The occurrence of ecological effects was not a product of different species of tilapia, different global regions, the type of data used, or study design. It is unequivocal that tilapia are frequently associated with, and a demonstrated cause of, undesirable ecological changes in many areas.
Results also illustrate that increasing research efforts leads to increasingly ambivalent perspectives about the net socioeconomic value of tilapia introductions, as undesirable ecological impacts become as apparent as the socioeconomic benefits of tilapia production. In some cases, perspectives are regionally determined. There is not, nor is there likely ever to be, a global consensus on the socioeconomic merits of tilapia introduction. Rather, we recommend that decisions be informed by comparisons of the regional and local economic benefits to the regional and local ecological costs now and in the future. While the ecological effects may be similar over much of the introduced range of tilapia, as results demonstrate, there is no reason to expect uniform socioeconomic benefits. The work of managers, decision and policy makers, and other stakeholders is therefore made all the more relevant in the careful consideration of local context in decisions about tilapia introductions. And there will be ample future opportunities for informed decisions.
Only about half of all tropical countries have at least one established, feral, tilapia population. This number is probably an underestimate due to underreporting in global datasets (Welcomme, Citation2011) and limited resources for research in areas where tilapia are prominent (UNESCO, Citation2007; Josupeit, Citation2010; Skelton and Swartz, Citation2011). Nonetheless, an important opportunity clearly exists for careful decisions in the many countries that are suitable for tilapia production, but are as yet uncolonized by tilapia.
Tilapia review supplement
Download MS Word (627.5 KB)Acknowledgments
B. Althouse, A. K. Baldridge, M. A. Barnes, N. Dorn, Z. Feiner, C. Gantz, D. Hayes, L. Sargent, and R. Wright contributed to the design and implementation of the data collection. The Lodge Lab contributed useful comments on analyses and early versions of this manuscript.
Funding
Funding for this research has been provided by GLOBES (NSF DGE-0504495), NSF DDEP award #1046682, US EPA, NOAA, and the Center for Sponsored Coastal and Ocean Research (CSCOR Award # NA09NOS4780192, NA10NOS4780218),and the Environmental Change Initiative at the University of Notre Dame. In particular, the staff of the interlibrary loan office at the University of Notre Dame Libraries made this literature review possible.
References
- Abell, R., Thieme, M. M. L., Revenga, C., Bryer, M., Kottelat, M., Bogutskaya, N., Coad, B., Mandrak, N., Balderas, S. C., Bussing, W., Stiassny, M. L. J., Skelton, P., Allen, G. R., Unmack, P., Naseka, A., Ng, R. Sindorf, N., Robertson, J., Armijo, E., Higgins, J. V., Heibel, T. J., Wikramanayake, E., Olson, D., López, H. L., Reis, R. E., Lundberg, J. G., Párez, M. H. S. and Petry, P. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience, 58(5): 403–414 (2008).
- Abrams, P. The Theory Of Limiting Similarity. Ann. Rev. Ecol. Systemat., 14(1983): 359–376 (1983).
- Akin, S., Buhan, E., Winemiller, K. O., and Yilmaz, H. Fish assemblage structure of Koycegiz Lagoon-Estuary, Turkey: Spatial and temporal distribution patterns in relation to environmental variation. Estuarine, Coastal and Shelf Sci., 64: 671–684 (2005).
- Amano, T. and Sutherland, W. J. Four barriers to the global understanding of biodiversity conservation: wealth, language, geographical location and security. Proceedings of the Royal Society B, 280: (2013). DOI: 20122649.
- Arthur, R. I., Lorenzen, K., Homekingkeo, P., Sidavong, K., Sengvilaikham, B., and Garaway, C. J. Assessing impacts of introduced aquaculture species on native fish communities: Nile tilapia and major carps in SE Asian freshwaters. Aquaculture, 299(1–4): 81–88 (2010).
- Barnes, M. A., Deines, A. M., Gentile, R. M. and Grieneisen, L. E. Adapting to invasions in a changing world: invasive species as an economic resource. In Z. Lew & J. Dukes, eds. Invasive Species and Global Climate Change. CABI Publishing (2014).
