Abstract
The development of water management infrastructures, such as dams and canals, are important components of society’s response to feed a growing human population and to fight climate change. Yet, these changes in land use can also increase the transmission risk for waterborne diseases. Transmission risk associated with artificial reservoirs has been extensively documented for schistosomiasis, a parasitic disease of poverty that infects more than 240 million people worldwide. Over 90% of these cases are in sub-Saharan Africa, a region that is being steadily reshaped by climate change. Controlling the parasite’s obligate intermediate host snail is key to reducing transmission of this disease. Using commercial aquaculture to farm marketable species which predate upon these snails in vulnerable regions can have multiple positive effects, including the improved socioeconomic and nutritional health of surrounding communities. Here the authors assessed the viability of using the aquaculture of snail predators to simultaneously control schistosomiasis infection rates while alleviating economic and/or nutritional poverty in endemic regions of sub-Saharan Africa. A PRISMA-based 6-step systematic methodology was used to explore the primary literature using the case study of Côte d’Ivoire and two native species of snail predator to make evidence-based conclusions on the viability of this method for controlling schistosomiasis. This detailed thematic examination of the literature concluded that using specific approaches and species, aquaculture could be effective in reducing economic poverty and chronic malnourishment along with high levels of schistosomiasis infection. More current species-specific aquaculture data and consumer survey data are, however, needed to determine the economic and logistical effectiveness of farming native snail predators in-country. These and other opportunities for future research are highlighted.
Introduction
There is perhaps no continental expanse where interactions among humans, animals, and disease are more destabilized by climate change than sub-Saharan Africa (SSA). Home to over 1 billion people spread across 54 countries (World Bank Citation2019c), this vast region is especially vulnerable to the effects of climate change (Boko et al. Citation2016; Gan et al. Citation2016) and has been challenged by some of the most persistent tropical diseases in human memory, including HIV/AIDS, hemorrhagic fevers (including the Ebola virus disease) and snail-hosted parasitic infections including fascioliasis and schistosomiasis (Centers for Disease Control and Prevention Citation2019; Lu et al. Citation2018). Also called bilharzia, or snail fever (Obrist et al. Citation2006), schistosomiasis is a debilitating vector-borne aquatic disease caused by trematode flatworms of the genus Schistosoma (Sokolow et al. Citation2015). It is a widespread disease of poverty infecting more than 200 million people worldwide (World Health Organization Citation2013; Lai et al. Citation2015), the vast majority in SSA (Utzinger et al. Citation2009). Women and children are especially vulnerable to repeated infection when they step into infested water bodies (McManus et al. Citation2018). The two most important schistosome species in SSA are Schistosoma haematobium and Schistosoma mansoni (see Sokolow et al. Citation2015), although evidence of hybridization between these species and others (McManus et al. Citation2018) could change their epidemiology by allowing the hybrids to be transmitted through different and/or multiple intermediate hosts. An increase in potential reservoir hosts my also follow, with hybrids of S. curassoni/S.guineensis/S.bovis and S. haematobium affecting both cattle and small ungulates.
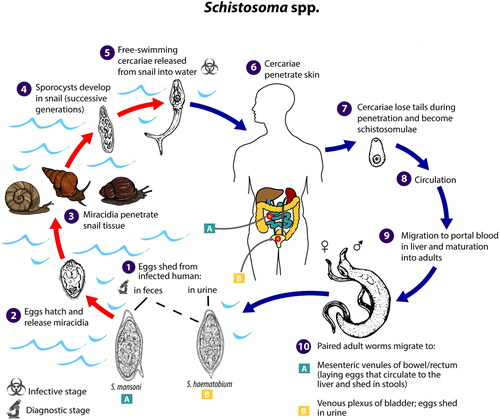
Schistosome parasites have a complex life-cycle which requires an obligate intermediate freshwater host snail of the genus Bulinus for S. haematobium and Biomphalaria for S. mansoni (see ). Parasite eggs are released via the urine (in the case of S. haematobium) and/or feces (S. mansoni) of infected people (both parasites) and other mammalian hosts (S. mansoni only). Upon contact with water, the eggs hatch into a free-swimming larval stage called a miracidium (Amberson and Schwarz Citation1953) which seeks out and penetrates a suitable freshwater snail. Schistosomes reproduce asexually in freshwater snails which, three to five weeks after the onset of infection, start to shed hundreds of cercariae per day, a second free-swimming, non-feeding larval stage (McCreesh and Booth Citation2013). Cercariae may infect a definitive vertebrate host by burrowing through the skin. Once in the bloodstream, schistosomes migrate to the vein plexus of major organs, often the ureters, bladder and kidneys (S. haematobium), or intestines (S. mansoni) where, three to four weeks after the onset of infection, they mate and release hundreds of eggs per day for years (McCreesh and Booth Citation2013; McManus et al. Citation2018). The host’s immune response to the transition of the eggs from the bloodstream to the organs can cause significant long-term debilitating damage to the body and, if left untreated, the potential death of the host (van der Werf et al. Citation2003).
Figure 1. Schistosoma spp. life cycle. Source: CDC. Please note: Use of this material does not imply endorsement by CDC, ATSDR, HHS or the United States Government of this article.
The most common method of controlling schistosomiasis in affected communities is with preventative chemotherapy, typically in the form of mass drug administration (MDA) by using praziquantel (PZQ), the drug of choice for schistosomiasis (World Health Organization Citation2013). A single PZQ dose rapidly kills adult worms but does not kill the juvenile worms nor prevent re-infection (Steinmann et al. Citation2006; Colley et al. Citation2017). Rapid re-infection from parasite-contaminated waters, evidence of drug resistance, and the logistics/cost of administering a second PZQ dose 3–4 weeks after the first one, require that MDA is complemented with intervention targeting the environmental stages of the parasites (Lo et al. Citation2017). Snail control was the primary historical approach to schistosomiasis control before PZQ became widely available in the 1990s (Rollinson et al. Citation2013; Sokolow et al. Citation2016; Lo et al. Citation2017; Knopp et al. Citation2019; Hoover et al. Citation2020). Snail habitat modification and especially chemical molluscicides have often been used to control vector snails in endemic regions (World Health Organization Citation2017; Sokolow et al. Citation2018). The high cost of snail habitat control, and the detrimental side effects of repeated used of molluscicides on water quality and aquatic fauna (Cowie Citation2001; Fenwick et al. Citation2006; Swartz and Rickards Citation2015), hinder the wide-scale implementation of these approaches in SSA (Sokolow et al. Citation2018; Allan et al. 2020).
More recently it has been argued that the biological control of the schistosome parasites’ intermediate snail host can be an additional weapon in the fight against schistosomiasis (Sokolow et al. Citation2015, Citation2019; Hoover et al. Citation2020) Biological control can be achieved by introducing (a) competing snail species that are immune to schistosome infection (Lee et al. Citation1982; Cowie Citation2001; Anto et al. Citation2005); (b) sterilizing/lethal microparasites and pathogens of snails (Pointier and Jourdane Citation2000; Coustau et al. Citation2015); and/or (c) snail predators (Madsen Citation1990; Gashaw et al. Citation2008; Hoover et al. Citation2020). The latter can be very effective in rapidly reducing local snail populations (Sokolow et al. Citation2014), although they are generally unable to extirpate the snail host population in larger unenclosed water bodies (Slootweg et al. Citation1994). A recent field experiment in the lower basin of the Senegal river, a hotspot of schistosomiasis transmission, provided supportive evidence that the African native molluscivorous prawns Macrobrachium vollenhovenii can be used to reduce snail abundance at transmission sites and hinder reinfection in schoolchildren (Sokolow et al. Citation2015). A similar outcome was reported two decades earlier in Kenyan water reservoirs hosting an invasive predator, the Florida crayfish Procamabrus clarkii (see Hofkin et al. Citation1991; Mkoji et al. Citation1999). Evidence of the importance of top-down control of snail populations by natural predators also emerges when predator removal leads to increased snail abundance and, ultimately, higher prevalence and intensity of infection rates in nearby human populations. In Lake Victoria, for instance, the declines of native molluscivorous fish due to the introduction of the Nile perch Lates niloticus and intensive overfishing has been associated with increased snail numbers and schistosomiasis transmission (Goudswaard and Witte Citation1997). As natural predators can be effective biological control agents of snails of medical importance, there is an untapped potential for aquaculture to restore and/or support molluscivorous species of commercial interest to improve disease control, nutrition, and local livelihoods, a win-win-win solution for health and human wellbeing.
The importance of aquaculture for disease control and improving livelihoods is further amplified under climate change. Climate change has already had significantly adverse impacts in West Africa on both marine and freshwater fish stocks, with smaller rivers and water bodies being most impacted by the increasing number and severity of droughts and flooding events experienced by the region (Dixon et al. Citation2003). Infrastructural attempts by national governments to hold and redirect water to increase national climate resilience, including the installation of dams, reservoirs, and irrigation canals, have been shown to exponentially increase local infection rates of schistosomiasis or allow additional species to thrive (Mouchet and Carnevale Citation1997; Lerer and Scudder Citation1999; Perez-Saez et al. Citation2016). Despite these increases the predictions for climate change’s effect on schistosomiasis are somewhat mixed, as increasing temperatures will open up new areas for the intermediate host snails to populate while closing other areas off, but both the disease and these snails are resilient to drought and to flooding, making accurate predictions beyond general statements for specific regions a particular challenge (Kalinda et al. Citation2017).
The use of aquaculture to grow native snail predators for restoring fisheries and for direct deployment in endemic areas may be an effective and climate-resilient approach to schistosomiasis control. The use of native species avoids the often-deleterious effects non-native species can have on local ecosystems and communities (which is explored as part of the history of biological control later in this review). Such a control strategy, coupled with chemical MDA and/or improvements to sanitation infrastructure and practice, could also fit within the One Health Initiative (OHI)’s aims of controlling and preventing disease emergence through a multidisciplinary approach (Hoover et al. Citation2020; CDC 2020). The OHI approach to improving disease control and prevention methods acknowledges the epidemiological interdependence between animals and humans; it encourages more comprehensive thinking on how to prevent disease emergence and combat historical threats to both human and animal populations (CDC 2020). This integrated strategy is considered essential by many organizations to achieving the interrelated Sustainable Development Goals (SDGs) adopted by all United Nations member states in 2015 (UNDP 2021).
The use of freshwater aquaculture to combat waterborne disease is a strategy which could be used to make progress in many of the seventeen different SDGs, including #1 (No Poverty) and #3 (Good Health and Wellbeing), by using the OHI approach to fighting disease in a sustainable and integrated way. Recognized as a neglected disease of poverty, one key element of schistosomiasis control is poverty alleviation, which small-scale commercial aquaculture has proven able to promote via profit generation and increased local access to nutritious foods by both farming families and surrounding communities (Arthur et al. Citation2013; Marques et al. Citation2016). With the recent identification of a range of native fish which are potential predators of host snails (Arostegui et al. Citation2019), there is an interest for these species to be trialed in the field in significant numbers. Species considered to be more robust and resilient to more extreme environments are of particular interest as the climate in sub-Saharan Africa changes (Goula et al. Citation2009; Yapo et al. Citation2019).
One of these species identified is the West African lungfish Protopterus annectens (see Daffalla et al. Citation1985; Arostegui et al. Citation2019). Preliminary work has been conducted in Uganda toward the domestication and marketing potential of P. annectens’ sister species the marbled lungfish Protopterus aethiopicus (see Molnar et al., Citation2012; Walakira and Molnar Citation2014), and the significant rate of vector snail consumption by wild-caught P. annectens individuals has been documented (Mahdi and Amin Citation1966; Daffalla et al. Citation1985; Arostegui et al. Citation2019), but no work has yet been done on the farming and market potential of this species in a schistosomiasis-prone country for the purpose of sustained intermediate host snail control. Similarly, more work is needed to evaluate the farming potential of a native proven snail predator, the West African freshwater prawn M. vollenhovenii (see Hoover et al. Citation2020).
Côte d’Ivoire, a relatively large, primarily French-speaking country located on Africa’s western coast, has multiple regions of both endemic schistosomiasis and elevated poverty. It also has one of SSA’s highest per capita rates of fish consumption (Ayilu et al. Citation2016) as well as multiple natural fisheries for both M. vollenhovenii and P. annectens (see Teugels et al. Citation1988; Konan et al. Citation2010), making Côte d’Ivoire an appropriate focus of this review. In recent decades the exponential increase in dam installations across the region for hydroelectric and agricultural utility has also been shown to greatly increase the incidence of this disease by increasing vector snail habitat while blocking natural predation by catadromous predators including M. vollenhovenii (see Sokolow et al. Citation2017). Many more dams are planned for installation in Côte d’Ivoire (Esri Citation2019) and with them will likely come a spike in schistosomiasis infections (Parent et al. Citation1997; Mortey et al. Citation2019). This systematic literature review attempts to explore the viability of using aquaculture of vector snail predators for simultaneous schistosomiasis control and poverty alleviation in Côte d’Ivoire as climate change continues to intensify and snail ranges increase.
Methods
The method used in this paper was a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-based systematic review (see for workflow diagram), derived from a scoping review method developed by Arthur et al. (Citation2013) for the UK Department for International Development (DFID). Those researchers used a six-step methodological protocol to assess the existing evidence of the contribution fisheries and aquaculture activities make to economic growth, food security, and nutrition in emerging economies. Their goal was to first identify all existing and pre-published evidence, exclude studies not meeting a threshold of quality, compile remaining studies into common sections, then evaluate the strength of evidence for each section and identify gaps in knowledge. Taking a similar approach with this review allows the thorough evaluation of this novel One Health-based approach to schistosomiasis control via aquaculture which had to cover a broad range of evidence, including epidemiological studies, climate models, species biology, and socioeconomic survey data. The identification of weak/insufficient evidence and gaps in the literature indicate where more research is required and/or where more detailed investigations need to occur. The six-step methodological protocol is described here below:
Sourcing of literature
A curated online database of scientific literature was used to obtain the evidence assessed in this review. Scopus (Elsevier) has been identified as an appropriate database for use in broad systematic reviews (Gusenbauer and Haddaway Citation2020). This database was selected over 15 other academic search databases that Gusenbauer and Haddaway (Citation2020) identified as being sufficient for use in systematic reviews because Scopus is science-focused yet multidisciplinary, includes a large set of French-language publications (with indexed English-translated abstracts), and has search results that can be filtered by country and topic, saved into lists and then bulk exported in multiple formats. Exported citations and abstracts were saved in as separate article entries in the desktop-based Mendeley (Elsevier) reference manager application.
Selection of search terms
Multiple search terms were selected from keywords the authors extracted from an initial review of core publications (see Appendix I) which were obtained via a ‘snowballing’ approach (<50 papers) (Wohlin Citation2014). Commonly used key words were extracted from this set of publications to be used as search terms in this review. To help ensure the entire breadth of peer-reviewed knowledge for this broad topic would be captured in the search results, the authors considered four primary search terms, with a further six secondary search terms to be paired with each primary term (see for the complete list of terms). Each primary term was also paired with every other primary term. Terms were separated using “AND,” for example the first query entered was “schistosomiasis AND aquaculture.” Core articles related to this review and cited in the core literature but that did not turn up in the systematic search results due to having a focus outside the scope of this review (especially those studies being mainly classified as topical papers in medicine) were also included. Relevance was determined by their appearance in the initial ‘snowball’ approach and citation by more than three other key articles. A total of 36 literature queries via Scopus were executed with 898 articles retrieved.