- Beveridge, M. C. M., and Baird, D. J. Diet, feeding and digestive physiology, pp. 59–87. In: Beverage, M. C. M., and B. J. McAndrew, eds. Tilapias: Biology and Exploitation. Boston: Kluwer Academic Publishers (2000).
- Bianco, P. G., and Turin, P. Record of two established populations of Nile tilapia, Oreochromis niloticus, in freshwaters of northern Italy. J. Appl. Ichthyol., 26: 140–142 (2010).
- Black, G. F. Status of the desert pupfish, Cyprinodon macularius (Baird and Girard), in California., p.42. (1980).
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., and Rothstein, H. R. Introduction to Meta-analysis, West Sussex, UK: John Wiley & Sons (2009).
- Bostock, J., McAndrew, B., Richards, R., Jauncey, K., Telfer, T., Lorenzen, K., Little, D., Ross, L., Handisyde, N., Gatward, I., and Corner, R. Aquaculture: global status and trends. Philos. Trans. Royal Soc. B, 365(1554): 2897–912 (2010).
- Brummett, R. E., and R. W. Ponzoni. Concepts, alternatives, and environmental considerations in the development and use of improved strains of tilapia in African aquaculture. Rev. Fisheries Sci., 17(1): 70–77 (2009).
- Bruno, J. F., Fridley, J. D., Bromberg, K. D., and Bertness, M. D. Insights into biotic interactions from studies of species invasions. In Sax, D. F., J. J. Stachowicz, & S. D. Gaines, eds. Species Invasions: Insights in ecology, evolution, and biogeography. Sunderland, MA: Sinauer Associates, Inc, pp. 13–40 (2005).
- Bruton, M. N., and J. van As. Faunal invasions of aquatic ecosystem in southern Africa, with suggestions for their management. In Proceedings of the National Synthesis Symposium on the Ecology of Biological Invasions. Stellenbosch, South Africa: Oxford University Press, pp. 47–61 (1986).
- Cadotte, M. W., L. R. Mehrkens, and D. N. L. Menge. Gauging the impact of meta-analysis on ecology. Evolut. Ecol., 26(5): 1153–1167 (2012).
- Canonico, G. C., Arthington, A., McCrary, J. K., and Thieme, M. L. The effects of introduced tilapias on native biodiversity. Aquat. Conservation: Mar. Freshwater Ecosystems, 15(5), 463–483 (2005).
- Coward, K., and Little, D. C. Culture of the “aquatic chicken”: present concerns and future prospects. Biologist, 48(1): 12–16 (2001).
- Crutchfield, J. U. J. Establishment and expansion of redbelly tilapia and blue tilapia in a power plant cooling reservoir. Am. Fish. Soc. Symp., 15: 452–461 (1995).
- Davis, M. A., Chew, M. K., Hobbs, R. J., Lugo, A. E., Ewel, J. J., Vermeij, G. J., Brown, J. H., Rosenzweig, M. L., Gardener, M. R., Carroll, S. P., Thompson, K., Pickett, S. T. A., Stromberg, J. C., Del Tredici, P., Suding, K. N., Ehrenfeld, J. G., Grime, J. P., Mascaro, J., and Briggs, J. C. Don't judge species on their origins. Nature, 474: 153–154 (2011).
- De Silva, S. S., Nguyen, T. T. T., Turchini, G. M., Amarasinghe, U. S, and Abery, N. W. Alien species in aquaculture and biodiversity: A paradox in food production. Ambio, 38(1): 24–28 (2009).
- De Silva, S. S., Nguyen, T. T. T., Wabery, N., and Amarasinghe, U. S. An evaluation of the role and impacts of alien finfish in Asian inland aquaculture. Aquacult. Res., 37(1): 1–17 (2006).
- De Silva, S. S. Status of Introduced species in Sri lanka, p. 99. In: De Silava, S. S. ed. Exotic Aquatic Orgnaisms in Asia. Proceedings of the workshop on Introduction of Exotic Aquatic Organisms in Asia.. Manila, Philippines: Asian Fishery Soceity, (1989).
- Diamond, J. Overview : field experiments, and natural experiments. pp. 3–22. In: Diamond, J., & T. J. Case, eds. Community Ecology. New York: Harper and Row, (1986).
- Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Leveque, C., Naiman, R. J., Prieur-Richard, A.-H. A.-H. , Soto, D., Stiassny, M. L. J., C. A. Sullivan, C. A., and L´vêque, C. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Cambridge Philos. Soc., 81(2): 163–82 (2006).
- FAO, Database of Introduced Aquatic Species. Available at: www.fao.org/fishery/dias/en [Accessed September 2, 2012].
- FAO. Fishery and Aquaculture Statistics 2009, ROME: Food and Agriculture Organization of the United Nations (2011).
- FAO Fisheries and Aquaculture Department. Fisheries Global Information System (FAO-FIGIS). Fisheries Global Information System (FIGIS). Available at: http://www.fao.org/fishery/figis/en (2014).
- Figueredo, C. C., and Giani, A. Ecological interactions between Nile tilapia (Oreochromis niloticus, L.) and the phytoplanktonic community of the Furnas Reservoir (Brazil). Freshwater Biol., 50(8): 1391–1403 (2005).
- Froese, R., and Pauly, D. FishBase version 2/2015. Available at: www.fishbase.org [Accessed January 1, 2015] (2012).
- Germany, R. D., and Noble, R. L. Population dynamics of blue tilapia in trinidad lake, Texas. Proce. 31st Ann. Conf. South East Assoc. Fish and Wildlife Agencies, 31: 412–417 (1977).
- Gindelberger, B. Why Sinarapan almost Disappered from Lake Buhi. Naga, 4: 3–5 (1981).
- Gleditsch, J. M., and Carlo, T. A. T. a. Fruit quantity of invasive shrubs predicts the abundance of common native avian frugivores in central Pennsylvania. Divers. Distribut., 17(2): 244–253 (2011).
- Goudswaard, P. C., F. Witte, and E. F. B. Katunzi. The tilapiine fish stock of Lake Victoria before and after the Nile perch upsurge. J. Fish Biol., 60: 838–856 (2002).
- Gozlan, R. E. Introduction of non-native freshwater fish: is it all bad?. Fish and Fish., 9: 106–115 (2008).
- Gurevitch, J., and Hedges, L. Meta-analysis: combining the results of independent experiments, pp. 347–369. In: Scheiner, S. M., and J. Gurevitch, eds. Design and Analysis of Ecological Experiments. New York: Oxford University Press, (2001).
- Hauser, W. J., E. F. Legnar, and F. Robinson. Biological control of aquatic weeds by fish in irrigation channels. Proc. water management for irrigation and drainage., ASCE/Reno, pp. 139–145 (1977).
- Hooper, D. U., Chapin, F. S., Ewel, J. J., Hector, A., Inchausti, P., Lavorel, S., Lawton, J. H., Lodge, D. M., Loreau, M., Naeem, S., Schmid, B., Setala, H., Symstad, A. J., Vandermeer, J., and Wardle, D. A. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr., 75(1): 3–35 (2005).
- Josupeit, H. World supply and demand of tilapia 2010. In INFOFISH Tilapia Conference. Kuala Lumpur: FAO GlobeFish, p. 6 (2010).
- Keller, R. P., Lodge, D. M., Lewis, M. A., and Shogren, J. F. Bioeconomics of invasive species: integrating ecology, economics, and management, New York: Oxford University Press US. (2009).
- Klett, V., and A. Meyer. What, if anything, is a Tilapia?-mitochondrial ND2 phylogeny of tilapiines and the evolution of parental care systems in the African cichlid fishes. Mol. Biol. Evol., 19(6): 865–83 (2002).
- Kolar, C. S., and D. M. Lodge. Progress in invasion biology: predicting invaders. TRENDS in Ecol. Evol., 16(4): 199–204 (2001).