Table 1. Search terms used in the systematic review of the literature via the online database Scopus in June 2020.
Screening and selection
Four inclusion/exclusion criteria were applied to the search results from each query:
Topical relevance: Results in ‘Medicine’, ‘Biochemistry, Genetics and Molecular Biology’ and ‘Planetary Sciences’ were excluded, as the focus of this review is on the potential of aquaculture to eliminate schistosomiasis via snail predation, not the schistosomiasis disease itself outside of this context.
Filtering of excessive results: Keyword pair search results totaling more than 100 articles were individually filtered in the following ways:
‘Schistosomiasis AND poverty’ = searched within results for ‘Africa’
‘Schistosomiasis AND snail*’ = searched within results for ‘Africa’ and limited results to last 20 years.
‘”Côte d’Ivoire” OR “Ivory Coast” AND market*’ = searched within results for ‘fish* OR seafood’.
‘Macrobrachium AND aquaculture’ = searched within results for ‘Africa’
‘Macrobrachium AND market*’ = searched within results for ‘Africa’
Relevance to review: The title and abstract of each article were read to determine the article’s relevance to the review. An article was deemed relevant if the article’s focus was within the scope of this review.
Reliability: The article was considered reliable if it was listed by the curated database and, if published before 2020, cited by other articles more than three times. Articles cited fewer than three times were considered on a case-by-case basis for inclusion.
Sorting articles into thematic clusters
The source of the data each article used or referenced was considered in their evaluation, with studies that collected primary data considered more impactful than articles interpreting secondary data. Similarly, the scale of each study was considered, with studies covering a geographic area larger than a single village, catchment or reservoir were considered stronger. Articles sharing a common subject were then sorted into thematic clusters. The 261 articles identified as relevant to this review were sorted into eight thematic clusters based upon shared topics related to the overall research question. Each thematic cluster was evaluated for overall technical quality using definitions given in Arthur et al. (Citation2013) (see ). Stronger and/or more impactful papers increased the technical quality score of the cluster in support of their respective evidential conclusions. The number of articles within each thematic cluster was also considered, using the following rating system:
Table 2. Definitions of technical evidential quality for a given body of evidence (from Arthur et al. Citation2013).
Large = >50 articles
Medium = 10–49 articles
Small = less than 10 articles
Each thematic cluster was then rated on whether the individual articles came to the same conclusions concerning the subject matter of the cluster, where a consistent cluster had all studies in agreement on the topic, a mixed cluster had most studies in agreement, and an inconsistent cluster had no clear agreement between studies.
Finally, a summary three-part cluster grade based upon quality, size and consistency was produced, with a summarizing paragraph for each cluster explaining the grade and summarizing the evidence within the cluster. This grade and summary paragraph was called the ‘General summary of evidence’.
Grouping clusters into main themes
Eight thematic clusters were organized into three main themes, where the findings of the literature were discussed in detail:
Epidemiology of schistosomiasis and of poverty in Côte d’Ivoire during the era of climate change
The potential biological control of obligate host snail species for long-term schistosomiasis control in Côte d’Ivoire: Evaluating two different predators
Economically sustainable disease control via marketing farmed native snail predators in Côte d’Ivoire
Evaluating the main themes
Each theme was explored via discussion of the graded thematic clusters, with overall conclusions shared by multiple articles given priority as a narrative develops within each thematic cluster.
Overall themes via thematic clusters
Poverty and health in Côte d’Ivoire during the era of climate change
Schistosomiasis and poverty: general summary of evidence
Quality: High
Size: Medium
Consistency: Consistent
The number of quality articles on schistosomiasis in sub-Saharan Africa published in the past two decades is high, but those that specifically explored poverty’s role in endemicity were not as numerous. Increased international investment over the past decade on neglected tropical diseases (NTDs) that strongly affect the most poverty-stricken, isolated and often stigmatized communities in Africa (Hotez and Fenwick Citation2009) supported much of this research. Papers discussing the impact of climate change on schistosomiasis epidemiology and on poverty-stricken regions in Côte d’Ivoire were even fewer in number, but clearly outlined how ‘climate vulnerability’ is a growing problem for many coastal and inland communities.
Schistosomiasis and poverty: the recognition of neglected tropical diseases
Many bacterial, protozoal, viral and helminthic diseases mainly found in the least developed countries, including schistosomiasis, collectively gained official recognition as Neglected Tropical Diseases in 2003 (Hotez and Fenwick Citation2009). NTDs are described as primarily infectious diseases that are especially common in resource-poor populations, mostly in tropical areas (Quansah et al. Citation2016). Most NTDs were systematically undertreated before the 2000s, with fewer than 10% of infected populations receiving consistent annual treatment for their often multiple helminth infections (Schur et al. Citation2011) despite schistosomiasis being recognized as second only to malaria for parasite-induced morbidity and mortality (Fenwick et al. Citation2009). Encouraging results from control efforts in Egypt, China and Brazil in the 1990s did lead to renewed interest and political commitment from countries in sub-Saharan Africa, with the Schistosomiasis Control Initiative (SCI) started by the World Health Organization (WHO) in 2002 with major support from the Bill and Melinda Gates Foundation (Brooker et al. Citation2004; Hotez and Fenwick Citation2009). The five-year initiative of SCI was based in six African countries and was chemotherapy-focused, using praziquantel as the treatment and targeting both school-aged children and at-risk occupational groups (cleaners, fish farmers, etc,) (Brooker et al. Citation2004). SCI also funded research into the effectiveness of chemotherapy for acute and preventative schistosomiasis control (Fenwick et al. Citation2009). Following SCI’s transition into the SCI foundation in 2018, the formation of the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) group continued SCI’s original mandate by developing evidence-based policy recommendations for future control programmes (The SCI Foundation Citation2020). Many SCORE-funded research projects have highlighted the important effect that poverty in its many forms has on schistosomiasis endemicity, which requires any effective control program for this disease to consider this relationship between poverty and schistosomiasis (Coulibaly et al. Citation2017; Lo et al. Citation2017, Citation2018; Lund et al. Citation2019).
Schistosomiasis and poverty: the influence of poverty on disease control
Poverty is a complex term to define, as there are many different manifestations. Poverty is recognized globally as more than simply a lack of income and productive resources required to ensure a sustainable livelihood; it can manifest as hunger and malnutrition (nutritional poverty), limited access to education and basic services, social discrimination and exclusion, and a lack of participation in decision-making (United Nations Development Program Citation2020). Schistosomiasis is common in tropical and subtropical regions lacking sanitation, access to clean water, and access to affordable health care. Appropriate, culturally sensitive, and evidence-based primary health education about basic personal and community hygiene, parasite control, and the use of medication is required (Sow et al. Citation2004, Citation2011; Palmeirim et al. Citation2018; N’Goran et al. 2019). When education is lacking, water and sewer infrastructure that ensures untreated sewage does not come into contact with water used for drinking, washing or recreation is essential, as it keeps any schistosome eggs excreted from an infected individual from infesting any vector snails inhabiting nearby water bodies (Kulinkina et al. Citation2016). Equally essential in every successful control strategy has been effective public health infrastructure that ensures comprehensive treatment of both infected individuals and communities (Xu et al. Citation2016). A lack of an effective health infrastructure is especially conducive to schistosomiasis endemicity (WHO Citation2015; N’Goran et al. 2019), as effective treatment delivery and intervention for chronically infected individuals requires comprehensive service access and often repeated treatments (Utzinger et al. Citation2003; Bonate et al. Citation2018). There are worryingly high instances of co-infections with other often serious chronic diseases including malaria, HIV and cancer (van der Werf et al. Citation2003; Muller et al. Citation2011; Colombe et al. Citation2018), and without adequate access to health care the overall effect of co-infections with chronic schistosomiasis is that endemic poverty is entrenched as a result of community-wide chronic disablement (King and Bertino Citation2008). Furthermore, poor health infrastructure keeps this knowledge from being captured and prevents other research about the disease from being effectively conducted (Rollinson et al. Citation2013; N’Goran et al. 2019). The presence of poverty is, therefore, both a cause and an effect of chronic schistosomiasis within afflicted communities. It is often embedded in the social-ecological system of endemic communities, becoming intimately connected with habituation, poverty and general neglect (Utzinger et al. Citation2011; Grimes et al. Citation2015).
Poverty, malnutrition and climate vulnerability in Côte d’Ivoire: general summary of evidence
Quality: High
Size: Medium
Consistency: Consistent
The subject of poverty in Côte d’Ivoire in all its manifestations, including vulnerability to climate change, has become a well-studied topic in the past decade. Years of civil war before 2012 gave way to relative stability of government and rapid economic progress. Combined with several decades of robust national statistics on poverty indicators at a household-level granularity, these factors were likely responsible for the large number of robust papers examining Ivorian poverty and the reasons why it has stagnated even as national economic indicators improved. Multiple studies in this cluster model the physical and economic effects of climate change across sub-Saharan Africa while a few have looked at Côte d’Ivoire in particular; all show an increasing trend toward a hotter and drier climate with more frequent and intense bouts of drought or flooding events, with moderate to severe economic impacts on this primarily agrarian-based country. The measures taken in recent decades to mitigate such problems, including the construction of dams, have been shown by multiple cluster studies in the cluster to present other unintended consequences including sustained outbreaks of snail-borne diseases including schistosomiasis in local communities.
Poverty, malnutrition and climate vulnerability in Côte d’Ivoire: economic poverty
The current poverty and economic outlook for Côte d’Ivoire is very uncertain due to the ongoing COVID-19 pandemic, as the severe global slowdown combined with the effects of local efforts to contain the coronavirus take their toll on the economy at every level (World Bank Citation2020). As of 2019, the last 8 years of rapid economic growth has not translated to increased levels of social inclusion and reductions in the Ivorian national poverty headcount ratio, which has been hovering around 46% (28% under the international standard poverty line of $1.90 per person per day) since 2015 (World Bank Citation2019a). The average rate of fiscal poverty among emerging economies in 2016 was 20% (Czajka Citation2017). This is mainly due to GDP growth being driven by retailers and construction, while employment is highly concentrated in agriculture (family farms) and nonagricultural self-employed workers, mostly women with no formal education (World Bank Citation2019b). There are still significant differences in income between regions (Sanogo Citation2019). Adopting aquaculture as an additional or primary income stream has shown to reduce poverty among African farming households while having a positive impact on their health mainly due to increased purchasing power (Jamu et al. Citation2002; Aiga et al. Citation2009).
Poverty, malnutrition and climate vulnerability in Côte d’Ivoire: nutritional poverty
Food insecurity and malnutrition is a continuing problem in Côte d’Ivoire for the roughly 46% of the population which is experiencing poverty, especially in rural communities in the north and west of the country (World Food Programme Citation2021). This starts at birth, with 23% of new-borns not receiving the important colostrum-laden breastmilk within an hour of delivery, and infants in Côte d’Ivoire receiving the lowest average rate of WHO-recommended exclusive breastfeeding (4%) in their first 6 months of life for any sub-Saharan African country (Alles et al. Citation2013; United Nations Children’s Fund (UNICEF)) Citation2018). Poor health, especially malnutrition, in early childhood causes irreversible physical and mental damages (Welander et al. Citation2015). Research into Ivorian infant malnutrition and breastfeeding has shown a worrying trend among poorer rural families toward feeding bigger, stronger babies breast milk with greater frequency and for longer than smaller, less healthy babies, who are more likely to receive lower quality liquid diets and move onto soft, semisolid and solid foods earlier than their stronger siblings (Issaka et al. Citation2015; Ezeh et al. 2019). Foods available to the impoverished families of West African children when weaning them off breastmilk are often inexpensive, carbohydrate-heavy, and of poor nutritional quality, with animal-derived proteinaceous foods only occasionally included in their diets, keeping children from receiving the essential fats, micronutrients and amino acids they require to optimally grow (Onyango et al. Citation2019). This triple burden of under- and over-nutrition with micronutrient deficiencies is increasingly common across many other low- and middle-income populations across the world, with recent global reductions in undernutrition and micronutrient deficiency being largely offset by increases in disease associated with obesity (Ridoutt et al. Citation2019). This is a challenge which aquaculture has been known to address, with even smaller fish grown amongst rice paddies or spare reservoirs in polyculture with other species effectively reducing the micro- and macro-nutrient deficiencies of those who harvest them for food or profit (Arthur et al. Citation2013). Ivorian food fortification programs, such as the Infant and Young Children Nutrition Program (IYCNP) were set up to reduce childhood deficiencies in iron (Glinz et al. 2017), iodine, and zinc (Alles et al. Citation2013), but problems with the price of iodine, population coverage, product storage, a lack of local production interest, and some cultural barriers (i.e., goiter not being recognized as a negative condition in many regions) have kept fortified foods from becoming significantly effective across the country (Alles et al. Citation2013; Leyvraz et al. Citation2016). There are multiple recent government- and NGO-sponsored programs working to overcome these hurdles and reduce the 834,000 Ivorian children under five that were still suffering from stunting due to acute malnutrition in 2019 (Global Nutrition Report Citation2020; World Food Programme Citation2021).
The interruption to food supplies and transport networks during the series of conflicts suffered by the country since independence highlights the need for more locally produced foodstuffs (Dabalen and Paul Citation2014). The lack of democratic rule during certain periods in the country’s history has been linked to significantly higher infant mortality due to malnutrition as value chains broke apart (Welander et al. Citation2015). Current trends in production under successive elected governments are more outward-facing, and the practice of export farming has worked out modestly well for both the families of export farmers (Sahn Citation1990) and for the government in terms of revenue under past and current international trade schemes (Bouët et al. Citation2018), with trade policies in Côte d’Ivoire being measured as generally pro-poor (Nicita et al. Citation2014). For local consumption, rice continues to be the main farmed and consumed food source for the average Ivorian, with consumption increasing from 27 kg per person in 1958 to almost 85 kg in 2006 as people moved to cities and urban dwellers increasingly preferred rice over cassava and yam (Becker and Yoboue Citation2009). Fish is the primary source of animal protein, with over 15 kg annually consumed per person; however, up to 67% of this fish is imported (FAO 2019a). The dangers of over-reliance on imported rice and fish for feeding millions revealed themselves in the 2007–2008 global food crisis, when the doubling of prices almost overnight quickly led to widespread violence in the streets of Abidjan, Côte d’Ivoire’s largest city. The resulting flood of relatively cheap imports, including frozen fish, from Asia into local markets combined with historical changes in policy across SSA which favor Asian importers over West African producers has gradually led to an agrarian crisis across the region (Moseley et al. Citation2010). Work is urgently needed to make nutritious protein sources more affordable, available, and desirable for lower- to middle-income Ivorians (Ridoutt et al. Citation2019) as Ivorian agriculture becomes an increasingly uncertain occupation as local climates change (Yapo et al. Citation2019).