- Leveque, C. Out of Africa: the success story of tilapias. Environ. Biol. Fishes, 64: 461–464 (2002).
- Lodge, D. M., Williams, S., Macisaac, H. J., Hayes, K. R., Leung, B., Reichard, S., Mack, R. N., Moyle, P. B., Smith, M., Andow, D. A., Carlton, J. T., and McMichael, A. Biological invasions: Recommendations for U.S. policy and management. Ecol. Appl., 16(6): 2035–2054 (2006).
- Lodge, D. M., Deines, A. M., Gherardi, F., Yeo, D. C. J., Arcella, T., Baldridge, A. K., Barnes, M. A., Chadderton, W. L., Feder, J. L., Gantz, C. A., Howard, G. W., Jerde, C. L., Peters, B. W., Peters, J. A., Sargent, L. W., Turner, C. R., Wittmann, M. E., and Zeng, Y. Global introductions of crayfishes: Evaluating the impact of species invasions on ecosystem services. Ann. Rev. Ecol. Evol. Syst., 43: 449–472 (2012).
- Lodge, D. M., and K. Shrader-Frechette. Nonindigenous species: Ecological explanation, environmental ethics, and public policy. Conser. Biol., 17(1): 31–37 (2003).
- Lorenzen, K. Population dynamics and management, pp. 163–225. In: Tilapias: Biology and Exploitation (Beverage, M. C. M., and B. J. McAndrew, eds.) Boston: Kluwer Academic Publishers (2000).
- Lövei, G. L., and T. M. Lewinsohn. Biological invasions in megadiverse regions network Megadiverse developing countries face huge risks from invasives. Trends Ecol. Evol., 27(1): 2–3 (2012).
- Lowe, S., Browne, M., Boudjelas, S., and D. P. M. 100 of the World's Worst Invasive Alien Species A selection from the Global Invasive Species Database., The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN) (2000).
- McCrary, J. K. J. K., Murphy, B. R., Stauffer, J. Jay R., Hendrix, S. S., and Stauffer, J. R. Tilapia (Teleostei: Cichlidae) status in Nicaraguan natural waters. Environ. Biol. Fishes, 78(2): 107–114 (2007).
- McKaye, K. R., Ryan, J. D., Stauffer, J. Jay R., Perez, L. J. L., Vega, G. I., and van den Berghe, E. P. African Tilapia in Lake Nicaragua. Bioscience, 45(6): 406–411 (1995).
- Millennium Ecosystem Assessment and Assessment, M. E. Ecosystems and Human Well-being: Synthesis, Washington, D.C.: Island Press (2005).
- Mkumbo, O. C., and Ligtvoet, W. Changes in the diet of Nile perch, lates niloticus (L), in the Mwanza Gulf, Lake Victoria. Hydrobiologia, 232: 79–83 (1992).
- Mouchet, M. a., Burns, M. D. M., Garcia, A. M., Vieira, J. P., and Mouillot, D. Invariant scaling relationship between functional dissimilarity and co-occurrence in fish assemblages of the Patos Lagoon estuary (Brazil): environmental filtering consistently overshadows competitive exclusion. Oikos, 122(2): 247–257 (2013).
- Moyle, P. B., and M. P. Marchetti. Predicting invasion success: freshwater fishes in California as a model. BioSci., 56(6): 515 (2006).
- Norrgren, L., Pettersson, U., Orn, S., and Bergqvist, P.-A. Environmental monitoring of the kafue river, located in the Copperbelt, Zambia. Arch. Environ. Contam. Toxicol., 341: 334–341 (2000).
- Nuñez, M. A., Simberloff, D. Invasive species and the cultural keystone species concept. Ecol. Soc., 10(1): 4–7 (2005).
- Osenberg, C. W., Sarnelle, O., Cooper, S. D., Holt, R. D., and Jun, N. Resolving ecological questions through meta-analysis: goals, metrics, and models. Ecol., 80(4): 1105–1117 (1999).