Poverty, malnutrition and climate vulnerability in Côte d’Ivoire: climate vulnerability
The Earth’s climate has warmed by 0.6 °C over the last 100 years (Walther et al. Citation2002). Coastal states of West Africa have seen an even bigger increase in both average temperatures and of sea levels, but what has been most devastating to communities are the increasingly long periods of drought and (to a lesser extent) of torrential rain that have measurably increased in severity and frequency over the past 50 years (Gan et al. Citation2016; Yapo et al. Citation2019) as a result of global climate change (Walther et al. Citation2002). Africa itself is already the hottest and driest continent on average, and so water availability is a key factor in all ecosystems and biomes as well as being essential for maintaining biodiversity; any change in historical patterns of rainfall, stream reach and aquifer levels triggers disaster for millions, especially in regions where water is already in short supply (Gan et al. Citation2016). Research has shown fisheries in West Africa are experiencing a substantial decline in landed value resulting in a 50% decline in fisheries-related jobs by 2050 from levels seen in the early 2000s (Lam et al. Citation2012).
Water resources are more complex to collate, but significant changes in annual runoff are predicted within Côte d’Ivoire, with increased interannual variability of overall decreasing amounts of precipitation (Dixon et al. Citation2003; Coulibaly et al. Citation2018; Kouame et al. Citation2019). Increases of mean daily temperatures experienced in the sparsely populated north of the country will be accompanied by decreases in the more populous south, but the temperature increases will generally be of greater magnitude (Yapo et al. Citation2019). This trend of increasing temperatures and decreasing rainfall has significantly raised the likelihood of having two successive drought years, a phenomenon considered the greatest impact of climate change in SSA (Gan et al. Citation2016; Santé et al. Citation2019). Projections for the Sahel region conducted by Diba et al. (Citation2019) concur, predicting decreasing yearly numbers of consecutive wet days and increasing numbers of consecutive dry days, along with an increase of very warm days and nights due to changes in the monsoon system driven by warming temperatures. These predicted changes could be even more profound than what can be modeled due to a mismatch between average meteorological data before and after 1950, the point at which measurable global climate change began to take effect (Paturel et al. Citation2003). Both droughts and flooding are posing increasing challenges to many Ivorian commercial fish farmers who often site their ponds along rivers that have increasingly experienced flood events, while extensive farmers who often grow their fish in polyculture with rice are experiencing more frequent eutrophication and water loss (2020 April 4th virtual conversation with R. Koumi). The African Development Bank forecasts all these changes pointing toward a moderately negative overall economic impact on Cote d’Ivoire directly due to the effects of climate change, with a minimum 6.4% drop in macroeconomic output by 2050 (Baarsch and Schaeffer Citation2019).
Poverty, malnutrition and climate vulnerability in Côte d’Ivoire: dams, climate change and schistosomiasis
Hydroelectric dams, along with artificial reservoirs and underground aquifers, are seen as significant examples of economic progress among emerging countries (Southgate Citation1997; Adenowo et al. Citation2015). Hundreds of dams have been constructed in SSA over the past 50 years, with hundreds more planned for completion in the next decade (Esri Citation2019). With dozens planned for Côte d’Ivoire, this makes the need for schistosomiasis and vector snail distribution models which can be used during the planning process of these dams more imperative than ever, as dam installation has been clearly linked to jumps in schistosomiasis infection rates among nearby populations (Sokolow et al. Citation2015; Lund et al. Citation2019).
Dams collect water upstream of their location for use in irrigation, livestock, hydroelectric power generation, and flood control (Adenowo et al. Citation2015; Diakité et al. Citation2017). This creates a new, often ideal habitat for vector snails in SSA as the dams slow the waters and inundate the land upstream while also blocking the passage of natural vector snail predators such as giant river prawns and various fish species (Arostegui et al. Citation2019). Steinmann et al. (Citation2006) estimated that 13.6% of people vulnerable to schistosomiasis infection reside close to large dam reservoirs and irrigation schemes, which lends credence to the observed surge in S. haematobium infection rates in these communities following construction of large hydroelectric projects in Senegal, Ghana, Mali, Namibia, Cameroon and Côte d’Ivoire (Olveda et al. Citation2013). Often these dam projects were led by government officials and private contractors external to local communities, resulting in little to no engagement or risk assessments completed regarding long-term impacts of the project. These actions can lead to the negative ecological and social effects of a new dam canceling out any benefits for local populations (Lerer and Scudder Citation1999; Boelee et al. Citation2013). However, with monthly rainfall in parts of Côte d’Ivoire projected to decrease by anywhere from 3% to 47% in the next 50 years while surface runoff increases due to more intense precipitation events, large infrastructure projects in Côte d’Ivoire will continue to be built to lessen the impact of successive drought years by regulating and storing the water runoff, as well as increasing the renewable energy portfolio of the countries involved (Kouame et al. Citation2019; Mortey et al. Citation2019). These dams will preserve or enhance local livelihoods by protecting downstream communities from flooding and upstream communities from the effects of drought (Kouame et al. Citation2019). This preservation has not already been without other costs, illustrated in Hunter’s (Citation2003) documenting of the installation of hundreds of small dams which improved the lives of local communities yet allegedly tripled their schistosomiasis infection rates and entrenched endemicity over successive generations.
Schistosome-vector snails have been shown to be significantly affected by changes in temperature related to climate change (Kalinda et al. Citation2017). Temperature affects both the infectivity and size of vector snails along with the rate of schistosome development within the snail, with higher temperatures significantly reducing the period between infection and transmission while elevating the rate of parasitical cercariae output up until 33 °C, the point beyond which output drops and snail mortality increases (Kulinkina et al. Citation2016; Kalinda et al. Citation2017). Some hotspots of schistosome infection will decrease under certain climate change scenarios while other areas will experience surges of infection, making habitat suitability and disease modeling essential in predicting how progressing climate change will affect schistosomiasis transmission within a specific region (Wrable et al. Citation2019). Nelson et al. (Citation2016) did find evidence of acclimatization to higher temperatures within challenged laboratory Biomphalaria glabrata populations, indicating negative effects of climate change on snails may be short-lived in certain populations (Nelson et al. Citation2016). Studies on the effects climate change has on the parasite itself have shifted the scientific consensus recently, from thinking they were scarcely affected compared to other how other microparasite diseases respond to changes in climate, to now believing the life-cycle lengthens as their temperature rises (Mas-Coma et al. Citation2008). They experience longer generation times, slower population growth rates and a longer time before infected hosts exhibit symptoms (Mas-Coma et al. Citation2011). This is a mixed bag for affected communities; as the spread of the disease slows, the lengthening time between an individual becoming infected and exhibiting symptoms can delay treatment and confound any attempts to trace the contaminated source. Further fine-grain modeling of changes to disease epidemiology in response to fluctuations in climate is required to determine how infections rates and endemicity will change under various scenarios.
The potential biological control of obligate host snail species for long-term schistosomiasis control in Côte d’Ivoire
Types of intermediate host species
Types of intermediate host species: general summary of evidence
Quality: High
Size: Large
Consistency: Consistent
Articles relevant to this thematic cluster mainly discussed the role Biomphalaria spp. and Bulinus spp. vector snails have in the schistosomiasis life-cycle, using both secondary data in the form of literature reviews and book chapters, and primary data from the large number of studies on African schistosomiasis vector species over the last 60 years.
Types of intermediate host species: Bulinus spp
Snails within the genus Bulinus are the obligate intermediate hosts for S. haematobium, causing urogenital schistosomiasis (Rollinson et al. Citation2013). They live in a range of habitats, from small streams to large lakes (Woolhouse and Chandiwana Citation1990). Bulinus globosus is by far the most studied of all Bulinus species in Africa (Kalinda et al. Citation2018); it can live for several months under laboratory conditions, but this reduces to only a few weeks in the field (Woolhouse and Chandiwana Citation1990). Bulinus spp. are generally more tolerant of higher temperatures compared to S. mansoni-carrying Biomphalaria species, with B. globosus experiencing progressive shell growth from 15.5 °C, peaking at 25.8 °C and halting over 31 °C, while surviving longer in cooler temperatures (Kalinda et al. Citation2017). Bulinus spp. are also more adapted to prolonged dry spells than Biomphalaria spp., allowing them to survive for a time beneath dry ephemeral ponds and streams via estivation (Perez-Saez et al. Citation2017). This makes modeling Bulinus spp. population dynamics in response to changes in precipitation and temperature more challenging than for Biomphalaria spp., which generally are only found within permanent and quasi-permanent water flows and bodies, although certain species within each genus can estivate for short periods (Perez-Saez et al. Citation2017). Akande et al. (Citation2010) describe the molecular process of estivation within two Bulinus species as an economical utilization of stored metabolites, which allows schistosome infection to persist upon revival albeit with a lower survival rate of infected snails. In Côte d’Ivoire, both B. globosus and B. truncatus can transmit S. haematobium (Diakité et al. Citation2017). Populations of Bulinus spp. have been found throughout Côte d’Ivoire (Tian-Bi et al. Citation2018), with most recent infected hotspots of S. haematobium endemicity having been found in the southern and central regions of the country (Swiss T P H 2020).
Types of intermediate host species: Biomphalaria spp
Snails in the genus Biomphalaria are the obligate intermediate hosts of S. mansoni, which causes intestinal schistosomiasis (World Health Organization Citation2006). Schistosoma mansoni’s geographic distribution is therefore closely tied with Biomphalaria abundance, especially with that of Biomphalaria pfeifferi due to its widespread distribution (Morgan et al. Citation2001). Biomphalaria pfeifferi snails are found primarily in very shallow water (0–7 cm) at the edge of small pools, in slow streams (water flow = 12–21 cm/s), and in lakes and irrigation canals (McManus et al. Citation2018). There are 12 Biomphalaria species in SSA and all are susceptible to infection, but transmission is predominantly through B. pfeifferi (see Morgan et al. Citation2001). Thus, most research into Biomphalaria spp. in Côte d’Ivoire has concentrated on this snail. The optimal water temperature window for B. pfeifferi is 20 to 29 °C, with fecundity and survival in the laboratory and observed in the field quickly falling when the temperature exceeds 27 °C, with shorter periods of intensely hot weather being more damaging to B. pfeifferi populations than longer periods of above-optimal temperatures (de Kock and Joubert Citation1991). This sensitivity to temperature and to changes in conductivity cause Biomphalaria populations to vary significantly by season in many areas of SSA, but their high tolerance of, and even preference for, high levels of organic matter (60–1060 mg/L total suspended solids) has been widely observed and allows for year-round dense populations in certain areas near human settlements (Rollinson Citation2011). Seasonal variation in rates of S. mansoni transmission has been observed, with most transmission occurring between May and August in Senegal (Southgate et al. Citation2001). Biomphalaria species infected with S. mansoni experience decreased speed and range of movement, a drop in growth rate, reproduction shutdown and increased hiding under substrate (Swartz et al. Citation2015). Biomphalaria pfeifferi’s relative intolerance for shade has also been noted, mainly due to behavioral observations possible related to shade inhibiting the growth of their diatomaceous algal food (Loreau and Baluku Citation1991). In Côte d’Ivoire, B. pfeifferi is the sole intermediate host of S. mansoni (see Assaré et al. Citation2016; Diakité et al. Citation2017). The current country-wide distribution of B. pfeifferi and accompanying rate of S. mansoni infection is unknown, but S. mansoni has been endemic to villages in Des Montagnes in Western Côte d’Ivoire since the 1980s, and is currently spreading into territory that has only known S. haematobium (see Assaré et al. Citation2016).
The history of biological control
The history of biological control: general summary of evidence
Quality: High
Size: Small
Consistency: Consistent
Literature on the biological methods used to reduce schistosomiasis transmission by controlling the intermediate snail host is small yet of good quality. Despite the fact that this literature originates from multiple geographical locations, is it quite consistent in demonstrating the importance of a multipronged approach to sustained control that includes both mass drug administration and a long-term method of controlling vector snail populations.
The history of biological control: competitors, parasites, and predators
There has been a long history of using biological methods to control or eliminate schistosomiasis, usually via controlling the intermediate host snail populations (Oliver-González Citation1946; Pointier et al. Citation1991; Arostegui et al. Citation2019). These methods include the use of trophically similar, non-susceptible competitor snail species (Lee et al. Citation1982; Cowie Citation2001; Anto et al. Citation2005), sterilizing/lethal microparasites and pathogens (Pointier and Jourdane Citation2000; Coustau et al. Citation2015), and molluscivorous predators (Madsen Citation1990; Slootweg et al. Citation1994; Hoover et al. Citation2020).
The introduction of competitive snail species, not susceptible to schistosome parasitism, into areas where both schistosomiasis and vector snail species are prevalent has been successfully used for sustained control in parts of the West Indies, Caribbean, Puerto Rico, Venezuela and the Dominican Republic in the late 20th Century (Madsen Citation1990; Pointier and Giboda Citation1999). Anto et al. (Citation2005) even observed predation on individuals and egg masses in addition to competition by the ampullariid snail Lanistes varicus on B. pfeifferi in laboratory conditions (Anto et al. Citation2005). Preliminary work has also been done on the possibility of developing transgenic strains of vector snail species which are immune to infestation to outcompete vulnerable strains (Coustau et al. Citation2015; Famakinde Citation2018). Toward this end, snail hematopoietic organ transplantation (which transfers resistance to infection into vector snails) has been successfully attempted (Barbosa et al. Citation2006). Development of new genetic techniques in recent years, such as CRISPR, will increase effectiveness of similar attempts, but research in relation to snail molecular research is underdeveloped (Famakinde Citation2018). Competitor snail species attributes are selected based upon having parasite resistance, high fecundity, voracious feeding habits, longer lifespans, and (ideally) being harmless to non-target plant and animal species (Gashaw et al. Citation2008). Specific species used depends upon location and availability, but popular snail families include ampullariids for additional control of aquatic weeds frequented by carrier snails and thiarids for direct competition and incidental predation (Pointier and Jourdane Citation2000; Cowie Citation2001). Most of the first competitive snail introductions were initially accidental, but often led to intentional stocking in other areas after their positive effects on local schistosomiasis infection rates were observed (Slootweg et al. Citation1994). Their efficacy has been confirmed based upon long-term studies and observed correlations between competitor population rises and the simultaneous decrease in schistosome-susceptible snail populations, often along with the disappearance of their preferred habitat such as the water lettuce Pistia stratiotes (see Pointier and Jourdane Citation2000; Cowie Citation2001; Pointier and David Citation2004). Considering their invasive potential, researchers highlight the generalist feeding habits of these competitor species, along with their tendency to also prey upon commercially important rice cultivars and other aquatic plants; little to no attention has been given to many of these species’ long-term effects on the ecosystems to which they were introduced (Rollinson Citation2011). Proper use of competitor species must include post-release monitoring and a genuine analysis of their impacts on native biodiversity (Madsen Citation1990; Cowie Citation2001).
The use of other parasites and of land-dwelling predators to target the intermediate host snails has also been examined. There has been substantial work on describing parasitic non-schistosome trematodes that infect Biomphalaria spp., with the members of the trematode genus Echinostoma having the most extensive body of literature due to their reproductive similarity (and sometimes lethal effect) to Schistosoma spp. in the laboratory (Rollinson Citation2011). Despite this deep knowledge of the genus, little research has been done on their actual use, along with bacteria, fungi and protozoans, in the sterilizing or killing of schistosome-carrying snails (Madsen Citation1990), even with a wide variety of these trematodes co-existing with both wild Biomphalaria and Bulinus species (Rollinson Citation2011). Predators of freshwater snails have been studied much more extensively, encompassing almost all groups of the animal kingdom in different life stages, but in particular birds, crustaceans and fish (Rollinson Citation2011; Sokolow et al. Citation2018). The use of the muscovy duck Cairina moschata, for example, can be win-win for schistosomiasis-endemic communities; they eat the host snails while often harboring the aforementioned Echinostoma parasites whose miracidia fatally infect the snails, and are marketable poultry, but concerns about theft and the effective number required limit their practical use for host snail control (Kloos et al. Citation2001; Sokolow et al. Citation2018).
The use of aquatic snail predators to target host snails has been studied in the laboratory for decades (Amberson and Schwarz Citation1953; Daffalla et al. Citation1985), but only recently have robust field trials taken place (Sokolow et al. Citation2015). Aquatic species which predate upon snails can dramatically reduce the population dynamics of a single snail species, but predation pressure rarely reaches levels where a snail population completely disappears in response (Slootweg et al. Citation1994). Regardless, the release of molluscivorous aquatic predators in areas of high endemicity for schistosomiasis has led to significant reductions in infection rates amongst schoolchildren (Mkoji et al. Citation1999; Sokolow et al. Citation2015), and reducing the snail population below a threshold where schistosome-snail infection rates are lower than snail death rates is enough to control the schistosome life-cycle (Sokolow et al. Citation2015). The use of a more generalist fish or crustacean predator that prefers intermediate host snails but can switch to other foods as they become rare allows for sustained control of the snail population without endangering the predator population (Sokolow et al. Citation2015). Also preferable are intraguild predators, which directly feed upon thefree-swimming life stage of the parasite and compete via consumption of the target intermediate snail host (Lodge et al. Citation2005; Sokolow et al. Citation2015). Individual laboratory evaluations of fish or crustacean species for snail predation are numerous (Madsen Citation1990; Stauffer et al. Citation2006; Gashaw et al. Citation2008; Hoover et al. Citation2020; Savaya-Alkalay et al. Citation2020), but as illustrated by recent and historic invasions of snail predators into non-target waterbodies and trophic niches, evaluating these predators in the field accompanied by thorough ecological investigations are essential to allay concerns about generalist predation, habitat destruction and competition with related species which may outweight potential benefits to local communities (Lodge et al. Citation2005; Andriantsoa et al. Citation2019; Marshall Citation2019; Oficialdegui et al. Citation2020).
One strategy to avoid many of these problems is to use native species that may have been overfished in a particular area or had their access blocked via environmental or geological changes and culture them locally via aquaculture to strategically increase populations while conveying nutritional and economic benefits (Savaya-Alkalay et al. Citation2014; Sokolow et al. Citation2015; Hoover et al. Citation2020). A recent study in Senegal used field, research, and model-based ecological and dietary evaluations to identify several potential candidates for use in sub-Saharan Africa for Biomphalaria and Bulinus spp. direct and/or indirect snail predation (Arostegui et al. Citation2019). Two of the most molluscivorous species identified amongst the ten candidate species were the giant freshwater prawn M. vollenhovenii and the West African lungfish P. annectens, previously identified as a suitable control agent (Daffalla et al. Citation1985; Arostegui et al. Citation2019).
Snail predator candidates for farming and deployment
Snail predator candidates for farming and deployment: general summary of evidence
Quality: High
Size: Small
Consistency: Consistent
Research articles discussing the ecology, range and regional fisheries of giant freshwater prawns across West Africa were plentiful, but there were relatively few papers on African lungfish ecology and ranges, with most research on this genus focused either on Lake Victoria where lungfish is considered a delicacy (Molnar et al., Citation2012), or on their use in evolutionary biology. Only a handful of papers explored the farming potential of African lungfish, and those papers that discuss culturing freshwater prawns do so in relation to techniques already used to culture the molluscivorous Malaysian prawn M. rosenbergii, which is widely farmed in Asia and South America but not native to Africa. There are no contradictory papers, but gaps in knowledge are present and identified.
Snail predator candidates for farming and deployment: the predator farming model
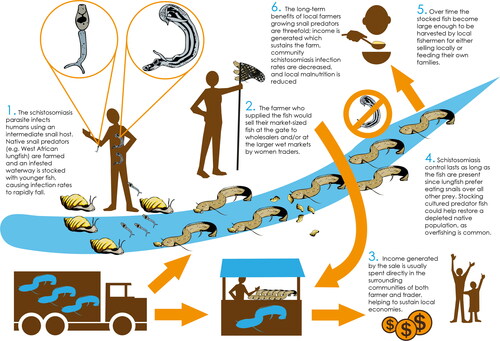
Farming of any species for the main purpose of schistosomiasis control must, like other aquaculture projects, generate a profit to the farmer to be considered sustainable (Arthur et al. Citation2013). Economic models exploring this method assume that farmers are responsible for growing relatively high-value native species to harvestable size either directly in water bodies containing infested snails (Hoover et al. Citation2020), or cohorts in ponds specifically built for aquaculture; these latter methods allow for more intensive farming, while a portion of each cohort is sold to the government before reaching harvest size so they can be deployed for snail predation in schistosomiasis-prone water bodies, bolstering depleted wild stocks or as new populations (Savaya-Alkalay et al. Citation2014). It is assumed these species could then be used by local community members to supplement their diets and/or incomes after this stage, ideally when the snail population has been reduced below their reproduction rate to such an extent that the water body is safe to use once more (Sokolow et al. Citation2015) (see for a graphical summary of this model). This model has had limited success during field trials in Senegal using M. vollenhovenii (see Sokolow et al. Citation2018), but requires further practical demonstrations. A summary of the two species’ relative strengths and weaknesses for use in this model is shown via .
Table 3. A comparison of the relative strengths and weaknesses between two potential candidates for snail predation in the predator farming model.
Snail predator candidates for farming and deployment: Macrobrachium vollenhovenii (giant african freshwater prawn)
In SSA many small commercial fisheries have existed for freshwater shrimp (Miller Citation1971). The most common of these fisheries in West Africa is the native giant freshwater prawn M. vollenhovenii, which grows to a total length of more than 15 cm and has been previously been considered for commercial aquaculture production in West Africa due to its relatively high market value and wide availability of wild post-larvae (PL) resulting in lower production costs (Willführ-Nast et al. Citation1993). It is the largest species in the African branch of the Palaemonidae prawn family, and has commanded a consistently high market value in many SSA countries (Miller Citation1971; Makombu et al. Citation2014). Multiple individual field studies on its ecology, biology and distribution have been conducted in several countries (Willführ-Nast et al. Citation1993; Etim and Sankare Citation1998; Nwosu and Wolfi Citation2009), and its optimal growth requirements for aquaculture have been documented (Makombu et al. Citation2014). Macrobrachium vollenhovenii ranges from the Senegal river in the north to Angola in the south (Savaya-Alkalay et al. Citation2014) and prefers well-oxygenated yet slow-moving streams, lakes, canals, upper estuaries and reservoirs, as well as rice paddies (Diakité et al. Citation2018). Despite their shared preference for similar habitat in permanent streams and reservoirs, M. vollenhovenii and Biomphalaria spp. are rarely found in the same location, possibly due to predation of the prawns upon the snails (Camara et al. Citation2009; Diakité et al. Citation2018). Like other catadromous Macrobrachium species the ovigerous (berried) females must migrate to mildly brackish estuarine waters to release their larvae, where after 13 further stages the post-larvae (PL) individuals migrate back upriver to spawn (Willführ-Nast et al. Citation1993; Savaya-Alkalay et al. Citation2014). The males are non-migratory, and simply feed until the next spawning opportunity (Savaya-Alkalay et al. Citation2020). This migration requirement is one of the factors that causes spikes in schistosomiasis upstream of new dams as the prawns are unable to reach the area and establish populations in the newly created water bodies (Sokolow et al. Citation2015; Hoover et al. Citation2020). In Côte d’Ivoire each population, usually found in river systems, is exploited by a local small-scale fishery (Konan et al. Citation2010). While many of these fisheries in other countries in SSA have been found to be overexploited (Nwosu and Wolfi Citation2009), there are no recent Ivorian fishery surveys for this species. Relative host snail abundance between depleted and healthy prawn fisheries has only been explored in Senegal after the installation of dams which block the catadromous movement of the females (Sokolow et al. Citation2017). Fishery recruitment peaks in the midsummer across the region in both estuaries and rivers, with one spawning cycle in the wild per year (Etim and Sankare Citation1998; Nwosu and Wolfi Citation2009). Morphological variations between individuals in regional M. vollenhovenii fisheries point toward the need for Ivorian stock subdivision when considering overall exploitation potential to avoid overfishing in certain regions of a preferred regional fishery (Konan et al. Citation2010). General morphological similarity (at various life stages) to another Macrobrachium species with an overlapping population, M. macrobrachion, further complicates accurate population estimations for these different fisheries (Konan et al. Citation2008).
Snail predator candidates for farming and deployment: freshwater prawn farming potential
Despite small-scale research conducted on the numerous Ivorian M. vollenhovenii fisheries (Bony et al. Citation2013) and a few studies examining the growth parameters required to culture these prawns in neighboring countries, as well as an interest in this prawn for aquaculture since the 1960s, little research has been done in Côte d’Ivoire on the farming of this species for food production (Miller Citation1971; Savaya-Alkalay et al. Citation2014). This could be due to the already advanced state of culturing techniques for the similar yet faster-growing M. rosenbergii, widely farmed in Asia (Makombu et al. Citation2014). Combined with a lack of domestic interest in culturing this species due to historically robust fisheries (Lawal-Are Citation2012) and roadblocks to international export, there has been little regional interest in farming this species until recently as local demand for such a high-value prawn has increased while fisheries begin to shrink (New et al. Citation2009). The book by New et al. (Citation2009) on freshwater prawn biology and farming gives the most comprehensive overview available on the aquaculture potential of this species. As described in this book and multiple studies, the potential for M. vollenhovenii to thrive in polyculture with tilapia and to reduce schistosomiasis risk in nearby communities is significant (Sokolow et al. Citation2015; Hoover et al. Citation2020), but the technical parameters for farming M. vollenhovenii for these purposes, including localized hatching, growth and deployment at scale, have yet to be completely studied (Zimmerman et al. Citation2010; Makombu et al. Citation2014). Numerous technical issues with production have been identified when compared to rearing M. rosenbergii; there is very little research conducted on fertilization and embryonic development (Sintondji et al. Citation2020); larval rearing problems include a long development time (∼50 days compared to ∼35 days for M. rosenbergii) and the requirement of enriched live feed for early post-larval (PL) stages (generally relatively expensive Artemia nauplii) (Tayamen and Brown Citation1999). Wild stocks of PL individuals can be collected for grow-out, and economic analysis of even extensively stocked systems show M. vollenhovenii to be practical for the predator farm model in Senegal (Hoover et al. Citation2020). The hatchery phase, however, is considered both a prerequisite for sustained commercial prawn culture and is recognized as the most technically demanding stage of the production cycle; optimizing water quality, health and nutrition via efficient hatchery technology adapted to local conditions is essential for M. vollenhovenii to be a commercial success (Zimmerman et al. Citation2010; Makombu et al. Citation2014). This makes the commercial farming of prawns in an emerging economy such as Côte d’Ivoire especially challenging, as the capture of wild PLs for even field research is often impractical; wild stocks of berried females are often found to be depleted (Nwosu and Wolfi Citation2009), making the restocking of a depleted hatchery or pond very difficult. Myriad issues with setting up a pioneering hatchery for aquaculture have resulted in mothballed and failed hatchery operations in neighboring SSA countries—these include the usual problems of access to effective feed, seed and financing, but also a lack of consistently high-water quality and dependable electricity, a high risk of equipment theft, a lack of quality first feeds and grow-out feed, and lack of trained locally-based hatchery managers (Bostock et al. Citation2010; FAO 2019b). Additional economic research on the farming conditions, market value and potential returns for this species in Côte d’Ivoire is also needed before M. vollenhovenii can be considered a viable candidate for the predator farm model.
Snail predator candidates for farming and deployment: Protopterus annectens (west african lungfish)
Protopterus annectens is one of only six ancient species of air-breathing lobe-finned fishes in Africa (4 species), South America (1), and Australia (1) with reduced gills in adults, allowing them to breathe via air or water (Chew et al. Citation2004; Molnar et al., Citation2012). In Africa, there is substantially more research cited in the literature concerning P. aethiopicus, although ecological work along with work on the lungfish genome (which is unusually large) and on the ability of African species to estivate often encompasses both species. The four species of African lungfishes occur naturally in a variety of freshwater ecosystems including swamps, rivers, and lakes across SSA (Greenwood Citation1986). Both P. aethiopicus and P. annectens are obligate air-breathers during adulthood (Babiker Citation1979; Greenwood Citation1986), although other research shows aquatic respiration is adopted when avoiding predators or swimming vertically (Mlewa et al. Citation2007). These lungfish are dioecious, but it is very difficult to tell the sexes apart by sight (Molnar et al., Citation2012). African lungfish can spawn multiple times per year (Mlewa and Green Citation2004), with females reaching maximum fecundity during the rainy season (Mosille and Mmnoya Citation1988; Walakira et al. Citation2016). Males will court multiple females throughout this season (Budgett Citation1901); they dig shallow mud burrows (<40 cm) by biting the mud and excreting it out of their gills openings, eventually turning around to make a teardrop shaped burrow (Budgett Citation1901). Once the burrow is complete, the females will lay her eggs in the burrow where the male will keep the eggs well-oxygenated by circulating water with body and tail movements (Mlewa et al. Citation2010). Little is known about the early life stages of African lungfishes (Greenwood Citation1986). A factsheet developed for Ugandan farmers by Walakira et al. (Citation2016) on establishing farming protocols for P. aethiopicus show eggs hatching into larval fish of 1.2 cm average length, becoming 1–2 g. fry after 21 days and 7–10 g fingerlings within six weeks. Wild longevity of this species is unknown, but captive lungfish have been known to live up to 20 years and grow over 2 meters in length (Genade et al. Citation2005). The African lungfishes are all omnivorous carnivores, feeding primarily on mollusks, crustaceans and insect larvae, with a marked preference for mollusks (Ballantyne and Frick Citation2016). The ability of P. aethiopicus to eat hundreds of vector snails (Bulinus spp.) per day in a laboratory setting, with accompanying tilapia fry almost untouched, was first noted by Mahdi and Amin (Citation1966) in Sudan, and this voraciousness was confirmed to be shared by P. annectens by Daffalla et al. (Citation1985), who also conducted limited yet successful field trials. The idea that P. annectens could be cultured for use as a noninvasive biocontrol agent against schistosomiasis was also mentioned by those authors, and strongly suggested again by Arostegui et al. (Citation2019) based upon species presence and dietary niches in schistosomiasis-endemic areas of Senegal. Concerns regarding destruction of earthen dams, the lungfish’s omnivorous feeding habits (Slootweg et al. Citation1994), deployment logistics, and uncertainties surrounding market demand in West Africa will need to be examined in modern field trials and research.