- Perry, W. L., D. M. Lodge, and J. L. Feder. Importance of hybridization between indigenous and nonindigenous freshwater species: an overlooked threat to North American Biodiversity. Soc. Syst. Biol., 51(2): 255–275 (2002).
- Pullin, R. S. V., Palomares, M. L., Casal, C. V., Dey, M. M., and Pauly, D. Environmental impacts of tilapias. In Proceedings from the Fourth International Symposium on Tilapia in Aquaculture, Coronado Springs Resort, Walt Disney World. Orlando, Florida: International Center for Living Aquatic Resources management (ICLARM), pp. 553–570 (1997).
- R Core Team. R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing (2012).
- Ranoemihardjo, B. S. Nauru: Eradication of Tilapia from fresh- and brackishwater lagoons and ponds with a view to promoting milkfish culture, Rome: FAO (1981).
- Redpath, S. M., Young, J., Evely, A., Adams, W. M., Sutherland, W. J., Whitehouse, A., Amar, A., Lambert, R. a, Linnell, J. D. C., Watt, A., and Guti´rrez, R. J. Understanding and managing conservation conflicts. Trends Ecol. Evol., 28(2): 100–109 (2013).
- Schlaepfer, M. A., D. F. Sax, and J. D. Olden. The potential conservation value of non-native species. Conserv. Biol., 25(3): 428–437 (2011).
- Skelton, P. H., and Swartz, E. R. Walking the tightrope: trends in African freshwater systematic ichthyology. J. Fish Biol., 79(6): 1413–1435 (2011).
- Sugunan, V. V Ecology and fishery management of reservoirs in India. Hydrobiologia, 430: 121–147 (2000).
- Sugunan, V. V Reservoir fisheries of India. FAO Fisheries Technical Paper, no 345, p. 423p (1995).
- UNESCO. A global perspective on research and development. UNESCO Institute for Statistics Fact Sheet, 5, pp. 1–8 (2007).
- Varela-Romero, A., Ruiz-Campos, G., Yepiz-Velazquez, L. M., and Alaniz-Garcia, J. Distribution, habitat and conservation status of desert pupfish (Cyprinodon macularius) in the Lower Colorado River Basin, Mexico. Rev. Fish Biol. Fisheries, 12(2): 157–165 (2000).
- Vitule, J. R. S., A. Freire, carolina, and D. Simberloff. Introduction of non-native freshwater fish can certainly be bad. Fish and Fisheries, 10: 98–108 (2009).
- Walpole, M., Almond, R. E. A., Besançon, C., Butchart, S. H. M., Campbell-Lendrum, D., Carr, G. M., Collen, B., Collette, L., Davidson, N. C., Dulloo, E., Fazel, A. M., Galloway, J. N., Gill, M., Goverse, T., Hockings, M., Leaman, D. J., Morgan, D. H. W., Revenga, C., Rickwood, C. J., Schutyser, F., Simons, S., Stattersfield, A. J., Tyrrell, T. D., Vi´, J.-C., and Zimsky, M. Ecology. Tracking progress toward the 2010 biodiversity target and beyond. Science, 325(5947): 1503–1504 (2009).
- Welcomme, R. L. An overview of global catch statistics for inland fish. ICES J. Marine Sci., 68(8): 1751–1756 (2011).
- Zambrano, L., Martinez-Meyer, E., Menezes, N., and Peterson, A. T. Invasive potential of Common carp (Cyprinus carpo) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Canadian J. Fisheries Aquat. Sci., 63: 1903–1910 (2006).
- Zengeya, T. A., Robertson, M. P., Booth, A. J., and Chimimba, C. T. A qualitative ecological risk assessment of the invasive Nile tilapia, Oreochromis niloticus in a sub-tropical African river system (Limpopo River, South Africa). Aquat. Conservation: Marine and Freshwater Ecosyst., 23(1): 51–64. (2012).
- Zenni, R. D., and Nuñez, M. A. The elephant in the room: the role of failed invasions in understanding invasion biology. Oikos, (December 2012), p.DOI: 10.1111/j.1600–0706.2012.00254.x. (2013).