Snail predator candidates for farming and deployment: lungfish farming potential
This species’ ability to estivate for up to several years during a period of drought within a self-made cocoon of dried mucus, sometimes termed a ‘summer torpor’, enables people to remove them from their surroundings while still in their cocoon and store them indoors for later consumption (Fishman et al. Citation1992). Both of these attributes, along with their tolerance of a wide range of oxygen levels, temperatures, and salinities (Mlewa et al. Citation2010), show P. annectens can be classified as a ‘climate-resilient’ species with potential for filling nutritional deficits in Côte d’Ivoire. Masa et al. (Citation2011) showed that the muscle, liver and adipose tissue of P. aethiopicus are all rich sources of omega-3 fatty acids (Masa et al. Citation2011). Mohamed and Al-Sabahi (Citation2014) confirmed P. annectens could also be considered a semi-fatty fish, with varying concentrations of fat depending upon organ type (Mohamed and Al-Sabahi Citation2014). There has been no published work done on the explicit culturing of P. annectens, but the work that has been completed on farming other African lungfish species, including P. amphibious in Kenya (Baer et al. Citation1992) and P. aethiopicus in Uganda (Molnar et al., Citation2012; Walakira and Molnar Citation2014), could be used as guidance; the conditions found in tilapia hatcheries could be used in hatching P. annectens, and growing in polyculture with tilapia is possible, as long as the tilapia are graded to prevent piscivory by the lungfish (Molnar et al., Citation2012). Morphological, ecological, and behavioral similarities between the Protopterus species (Greenwood Citation1986; Otero Citation2011) indicates similar production methods could be effectively used for P. annectens as for P. aethiopicus. Capturing wild fingerlings is also possible, as lungfish do not burrow into the mud until they are sexually mature (Greenwood Citation1986). Grow-out in a larger tilapia farm would be a good strategy for these first cohorts, as Walakira and Molnar (Citation2014) experienced higher SGRs and final weights when raising them on similar feeding regimens but with higher-protein feeds, similar to other carnivorous fishes. This existing work on culturing a similar species, combined with the relative resilience of the species to changes in their environment, make P. annectens a potentially viable candidate for farming as a potential snail predator. Full-scale field trials are needed to answer problems surrounding omnivorous and especially piscivorous feeding habits in polyculture (Slootweg et al. Citation1994) and the potential for damage to earthen ponds via burrowing by sexually mature males. There is no evidence of existing large-scale consumer demand in Côte d’Ivoire for this species despite availability of local fisheries; this could be due to a variety of reasons, including cultural preferences, which cannot be known without further research into local markets and into the biocultural diversity of Côte d’Ivoire in the context of fishing, fish farming, and fish consumption.
Marketing and distributing farmed native snail predators in Côte d’Ivoire
Ivorian infrastructure and transport networks
Ivorian infrastructure and transport networks: general summary of evidence
Quality: Medium
Size: Small
Consistency: Consistent
There were relatively few peer-reviewed studies examining Ivorian infrastructure and general transportation networks in the context of aquaculture value chains, with contextual information from non-peer reviewed official websites being necessary to fill in gaps between papers. These websites include organizations such as the World Bank and the International Monetary Fund that do have national statistics on these topics for Côte d’Ivoire, but often these contain dated and/or overly generalized data. Reliable electrical grids and dense road networks of decent year-round quality between farms and markets are essential for the effective expansion and/or commercialization of fish farms, but very few papers captured in this review discussed this topic.
Ivorian infrastructure and transport networks: past and present infrastructure
During the 1990s Côte d’Ivoire made major strides with respect to infrastructure, resulting in broad coverage for electricity, sanitation, road networks and telephone lines. However, during the conflicts of the mid-2000s much ground was lost, and low investment in the years afterward caused these services to deteriorate. The use of private investment in public infrastructure (public-private partnerships [PPPs], unique to Côte d’Ivoire in SSA at that time) helped to assure commercial viability of these networks, which encouraged performance improvements and further investment, but funding for these partnerships dried up (Foster and Pushak Citation2010). After the political situation stabilized in 2011 PPPs resumed as the country’s growth rate increased, renovating airports, sea ports and the extensive but aging road and rail networks (Oxford Business Group Citation2018). Currently Côte d’Ivoire has generally good infrastructure compared to neighboring countries. The largest road network in West Africa and an extensive rail network connecting landlocked northern neighbors Burkina Faso and Mali to two major sea ports (Abidjan and San Pedro) (World Port Source Citation2020), along with three international airports (Abidjan, Yamoussoukro and Bouaké) and four additional regional paved airports (Airports Worldwide Citation2021) all enable the relatively smooth movement of goods (Oxford Business Group Citation2018). The condition of telecoms, electricity and water infrastructure is all above the African average, with the calculated amount of investment needed for maintaining these services relatively low, especially in the case of the electrical grid (Global Infrastructure Hub Citation2017).
Ivorian fish market structures
Ivorian fish market structures: general summary of evidence
Quality: Medium
Size: Very small
Consistency: Consistent
Almost no research regarding fish markets and overall aquaculture within Côte d’Ivoire was identified through the keyword search; these subjects were anticipated to be the primary areas in which new research could be useful. There were no contradictory conclusions made within the body of research that was identified, and subsequent correspondence with local researchers revealed only one additional paper on this topic (Yao et al. Citation2017) published in a journal not recognized by Scopus or Web of Science and now identified as a predatory journal as defined by Xia et al. (Citation2015). Unpublished maps of aquaculture across the country matched those that were published regarding national fish movements between market towns and neighboring countries.
Ivorian fish market structures: fish trade and movement across Côte d’Ivoire
Major, long-established fish markets exist in both the capital Yamoussoukro and in Abidjan, where the capture and processing of fish also occurs (Kouakou et al. Citation2013). The types of value-added fish product available in these markets are many and include fresh, frozen, smoked, dried, fermented and other value-added products (Kouakou et al. Citation2013; Shehu Jega et al. Citation2013; Fall et al. Citation2019). While local and regional markets can be large in scale, the majority of fish trading in West Africa is done informally, and mostly undertaken by women traders and other marginalized groups (Ayilu et al. 2016); these informal transactions are not well-documented and thus the true scale of fish markets in Côte d’Ivoire is not well understood or adequately supported by government. Perhaps consequently, there are very few studies on Ivorian fish markets or traders in the literature. One study does comprehensively examine the country’s fish market structures; Ayilu et al. (Citation2016) in cooperation with Worldfish attempted to document Ivorian informal markets and illustrate the flows of fish within and between three countries within West Africa, including Côte d’Ivoire. Using research and interviews with traders, fishermen and other stakeholders, the authors paint a detailed picture of how fish and seafood products move around and through this region (see for detailed map of these movements):
Figure 4. Movements of fish catch and imports within Côte d’Ivoire. Source: Worldfish, used with permission.
Abidjan, being a port city home to one of the biggest ports in West Africa, is where thousands of tons of imported frozen and processed (usually smoked) fish enter the regional distribution network via middlemen and retailers. While frozen fish products enter the country formally, the movements within the country and across the borders is completely informal. The decent Ivorian road network allows fairly long cold chains which transport frozen fresh fish as far west as Man city and as far north as Korhogo city, with some traveling still further to be processed in nearby Ferkessédougou. Most African imports come from Mauritania to Abidjan by sea, but some processed imports enter through Korogho city via land from Mali and Senegal, to often be reexported onward to Burkina Faso (by road) and Ghana (by canoe via Bouna city). The top ten most frequently traded fish in the region include shad (smoked), Sardinella (smoked), anchovy (dried and smoked), Atlantic bumper (smoked and dried), chub mackerel (smoked), pink shrimp (smoked), deepwater rose shrimp (smoked), black-chinned tilapia (salt dried, smoked), catfish (C. gariepinus) (smoked), and African moonfish (dried and smoked). (From Ayilu et al. (Citation2016) reproduced with permission from Worldfish)
During these interviews, traders described the various barriers and advantages they encounter; having a primary or basic education helped with their trade, as simple arithmetic is essential for this occupation. Shorter distances between purchase points and destinations enhanced cross-border trade, as did membership in a fish traders’ association as members can pool their knowledge of foreign markets and price fluctuations, as well as gain access to credit facilities and assistance during times of illness or economic hardship. Prohibitive taxes and high numbers of bribes, transport costs, export/import formalities, ignorance of regional trade regulations and harassment at border checkpoints, and inefficient/inadequate fish processing are all seen as barriers to effective fish trading. The problems with fish processing, coupled with poor handling practices during transport and display at market, can endanger the public who are often unaware or ignorant of which practices are essential for food safety in the marketplace (Chimatiro Citation1998; Essien et al. Citation2006).
Ivorian aquaculture practices
Ivorian aquaculture practices: general summary of evidence
Quality: Medium
Size: Very small
Consistency: Consistent
Most literature discussing Ivorian fish farms is either dated (i.e. from before the 2002 civil war) or focused on the demographics of the farmers themselves rather than on their farming practices. Some recent work has also been done on feed quality and nutrition within Ivorian fish farms, but most of this research has been conducted by a only single regional research institute (Centre de Recherches Océanologiques) whose work is funded by the Ivorian government and NGOs. Similarly, one article on the topic was published in an open-access journals that is not recognized by curated databases as meeting the quality standards for inclusion—the ‘predatory journal’ trap often encountered by younger researchers in non-English-majority developing countries may be responsible for this outcome (Xia et al. Citation2015).
Ivorian aquaculture practices: Ivorian fish farms and wet markets
Côte d’Ivoire farms relatively few fish compared to its neighbors, with 3750t harvested in 2015. Ghana and Nigeria grew roughly 10× and 110× that amount, respectively, that same year (FAO 2016). Statistics on current production are uncertain due to the ongoing pandemic, but it is estimated that output has not significantly changed in the past few years, with production levels hovering around 4500t since 2016 (FAO 2019b). There are few studies in the literature regarding Ivorian fish farms but Yao et al. (Citation2017) conducted extensive surveys (via personal interview) of 301 farmers across fifteen fish production regions in Côte d’Ivoire in 2013, and wrote two comprehensive articles on their findings (Yao et al. Citation2016, Citation2017). These are the only reports on these farmers that took place after the end of the most recent civil war, an event that drastically affected aquaculture production and farm data collection (FAO 2019b). Yao et al. (Citation2017) describe most of the farmers as having 5–10 years of experience, are male, are 40–60 years old, and use mostly extensive or semi-intensive earthen pond aquaculture methods to raise mainly Nile tilapia (Oreochromis niloticus), catfish (Clarias gariepinus, Heteroclarias spp.), bonytongue (Heterotis niloticus), bagrid catfish (Chrysichthys nigrodigitatus,), with occasionally snakehead (Parachanna africana) grown in polyculture with the tilapia. Feeds were generally agro-industrial by-products, with only intensive and semi-intensive farmers utilizing commercial feeds, either imported or formulated in-country. These local formulations were often of low protein content and nutritional quality, with elevated levels of anti-nutrients and fiber. The few farmers who operated intensive or semi-intensive farms were local to the region where their farm was situated, while most of the extensive rice-fish system farmers were migrants from other regions. There are very few farmers using intensive systems, and a majority of semi-intensive and extensive systems are run as secondary to the primary agricultural work of farming cocoa, coffee, palm oil, rubber, and other crops; it is thought that this keeps production levels from benefiting from improvements to efficiency and investment that farmers who primarily farm fish would put into their systems. The aquaculture sector in Côte d’Ivoire is beset by most of the usual problems affecting African fish farms in many other countries; lack of access to high quality feed and fingerlings, persistent water contamination from pesticide residues (Roche and Tidou Citation2009), limited access to monosexing technology or credit facilities, a lack of national feed mills and commercial hatcheries, and a need for extension services to educate farmers about methods of intensification and efficiency (Yao et al. Citation2017). The traditional roots, informal nature and uncertain contemporary regulations surrounding land tenancy and ownership is also a major barrier to commercial expansion for some farmers, particularly in what was formerly called the Pioneer Frontier (Montagnes and Moyen-Cavally regions in Southwestern Côte d’Ivoire) according to Babo and Droz (Citation2008): Policies established since independence encouraged migrants from outside these regions (and eventually even outside the country) to colonize land in mainly forested areas through the 1990s. These were areas where multiple tribes considered themselves indigenous, but practiced little agriculture; therefore, their forested lands were considered ripe for exploitation. Cultivation rights were informally purchased through ritual and gifts in a long-cherished system called “tutoring,” with very few exchanges being registered in any documented manner. After the economic crises starting in the 1980s, this lack of official records combined with increasing xenophobia and the increasing value of land for growing cash crops caused questions regarding who is considered most “native Ivorian” to become more critical than documentation when disputes over land rights were being settled. Regional major fish farming countries (especially Senegal, Ghana and Nigeria) have had over 15 years of relatively stable economies during which their technological knowledge and investment made in aquaculture has paid off with much higher comparative yields; with continuing political stability and both public and private investment in their sector, Ivorian fish farmers may yet see their production rates rival their neighbors (Babo and Droz Citation2008; Fallier et al. Citation2014).
Locally farmed fish products are currently considered luxury items in Côte d’Ivoire likely due to relatively high production costs making them expensive compared to frozen imports or smoked wild-caught species, but consumer preferences irrespective of relative cost are unknown. Farmed fish are mostly sold fresh and whole directly from the farm gate or from nearby markets due to a lack of reliable cold transport chains in most of the country (Yao et al. Citation2017). Producing an aquaculture product at a low enough price point for local Ivorians to consume will likely be a long-term goal, especially with a species newly introduced to the market. There are many ways this price point could be met through concerted effort, from increased farming efficiency, marketing campaigns leading to increasing adoption by consumers and farmers, advertising and sponsorship, and renewed interest and investment by the government (Ridoutt et al. Citation2019).
Discussion
The broad scope and interdisciplinary nature of this systematic review necessitated the sorting of the retrieved literature into three main themes, the first of which examined the neglected disease of poverty which is schistosomiasis. The second theme explored the strategy of using biological control to combat the spread of schistosomiasis during the era of increasing climate change by focusing on the specific strategy of farming two separate molluscivorous species. The third and final theme examined the application of this strategy to combat disease, poverty, and economic insecurity in the West African nation of Côte d’Ivoire. Each theme was explored in detail using thematic clusters to organize the individual articles into related groups, with the quality, size, and consistency of evidence from each cluster graded and summarized. This systematic review was conducted to determine, based purely upon the literature, whether two different freshwater species native to Côte d’Ivoire could be grown in Ivorian commercial aquaculture to be deployed as long-term biological vector snail control and/or bolster native populations, with the ultimate goal being to reduce the burden both of schistosomiasis disease and of poverty on vulnerable Ivorian communities during a time of climate change. The literature does indicate that this use of snail predator farming would be a practicable, climate-resilient method for the sustained control of schistosomiasis in Côte d’Ivoire, but with some caveats to this strategy which need clarification through physical trials and primary research.
The predator farming model does embody both One Health and pro-poor principles, as it uses collective knowledge and coordination between multiple disciplines (aquaculture, epidemiology, etc.) to find a general solution for effectively combatting such a widespread and persistent disease of poverty. But the caveats to this idea are substantial, and mainly relate to gaps in the literature and the practicalities in areas where the strategy is used, while ensuring it is paired with other schistosomiasis control measures such as chemical MDA and/or water infrastructure improvements. Between the two species evaluated, for example, the farming of M. vollenhovenii for this purpose will be quicker to set up in the shorter term due to an established market for large prawns, availability of wild PLs, and extensive knowledgebase on freshwater prawn farming compared to P. annectens. However, there is some evidence this species does not naturally share the same habitat as vector snails (Diakité et al. 2018), while the evidence for the relative ecological resilience, nutritional content, size, and potential ease of farming P. annectens is substantial and worth further investigation. The lack of a large, established market for lungfish in Côte d’Ivoire, no domestication effort, and little knowledge about farming even similar species relative to those already farmed in-country makes lungfish a “risky” candidate in the shorter term, however, even if the farming potential and nutritional impact of this resilient species is relatively high.
Other freshwater species already farmed and sold in Côte d’Ivoire that also display a degree of molluscivorous behavior, including Heterotis niloticus (African arowana), sterile monosex varieties of M. rosenbergii, and the non-native Ctenopharyngodon idella (grass carp) (see Arostegui et al. Citation2019), also warrant further investigation. Research conducted by the German NGO Bernhard Nocht Institute for Tropical Medicine (BNITM) on the host snail predation potential of H. niloticus began in Madagascar in April 2021, and so these investigations are already beginning (2021 May 3rd email with N. Jouanard).
This review revealed gaps in the literature surrounding (a) the effect climate change will have on Ivorian nutritional poverty, (b) the existing population levels of both P. annectens and M. vollenhovenii in Côte d’Ivoire, (c) the effectiveness of P. annectens host snail predation in Ivorian field conditions, (d) the possibility of growing out either wild-caught M. vollenhovenii or P. annectens in existing Ivorian semi-intensive/intensive aquaculture systems, (e) the Ivorian fish markets’ openness to farmed P. annectens, and (f) which deployment strategy should be used to reduce endemic schistosomiasis in the country, either pioneering/increasing natural populations or being deployed to specific sites. Primary data collection via survey in Ivorian fish markets and studies that explore the biocultural diversity of Côte d’Ivoire can help cement a more complete foundation of knowledge. The eventual deployment of field trials at select local farms should build upon this foundation and address many of the more practical unknowns. These farms would be best located in regions where schistosomiasis endemicity is high, hybridization between Schistosoma species has been documented, and where cattle, ungulates and/or other nonhuman mammals are likely to share the infested water. Preventing the potential infection of the wider reservoir of both human and non-human hosts that could be infected by hybrids would be very useful. Once the knowledge gaps begin to close and the farm trials start producing cohorts of snail predators, they will quickly lead to an integrated approach in agri-food research as described by Horton et al. (Citation2017) as the focus of this work shifts from effective implementation of the farming model to achieving sustainability of the model through commercial activity. Such an integrated strategy is vital, as climate change is projected to have an overall spreading effect on famine and the epidemiology of schistosomiasis in sub-Saharan Africa. Increasingly variable precipitation and weather patterns, combined with the higher temperatures already being experienced by millions of people in endemic regions across the continent, are affecting food supplies and already encouraging people to migrate in search of better, more stable living conditions; the disease can travel with them (McCreesh et al. Citation2015). Forced away from the coastlines by rising sea levels, slower, more frequent storms and increased instances of flooding, fishing communities who have already suffered from rapidly declining stocks will require food of at least the same quantity and quality to avoid further hardship, and sustainable economic opportunities for themselves and their children. Aquaculture is already producing over 50% of all fish consumed globally (FAO 2018), and farmed fish is a relatively rich source of dietary protein, fats and minerals grown more efficiently and with fewer carbon emissions compared to terrestrial animals (Norman et al. Citation2019). Integrating commercial-scale fish farming with schistosomiasis control can therefore lead to reductions in poverty, disease endemicity, and vulnerability to a changing climate, but only if such a win-win-win strategy is properly implemented.
Regardless of how we get there, this review does show a need for resilient, sustainable methods of significant long-term control of host snail populations amidst increasingly fluctuating climate factors, low financial resources, food poverty/insecurity and global NGOs’ single-minded focus on only repeated chemotherapy for schistosomiasis control. The use of aquaculture to achieve this goal seems to be a strategy that is worth pursuing.
Acknowledgements
The authors would like to thank Worldfish for use of their content and graphics, P. Nelson for assistance with artwork, and the entire Belmont Forum Schistosomiasis and Climate Change team.
Disclosure statement
The authors hereby declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. 2015. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis.19(2):196–205. doi:10.1016/j.bjid.2014.11.004
- Aiga H, Matsuoka S, Kuroiwa C, Yamamoto S. 2009. Malnutrition among children in rural Malawian fish-farming households. Trans R Soc Trop Med Hyg. 103(8):827–833.
- Airports Worldwide. 2021. Ivory coast airports. Lviv, Ukraine: Airports Worldwide [accessed 2021 February 3]. http://www.airports-worldwide.com.
- Akande IS, Odetola AA, Samuel TA. 2010. Profile of metabolite and biomolecule concentrations in the hemolymph of Bulinus globosus and Bulinus rohlfsi under aestivation and starvation. Nigerian Journal of Parasitology 31:60–67.
- Allan F, Ame SM, Tian-Bi Y-NT, Hofkin BV, Webster BL, Diakité NR, N’Goran EK, Kabole F, Khamis IS, Gouvras AN, et al. 2020. Snail-related contributions from the schistosomiasisc consortium for operational research and evaluation program including xenomonitoring, focal mollusciciding, biological control, and modeling. Am J Trop Med Hyg.103(1_Suppl):66–79. doi:10.4269/ajtmh.19-0831
- Alles M, Eussen S, Ake-Tano O, Diouf S, Tanya A, Lakati A, Oduwole A, Mauras C. 2013. Situational analysis and expert evaluation of the nutrition and health status of infants and young children in five countries in sub-Saharan Africa. Food Nutr Bull. 34(3):287–298.
- Amberson JM, Schwarz E. 1953. On African schistosomiasis. Trans R Soc Trop Med Hyg. 47(6):451–502.
- Andriantsoa R, Tönges S, Panteleit J, Theissinger K, Carneiro VC, Rasamy J, Lyko F. 2019. Ecological plasticity and commercial impact of invasive marbled crayfish populations in Madagascar. BMC Ecol. 19(1):1–10. doi:10.1186/s12898-019-0224-1
- Anto F, Bosompem K, Kpikpi J, Adjuik M, Edoh D. 2005. Experimental control of Biomphalaria pfeifferi, the intermediate host of Schistosoma mansoni, by the ampullariid snail Lanistes varicus. Ann Trop Med Parasitol. 99(2):203–209.
- Arostegui MC, Wood CL, Jones IJ, Chamberlin AJ, Jouanard N, Faye DS, Kuris AM, Riveau G, De Leo GA, Sokolow SH. 2019. Potential biological control of schistosomiasis by fishes in the lower Senegal river basin. Am J Trop Med Hyg. 100(1):117–126. doi:10.4269/ajtmh.18-0469
- Arthur R, Béné C, Leschen W, Little D. 2013. Fisheries and aquaculture and their potential roles in development: an assessment of the current evidence. Institute of Development Studies/DFID, June: 1–92.
- Assaré RK, Tian-Bi YNT, Yao PK, N’Guessan NA, Ouattara M, Yapi A, Coulibaly JT, Meïté A, Hürlimann E, Knopp S, et al. 2016. Sustaining control of schistosomiasis mansoni in western Côte d’Ivoire: results from a SCORE study, one year after initial praziquantel administration. PLoS Negl Trop Dis. 10(1):e0004329. doi:10.1371/journal.pntd.0004329
- Ayilu RK, Antwi-Asare TO, Anoh P, Tall A, Aboya N, Chimatiro S, Dedi S. 2016. Informal artisanal fish trade in West Africa: improving cross-border trade; Penang, Malaysia: Worldfish. Program Brief: 2016-37.
- Baarsch F, Schaeffer M. 2019. Western Africa – the Sahel and the coastal countries. In: Smith E, editor. Climate change impacts on Africa’s economic growth. Abidjan: African Development Bank. p. 55–67.
- Babiker MM. 1979. Respiratory behaviour, oxygen consumption and relative dependence on aerial respiration in the African lungfish (Protopterus annectens, Owen) and an air-breathing teleost (Clarias lazera). Hydrobiologia. 65(2):177–187. doi:10.1007/BF00017423
- Babo A, Droz Y. 2008. Land tenure conflicts: from moral ethnicity to the imagination of nation: interethnic relationships and "ivority" in South-West Ivory Coast. etudes africaines. 48(192):741–763. doi:10.4000/etudesafricaines.15489
- Baer S, Haller RD, Freyvogel TA, Freyogel TA. 1992. Growth of the African lungfish, Protopterus amphibious Peter, in aquaculture. Aquac Res. 23(2):265–267. doi:10.1111/j.1365-2109.1992.tb00617.x
- Ballantyne JS, Frick NT. 2016. The biology of lungfishes. Boca Raton (FL): CRC Press.
- Barbosa L, Caldeira RL, Carvalho OS, Vidigal THDA, Jannotti-Passos LK, Coelho PMZ. 2006. Resistance to Schistosoma mansoni by transplantation of APO Biomphalaria tenagophila. Parasite Immunol. 28(5):209–212.
- Becker L, Yoboue N. 2009. Rice producer-processor networks in Côte d’Ivoire. Geogr Rev. 99(2):164–185. doi:10.1111/j.1931-0846.2009.tb00425.x
- Boelee E, Yohannes M, Poda JN, McCartney M, Cecchi P, Kibret S, Hagos F, Laamrani H. 2013. Options for water storage and rainwater harvesting to improve health and resilience against climate change in Africa. Reg Environ Change. 13(3):509–519. doi:10.1007/s10113-012-0287-4
- Boko ANN, Cisse G, Kone B, Dedy SF. 2016. Local beliefs and strategies of adaptation to climate change in Korhogo (Ivory Coast). Tropicultura. 34:40–46.
- Bonate PL, Wang T, Passier P, Bagchus W, Burt H, Lüpfert C, Abla N, Kovac J, Keiser J. 2018. Extrapolation of praziquantel pharmacokinetics to a pediatric population: a cautionary tale. J Pharmacokinet Pharmacodyn. 45(5):747–762.
- Bony YK, Edia OE, Konan FK, Ouattra A. 2013. Spatial distribution pattern of freshwater mollusks in Mé, Agnéby and Banco basins (Ivory Coast; West Africa). Bull Environ Pharmacol Life Sci. 2:146–151.
- Bostock J, McAndrew B, Richards R, Jauncey K, Telfer T, Lorenzen K, Little D, Ross L, Handisyde N, Gatward I, et al. 2010. Aquaculture: global status and trends. Philos Trans R Soc Lond B Biol Sci. 365(1554):2897–2912.
- Bouët A, Laborde D, Traoré F. 2018. The European Union–West Africa economic partnership agreement: small impact and new questions. J Int Trade Econ Dev. 27(1):25–53. doi:10.1080/09638199.2017.1337803
- Brooker S, Whawell S, Kabatereine NB, Fenwick A, Anderson RM. 2004. Evaluating the epidemiological impact of national control programmes for helminths. Trends Parasitol. 20(11):537–545. doi:10.1016/j.pt.2004.08.012
- Budgett JS. 1901. On the breeding-habits of some West-African fishes, with an account of the external features in development of Protopterus annectens, and a description of the larva of Polypterus lapradei. Trans Zool Soc London. 16(2):115–136. doi:10.1111/j.1096-3642.1901.tb00028.x
- Camara IA, Konan MK, Diomandé D, Edia EO, Gourène G. 2009. Ecology and diversity of freshwater shrimps in Banco National Park, Côte d’Ivoire (Banco River Basin). Knowl Manag Aquat Ecosyst. 5:1–10.
- Centers for Disease Control and Prevention. 2019. Zoonotic diseases. Atlanta: Centers for Disease Control; [accessed 2019 August 25]. https://www.cdc.gov/onehealth/basics/zoonotic-diseases.html.
- Centers for Disease Control and Prevention. 2020. One health | CDC. In: Topics. Atlanta: Centers for Disease Control; [accessed 2020 June 1]. https://www.cdc.gov/onehealth/index.html.
- Chew SF, Chan NKY, Loong AM, Hiong KC, Tam WL, Ip YK. 2004. Nitrogen metabolism in the African lungfish (Protopterus dolloi) aestivating in a mucus cocoon on land. J Exp Biol. 207(5):777–786. doi:10.1242/jeb.00813
- Chimatiro SK. 1998. Aquaculture production and potential for food safety hazards in sub-Saharan Africa: with special reference to Malawi. Int J Food Sci Tech. 33(2):169–176. doi:10.1046/j.1365-2621.1998.3320169.x
- Colley DG, Andros TS, Campbell CH.Jr. 2017. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty. 6(1):63. doi:10.1186/s40249-017-0275-5
- Colombe S, Corstjens PLAM, de Dood CJ, Miyaye D, Magawa RG, Mngara J, Kalluvya SE, van Lieshout L, van Dam GJ, Downs JA. 2018. HIV-1 viral loads are not elevated in individuals co-infected with Schistosoma spp. After adjustment for duration of HIV-1 infection. Front Immunol. 9:1–10. doi:10.3389/fimmu.2018.02005
- Coulibaly N, Coulibaly TJH, Mpakama Z, Savané I. 2018. The impact of climate change on water resource availability in a trans-boundary Basin in West Africa: the case of Sassandra. Hydrology. 5(1):12–13. doi:10.3390/hydrology5010012
- Coulibaly JT, Panic G, Silué KD, Kovač J, Hattendorf J, Keiser J. 2017. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob Health. 5(7):e688–698. doi:10.1016/S2214-109X(17)30187-0
- Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G. 2015. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: a review of research progress over the last decade. Fish Shellfish Immunol. 46(1):5–16. doi:10.1016/j.fsi.2015.01.036
- Cowie RH. 2001. Can snails ever be effective and safe biocontrol agents? Int J Pest Manag. 47(1):23–40. doi:10.1080/09670870150215577
- Czajka L. 2017. Income inequality in Côte d’Ivoire: 1985–2014. World Inequality Lab, Paris School of Economics, Paris, 1–45.
- Dabalen AL, Paul S. 2014. Effect of conflict on dietary diversity: evidence from Côte d’Ivoire. World Dev. 58:143–158. doi:10.1016/j.worlddev.2014.01.010
- Daffalla AA, Elias EE, Amin MA. 1985. The lungfish Protopterus annectans (Owen) as a biocontrol agent against schistosome vector snails. J Trop Med Hyg 88:131–134.
- de Kock KN, Joubert PH. 1991. Life-table experiments with Helisoma duryi (Wetherby) and Biomphalaria pfeifferi (Krauss) at constant temperatures. South Afr J Zool. 26(4):149–152. doi:10.1080/02541858.1991.11448243
- Diakité NR, N’Zi KG, Ouattara M, Coulibaly JT, Saric J, Yao PK, Hattendorf J, Utzinger J, N’Goran EK. 2018. Association of riverine prawns and intermediate host snails and correlation with human schistosomiasis in two river systems in south-eastern Côte d’Ivoire. Parasitology. 145(13):1792–1800. doi:10.1017/S003118201800135X
- Diakité NR, Winkler MS, Coulibaly JT, Guindo-Coulibaly N, Utzinger J, N’Goran EK. 2017. Dynamics of freshwater snails and Schistosoma infection prevalence in schoolchildren during the construction and operation of a multipurpose dam in central Côte d’Ivoire. Infect Dis Poverty. 6(1):1–19. doi:10.1186/s40249-017-0305-3
- Diba I, Camara M, Diedhiou A. 2019. Investigating West African monsoon features in warm years using the regional climate model RegCM4. Atmosphere. 10(1):16.
- Dixon RK, Smith J, Guill S. 2003. Life on the edge: vulnerability and adaptation of African ecosystems to global climate change. Mitig Adapt Strateg Glob Chang. 8(2):93–113. doi:10.1023/A:1026001626076
- Elsevier. 2019. Scopus factsheet. Amsterdam: Scopus; [accessed 2020 June 2]. https://www.elsevier.com/research-intelligence/resource-library/scopus-fact-sheet.
- Esri. 2019. Global dams and reservoirs. West Redlands (CA): Esri; [accessed 2019 May 12]. https://hub.arcgis.com/maps/ef769752083b46dd8444f0d1b77defa9/explore.
- Essien JP, Ekwu A, Ekpo MA. 2006. Microflora dynamics and organoleptic properties of dried shrimps during storage in a Nigerian market centre. Pollut. Res. 25:209–212.
- Etim L, Sankare Y. 1998. Growth and mortality, recruitment and yield of the fresh-water shrimp, Macrobrachium vollenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fahe reservoir, Cote d’Ivoire, West Africa. Fish Res. 38(3):211–223. doi:10.1016/S0165-7836(98)00161-1
- Ezeh OK, Ogbo FA, Stevens GJ, Tannous WK, Uchechukwu OL, Ghimire PR, Agho KE, Global Maternal and Child Health Research Collaboration (GloMACH). 2019. Factors associated with the early initiation of breastfeeding in Economic Community of West African States (ECOWAS). Nutrients. 11(11):2765–2716. doi:10.3390/nu11112765
- Fall M, Diop MB, Montet D, Maiga AS, Guiro AT. 2019. Fermentation of fish in West Africa and societal challenges for qualitative improvement of the products (adjuevan, guedj and lanhouin): a review. Cah. Agric. 28:1–12.
- Fallier P, El Ayoubi H, Konan A. 2014. Industrie des pêches et de l’aquaculture en Côte d’Ivoire. Report no. 7, Conférence Ministérielle sur la Coopération Halieutique entre les États Africains Riverains de l’Océan Atlantique, Abidjan. pp. 1–99.
- Famakinde DO. 2018. Treading the path towards genetic control of snail resistance to schistosome infection. Trop. Med. 3(3):86–15. doi:10.3390/tropicalmed3030086
- Fenwick A, Rollinson D, Southgate V. 2006. Implementation of human schistosomiasis control: challenges and prospects. Adv Parasitol. 61:567–622.
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements ACA, et al. 2009. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 136(13):1719–1730. doi:10.1017/S0031182009990400
- Fishman AAP, Galante RJ, Winokur A, Pack AI. 1992. Estivation in the African lungfish. Proc Am Philos Soc. 136:61–72.
- Food and Agricultural Organization of the United Nations. 2016. The Republic of Ghana. In: United Nations, fishery and aquaculture country profiles. Rome: Food and Agricultural Organization of the United Nations; [accessed 2020 Jan 5]. https://www.fao.org/fishery/facp/GHA/en.
- Food and Agricultural Organization of the United Nations. 2019a. Challenges to coastal fisheries communities in Abidjan, Côte d’Ivoire. Food and Agriculture Organization of the United Nations; [accessed 2020 Feb 17]. https://www.fao.org/blogs/blue-growth-blog/challenges-to-coastal-fisheries-communities-in-abidjan-cote-divoire/en/.
- Food and Agricultural Organization of the United Nations. 2019b. Côte d’Ivoire. National Aquaculture Sector Overview; [accessed 2020 Feb 18]. https://www.fao.org/fishery/countrysector/naso_cotedivoire/en.
- Foster V, Pushak N. 2010. Côte d’Ivoire’s infrastructure: a continental perspective. In: World Bank. Africa’s infrastructure: a time for transformation. Washington, DC: The World Bank.
- Gan TY, Ito M, Hülsmann S, Qin X, Lu XX, Liong SY, Rutschman P, Disse M, Koivusalo H. 2016. Possible climate change/variability and human impacts, vulnerability of drought-prone regions, water resources and capacity building for Africa. Hydrol Sci J. 61:1209–1226.
- Gashaw F, Erko B, Teklehaymanot T, Habtesellasie R. 2008. Assessment of the potential of competitor snails and African catfish (Clarias gariepinus) as biocontrol agents against snail hosts transmitting schistosomiasis. Trans R Soc Trop Med Hyg. 102(8):774–779.
- Genade T, Benedetti M, Terzibasi E, Roncaglia P, Valenzano DR, Cattaneo A, Cellerino A. 2005. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell. 4(5):223–233.
- Glinz D, Wegmüller R, Ouattara M, Diakité VG, Aaron GJ, Hofer L, Zimmermann MB, Adiossan LG, Utzinger J, N’Goran EK, et al. 2017. Iron fortified complementary foods containing a mixture of sodium iron EDTA with either ferrous fumarate or ferric pyrophosphate reduce iron deficiency anemia in 12- to 36-month-old children in a malaria endemic setting: a secondary analysis of a cluster. Nutrients. 9 (7):759–715. doi:10.3390/nu9070759
- Global Infrastructure Hub. 2017. Cote d’Ivoire. Country profile. Sydney NSW: Oxford Economics; [accessed 2020 July 19]. https://infracompass.gihub.org/ind_country_profile/civ/.
- Global Nutrition Report. 2020. Inequalities in the global burden of malnutrition. 2020 Global Nutrition Report: action on equity to end malnutrition. Bristol: Development Initiatives Poverty Research.
- Goudswaard K, Witte F. 1997. The catfish fauna of Lake Victoria after the Nile perch upsurge. Environ Biol Fishes. 49:21–43.
- Goula BTA, Kouassi FW, Fadika V, Kouakou KE, Kouadio GB, Koffi K, Bamory K, Inza D, Savane I. 2009. Impact of climate change and variability on groundwater resources in humid tropical areas: a case study from the Ivory Coast. IAHS. 334:190–202.
- Greenwood PH. 1986. The natural history of African lungfishes. J Morphol. 190(S1):163–179. doi:10.1002/jmor.1051900412
- Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. 2015. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 156:1–16.
- Gusenbauer M, Haddaway NR. 2020. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res Synth Methods. 11(2):181–217. doi:10.1002/jrsm.1378
- Hofkin BV, Koech DK, Oumaj J, Loker ES. 1991. The North American crayfish Procambarus clarkii and the biological control of schistosome-transmitting snails in Kenya: laboratory and field investigations. Biol Control. 1(3):183–187. doi:10.1016/1049-9644(91)90065-8
- Hoover CM, Sokolow SH, Kemp J, Sanchirico JN, Lund AJ, Jones IJ, Higginson T, Riveau G, Savaya A, Coyle S, et al. 2020. Modelled effects of prawn aquaculture on poverty alleviation and schistosomiasis control. Nat Sustain. 2(7):611–620.
- Horton P, Banwart SA, Brockington D, Brown GW, Bruce R, Cameron D, Holdsworth M, Lenny Koh SC, Ton J, Jackson P. 2017. An agenda for integrated system-wide interdisciplinary agri-food research. Food Sec. 9(2):195–210. doi:10.1007/s12571-017-0648-4
- Hotez PJ, Fenwick A. 2009. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 3(9):e485–3. doi:10.1371/journal.pntd.0000485
- Hunter JM. 2003. Inherited burden of disease: agricultural dams and the persistence of bloody urine (Schistosomiasis hematobium) in the upper east region of Ghana, 1959–1997. Soc Sci Med. 56(2):219–234. doi:10.1016/S0277-9536(02)00021-7
- Issaka AI, Agho KE, Page AN, Burns PL, Stevens GJ, Dibley MJ. 2015. Factors associated with early introduction of formula and/or solid, semi-solid or soft foods in seven francophone West African countries. Nutrients. 7(2):948–969. doi:10.3390/nu7020948
- Jamu D, Kaunda K, Eiríksson G. 2002. Mitigation of Food Insecurity and Poverty: the role of Integrated Agriculture Aquaculture (IAA) Systems in Malawi. Aquaculture and Fisheries Technical Report. Bunda College of Agriculture, Lilongwe.
- Kalinda C, Chimbari MJ, Grant WE, Wang HH, Odhiambo JN, Mukaratirwa S. 2018. Simulation of population dynamics of Bulinus globosus: effects of environmental temperature on production of Schistosoma haematobium cercariae. PLoS Negl Trop Dis. 12(8):e0006651–15. doi:10.1371/journal.pntd.0006651
- Kalinda C, Chimbari M, Mukaratirwa S. 2017. Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: a systematic review. IJERPH. 14(1):80–18. doi:10.3390/ijerph14010080
- King CH, Bertino AM. 2008. Asymmetries of poverty: why global burden of disease valuations underestimate the burden of neglected tropical diseases. PLoS Negl Trop Dis. 2(3):e209–10. doi:10.1371/journal.pntd.0000209
- Kloos H, De Souza C, Gazzinelli A, Soares Filho BS, Da Costa Temba P, Bethony J, Page K, Grzywacz C, Lewis F, Minchella D, et al. 2001. The distribution of Biomphalaria spp. in different habitats in relation to physical, biological, water contact and cognitive factors in a rural area in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 96(suppl):57–66. doi:10.1590/S0074-02762001000900008
- Knopp S, Person B, Ame SM, Ali SM, Hattendorf J, Juma S, Muhsin J, Khamis IS, Mohammed KA, Utzinger J, et al. 2019. Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: a cluster-randomised trial. Lancet Glob Health. 7(8):e1118–1129. doi:10.1016/S2214-109X(19)30189-5
- Konan KM, Adépo-Gourène AB, Ouattara A, Nyingy WD, Gourène G. 2010. Morphometric variation among male populations of freshwater shrimp Macrobrachium vollenhovenii Herklots, 1851 from Côte d’Ivoire rivers. Fish Res. 103(1–3):1–8. doi:10.1016/j.fishres.2010.01.005
- Konan MK, Allassane O, Beatrice AGA, Germain G. 2008. Morphometric differentiation between two sympatric Macrobrachium Bates, 1868 shrimps (Crustacea: Decapoda: Palaemonidae) in West-African rivers. J Nat Hist. 42(31–32):2095–2115. doi:10.1080/00222930802254730
- Kouakou AC, Kouadio FN, Dadie AT, Montet D, Djè MK. 2013. Production and marketing of adjuevan, a fermented fish from Côte d’Ivoire. Cah Agric. 22:559–567.
- Kouame YM, Obahoundje S, Diedhiou A, François B, Amoussou E, Anquetin S, Didi RS, Kouassi LK, Bi VHN, Soro EG, et al. 2019. Climate, land use and land cover changes in the Bandama Basin (Côte D’Ivoire, West Africa) and incidences on hydropower production of the Kossou Dam. Land. 8(7):103–121. doi:10.3390/land8070103
- Kulinkina AV, Walz Y, Liss A, Kosinski KC, Biritwum NK, Naumova EN. 2016. Combining remotely sensed environmental characteristics with social and behavioral conditions that affect surface water use in spatiotemporal modelling of schistosomiasis in Ghana. Int Arch Photogramm Remote Sens Spat Inf Sci. 41:203–208.
- Lai Y-SS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, Mwinzi P, N’Goran EK, Raso G, Assaré RK, et al. 2015. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 15(8):927–940. doi:10.1016/S1473-3099(15)00066-3
- Lam VWY, Cheung WWL, Swartz W, Sumaila UR. 2012. Climate change impacts on fisheries in West Africa: implications for economic, food and nutritional security. Afr J Mar Sci. 34(1):103–117. doi:10.2989/1814232X.2012.673294
- Lawal-Are AO. 2012. Comparative biology of the prawns Macrobrachium macrobrachion (Herklots) and Macrobrachium vollenhovenii (Herklots) from two interconnecting fresh/brackish water lagoons in south-west Nigeria. J Mar Sci Res Dev. 2:1–9.
- Lee PG, Rodrick GE, Sodeman WA, Jr, Blake NJ. 1982. The giant Malaysian prawn, Macrobrachium rosenbergii, a potental predator for controlling the spread of schistosome vector snails in fish ponds. Aquaculture. 28(3–4):293–301. doi:10.1016/0044-8486(82)90071-0
- Lerer LB, Scudder T. 1999. Health impacts of large dams. Environ Impact Assess Rev. 19(2):113–123. doi:10.1016/S0195-9255(98)00041-9
- Leyvraz M, Rohner F, Konan AG, Esso LJCE, Woodruff BA, Norte A, Adiko AF, Bonfoh B, Aaron GJ. 2016. High awareness but low coverage of a locally produced fortified complementary food in Abidjan, Côte d’Ivoire: findings from a cross-sectional survey. PLoS One. 11(11):e0166295. doi:10.1371/journal.pone.0166295
- Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, Evans DS, Colley DG, Lin W, Coulibaly JT, Bustinduy AL, et al. 2017. A call to strengthen the global strategy for schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Inf Dis. 17:64–69.
- Lo NC, Gurarie D, Yoon N, Coulibaly JT, Bendavid E, Andrews JR, King CH. 2018. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc Natl Acad Sci USA. 115:584–591.
- Lodge DM, Rosenthal SK, Mavuti KM, Muohi W, Ochieng P, Stevens SS, Mungai BN, Mkoji GM. 2005. Louisiana crayfish (Procambarus clarkii) (Crustacea: Cambaridae) in Kenyan ponds: non-target effects of a potential biological control agent for schistosomiasis. Afr J Aquat Sci. 30(2):119–124. doi:10.2989/16085910509503845
- Loreau M, Baluku B. 1991. Shade as a means of ecological control of Biomphalaria pfeifferi. Ann Trop Med Parasitol. 85(4):443–446.
- Lu XT, Gu QY, Limpanont Y, Song LG, Wu ZD, Okanurak K, Lv ZY. 2018. Snail-borne parasitic diseases: an update on global epidemiological distribution, transmission interruption and control methods. Infect Dis Poverty. 7(1):1–16. doi:10.1186/s40249-018-0414-7
- Lund AJ, Sam MM, Sy AB, Sow OW, Ali S, Sokolow SH, Merrell SB, Bruce J, Jouanard N, Senghor S, et al. 2019. Unavoidable risks: local perspectives on water contact behavior and implications for schistosomiasis control in an agricultural region of northern Senegal. Am J Trop Med Hyg. 101(4):837–847. doi:10.4269/ajtmh.19-0099
- Madsen H. 1990. Biological methods for the control of freshwater snails. Parasitol Today. 6(7):237–241. doi:10.1016/0169-4758(90)90204-H
- Mahdi MA, Amin MA. 1966. An attempt to control bilharziasis by fish. Hydrobiologia. 28(1):66–72. doi:10.1007/BF00144939
- Makombu JG, Oben PM, Oben BO, Gaudin GLP, Motto IS, Makoge N, Syapze JK, Brown JH, Ngueguim JR, Mialhe E. 2014. Complete larval development of the fresh water prawn Macrobrachium vollenhovenii in Cameroon. J Appl Aquacult. 26(4):310–328. doi:10.1080/10454438.2014.934170
- Marques HLA, New MB, Boock MV, Barros HP, Mallasen M, Valenti WC. 2016. Integrated freshwater prawn farming: state-of-the-art and future potential. Rev Fish Sci Aquac. 24(3):264–293. doi:10.1080/23308249.2016.1169245
- Marshall BE. 2019. Crayfish, catfish and snails: the perils of uncontrolled biological control. Afr J Aquat Sci. 44(1):1–5. doi:10.2989/16085914.2019.1599810
- Masa J, Ogwok P, Muyonga JH, Kwetegyeka J, Makokha V, Ocen D. 2011. Fatty acid composition of muscle, liver, and adipose tissue of freshwater fish from lake Victoria, Uganda. J Aquat Food Prod Technol. 20(1):64–72. doi:10.1080/10498850.2010.539773
- Mas-Coma S, Valero MA, Bargues MD. 2008. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech OIE. 27(2):443–457. doi:10.20506/rst.27.2.1822
- Mas-Coma S, Valero MA, Bargues MD. 2011. Climate change effects on trematode and nematode diseases affecting children in rural areas of developing countries. Int Public Health J 2:405–430.
- McCreesh N, Booth M. 2013. Challenges in predicting the effects of climate change on Schistosoma mansoni and Schistosoma haematobium transmission potential. Trends Parasitol. 29(11):548–555. doi:10.1016/j.pt.2013.08.007
- McCreesh N, Nikulin G, Booth M. 2015. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors. 8(1):4–9. doi:10.1186/s13071-014-0617-0
- McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Primers. 4(1):1–19. doi:10.1038/s41572-018-0013-8
- Miller GC. 1971. Commercial fishery and biology of the freshwater shrimp, Macrobrachium, in the Lower St. Paul River, Liberia, 1952-53. Miami (FL): Tropical Atlantic Biological Laboratory, National Oceanic and Atmospheric Administration. Special Scientific Report-Fisheries No. 626.
- Mkoji GM, Hofkin BV, Kuris AM, Stewart-Oaten A, Mungai BN, Kihara JH, Mungai F, Yundu J, Mbui J, Rashid JR, et al. 1999. Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. Am J Trop Med Hyg. 61(5):751–759. doi:10.4269/ajtmh.1999.61.751
- Mlewa CM, Green JM. 2004. Biology of the marbled lungfish, Protopterus aethiopicus Heckel, in Lake Baringo, Kenya. Afr J Ecol. 42(4):338–345. doi:10.1111/j.1365-2028.2004.00536.x
- Mlewa C, Green JJM, Dunbrack RRL. 2010. The general natural history of the African lungfishes. In: Jorgensen JM, Joss J, editors. The biology of lungfishes. Sydney: CRC Press. p. 97–128.
- Mlewa CM, Green JM, Dunbrack R. 2007. Are wild African lungfish obligate air breathers? Some evidence from radio telemetry. Afr Zool. 42(1):131–134. doi:10.1080/15627020.2007.11407386
- Mohamed E, Al-Sabahi JN. 2014. (PDF) The composition of fatty acids stored in liver, muscle and fat tissues of the African lungfish Protopterus annectens (Owen, 1839). Afr J Biochem Res. 8:19–24.
- Molnar J, Walakira J, Terhune J. 2012. Prospects and potential of the African lungfish (Protopterus spp): results from a field survey in Uganda. In: Shriver AL, editor. Visible possibilities: the economics of sustainable fisheries, aquaculture and seafood trade. Proceedings of the Sixteenth Biennial Conference of the International Institute of Fisheries Economics and Trade, 2012 July 16–20, Dar Es Salaam, Tanzania. Corvallis (OR): International Institute of Fisheries Economics and Trade (IIFET).
- Morgan JAT, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. 2001. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 123(7):211–228. doi:10.1017/S0031182001007703
- Mortey EM, Kouassi KL, Diedhiou A, Anquetin S, Genoud M, Hingray B, Kouame DGM. 2019. Sustainable hydroelectric dam management in the context of climate change: case of the Taabo Dam in Côte d’Ivoire, West Africa. Sustainability (Switzerland). 11(18):4846–4832. doi:10.3390/su11184846
- Moseley WG, Carney J, Becker L. 2010. Neoliberal policy, rural livelihoods, and urban food security in West Africa: a comparative study of The Gambia, Côte d’Ivoire, and Mali. Proc Natl Acad Sci USA. 107:5774–5779.
- Mosille OIIW, Mmnoya JR. 1988. Reproductive biology of the East African lungfish Protopterus aethiopicus in Mwanza Gulf, Lake Victoria. African J Ecol. 26(2):149–162. doi:10.1111/j.1365-2028.1988.tb00965.x
- Mouchet J, Carnevale P. 1997. Impact of environmental changes on vector-bone diseases. Cahiers D’études et de Recherches Francophones. 7:263–269.
- Muller I, Coulibaly JT, Furst T, Knopp S, Hattendorf J, Krauth SJ, Stete K, Righetti AA, Glinz D, Yao AK, et al. 2011. Effect of schistosomiasis and soil-transmitted helminth infections on physical fitness of school children in Cote d’Ivoire. PLoS Negl Trop Dis. 5(7):e1239–10. doi:10.1371/journal.pntd.0001239
- Nelson MK, Cruz BC, Buena KL, Nguyen H, Sullivan JT. 2016. Effects of abnormal temperature and starvation on the internal defense system of the schistosome-transmitting snail Biomphalaria glabrata. J Invertebr Pathol. 138:18–23.
- New M, D’Abramo L, Valenti W, Singholka S. 2009. Sustainability of freshwater prawn culture. In: New M, Valenti W, Tidell J, D’Abramo L, Kutty M, editors. Freshwater prawns: biology and farming. London (UK): Wiley-Blackwell. p. 524–530.
- N’Goran E, David Aka NA, Ouattara M, Huber E, Bezuidenhout D, Kourany-Lefoll E, Pediatric Praziquantel C, Pediatric Praziquantel Consortium. 2019. Challenges and lessons from conducting a paediatric clinical trial in sub-Saharan Africa: the case of the praziquantel oral dispersible tablets phase II study in Cote d’Ivoire. Adv Parasitol. 103:75–89.
- Nicita A, Olarreaga M, Porto G. 2014. Pro-poor trade policy in Sub-Saharan Africa. J Int Econ. 92(2):252–265. doi:10.1016/j.jinteco.2014.01.001
- Norman RA, Crumlish M, Stetkiewicz S. 2019. The importance of fisheries and aquaculture production for nutrition and food security. Revue Scientifique et Technique (International Office of Epizootics). 38:395–407.
- Nwosu FM, Wolfi M. 2009. Population dynamics of the giant African river prawn Macrobrachium vollenhovenii Herklots 1857 (Crustacea, Palaemonidae) in the Cross River Estuary, Nigeria. West Afr J Appl Ecol. 9(1):1–14. doi:10.4314/wajae.v9i1.45681
- Obrist B, Cissé G, Koné B, Kouassi D, Dongo K, Granado S, Tanner M. 2006. Interconnected slums: Water, sanitation and health in Abidjan, Côte d’Ivoire. European Journal of Development Research. 18:319–336.
- Oficialdegui FJ, Sánchez MI, Clavero M. 2020. One century away from home: how the red swamp crayfish took over the world. Rev Fish Biol Fish. 30(1):121–135. doi:10.1007/s11160-020-09594-z
- Oliver-González J. 1946. The possible role of the guppy, Lebistes reticulatus, on the biological control of Schistosomiasis mansoni. Science. 104(2712):605–605. doi:10.1126/science.104.2712.605.c
- Olveda DU, Li Y, Olveda RM, Lam AK, Pchau TN, Harn DA. 2013. Bilharzia: pathology, diagnosis, management and control. Trop Med Surg. 1:135–135.
- Onyango AW, Jean-Baptiste J, Samburu B, Mahlangu TLM. 2019. Regional overview on the double burden of malnutrition and examples of program and policy responses: African region. Ann Nutr Metab. 75(2):127–130.
- Otero O. 2011. Current knowledge and new assumptions on the evolutionary history of the African lungfish, Protopterus, based on a review of its fossil record. Fish Fish. 12(3):235–255. doi:10.1111/j.1467-2979.2010.00389.x
- Oxford Business Group. 2018. Cote d’Ivoire revamps infrastructure in transport sector to support economic growth. In: The report: Cote d’Ivoire 2017. Oxford: Oxford Business Group; [accessed 2020 July 23]. https://oxfordbusinessgroup.com/cote-divoire-2017.
- Palmeirim MS, Ouattara M, Essé C, Koffi VA, Assaré RK, Hürlimann E, Coulibaly JT, Diakité NR, Dongo K, Bonfoh B, et al. 2018. Are schoolchildren less infected if they have good knowledge about parasitic worms? A case study from rural Côte d’Ivoire. BMC Public Health. 18(1):1–11. doi:10.1186/s12889-018-5776-z
- Parent G, Ouédraogo A, Zagré NM, Compaoré I, Kambiré R, Poda JN. 1997. Large dams, health and nutrition in Africa: beyond the controversy. Sante. 7(6):417–422.
- Paturel JE, Ouedraogo M, Servat E, Mahe G, Dezetter A, Boyer JF. 2003. The concept of rainfall and streamflow normals in west and central Africa in a context of climatic variability. Hydrol Sci J. 48(1):125–137. doi:10.1623/hysj.48.1.125.43479
- Perez-Saez J, Mande T, Larsen J, Ceperley N, Rinaldo A. 2017. Classification and prediction of river network ephemerality and its relevance for waterborne disease epidemiology. Adv Water Resour. 110:263–278. doi:10.1016/j.advwatres.2017.10.003
- Perez-Saez J, Mande T, Ceperley N, Bertuzzo E, Mari L, Gatto M, Rinaldo A. 2016. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proc Natl Acad Sci USA. 113:6427–6432.
- Pointier JP, David P. 2004. Biological control of Biomphalaria glabrata, the intermediate host of schistosomes, by Marisa cornuarietis in ponds of Guadeloupe: long-term impact on the local snail fauna and aquatic flora. Biol Control. 29(1):81–89. doi:10.1016/S1049-9644(03)00137-3
- Pointier JP, Giboda M. 1999. The case for biological control of snail intermediate hosts of Schistosoma mansoni. Parasitol Today. 15(10):395–397.
- Pointier JP, Jourdane J. 2000. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Trop. 77(1):53–60. doi:10.1016/S0001-706X(00)00123-6
- Pointier JP, Théron A, Imbert-Establet D, Borel G. 1991. Eradication of a sylvatic focus of Schistosoma mansoni using biological control by competitor snails. Biol Control. 1(3):244–247. doi:10.1016/1049-9644(91)90073-9
- Quansah E, Sarpong E, Karikari TK. 2016. Disregard of neurological impairments associated with neglected tropical diseases in Africa. eNeurologicalSci. 3:11–14.
- Ridoutt B, Bogard JR, Dizyee K, Lim-Camacho L, Kumar S. 2019. Value chains and diet quality: a review of impact pathways and intervention strategies. Agriculture (Switzerland). 9:1–18.
- Roche H, Tidou A. 2009. First ecotoxicological assessment assay in a hydroelectric reservoir: the Lake Taabo (Côte d’Ivoire). Bull Environ Contam Toxicol. 82(3):322–326. doi:10.1007/s00128-008-9572-9
- Rollinson D. 2011. Biomphalaria: natural history, ecology and schistosome transmission. In: Toldeo R, Fried B, editors. Biomphalaria snails and larval trematodes. New York: Springer. p. 57–79.
- Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, et al. 2013. Time to set the agenda for schistosomiasis elimination. Acta Trop. 128(2):423–440.
- Sahn DE. 1990. The impact of export crop production on nutritional status in Côte d’Ivoire. World Dev. 18(12):1635–1653. doi:10.1016/0305-750X(90)90060-B
- Sanogo T. 2019. Does fiscal decentralization enhance citizens’ access to public services and reduce poverty? Evidence from Côte d’Ivoire municipalities in a conflict setting. World Dev. 113:204–221. doi:10.1016/j.worlddev.2018.09.008
- Santé N, N’Go YA, Soro GE, Meledje NH, Bi TAG. 2019. Characterization of meteorological droughts occurrences in Côte d’Ivoire: case of the Sassandra watershed. Climate 7:1–17.
- Savaya-Alkalay A, Glassner H, Livne-Luzon S, Chishinski R, Molcho J, Aflalo ED, Zilberg D, Sagi A. 2020. Prawn monosex populations as biocontrol agents for snail vectors of fish parasites. Aquaculture. 520:1–8.
- Savaya-Alkalay A, Rosen O, Sokolow S, Faye Y, Faye D, Aflalo EDE, Jouanard N, Zilberg D, Huttinger E, Sagi A. 2014. The prawn Macrobrachium vollenhovenii in the Senegal River basin: towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis. 8(8):e3060–13. doi:10.1371/journal.pntd.0003060
- Schur N, Gosoniu L, Raso G, Utzinger J, Vounatsou P. 2011. Modelling the geographical distribution of co-infection risk from single-disease surveys. Stat Med. 30(14):1761–1776.
- Shehu Jega I, Magawata I, Ipinjolu JK, Jibir M. 2013. Evaluation of slurry formulations for Kilishi processing of African lungfish (Protopterus annectens, Owen). Pak J Nutr. 12(7):673–677. doi:10.3923/pjn.2013.673.677
- Sintondji SW, Adjahouinou DC, Djihinto GA, Fiogbe ED. 2020. Embryology of African giant freshwater shrimp Macrobrachium vollenhovenii. Biologia. 75(1):93–101. doi:10.2478/s11756-019-00280-5
- Slootweg R, Malek EA, McCullough FS. 1994. The biological control of snail intermediate hosts of schistosomiasis by fish. Rev Fish Biol Fish. 4(1):67–90. doi:10.1007/BF00043261
- Sokolow SH, Huttinger E, Jouanard N, Hsieh MH, Lafferty KD, Kuris AM, Riveau G, Senghor S, Thiam C, N’Diaye A, et al. 2015. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc Natl Acad Sci USA. 112(31):9650–9655. doi:10.1073/pnas.1502651112
- Sokolow SH, Jones IJ, Jocque M, La D, Cords O, Knight A, Lund A, Wood CL, Lafferty KD, Hoover CM, et al. 2017. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos Trans R Soc London B Biol Sci. 372:1–12.
- Sokolow SH, Lafferty KD, Kuris AM. 2014. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop. 132:64–74. doi:10.1016/j.actatropica.2013.12.013
- Sokolow SH, Nova N, Pepin KM, Peel AJ, Pulliam JRC, Manlove K, Cross PC, Becker DJ, Plowright RK, McCallum H, et al. 2019. Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos Trans R Soc London B Biol Sci. 374:1–10.
- Sokolow SH, Wood CL, Jones IJ, Lafferty KD, Kuris AM, Hsieh MH, Leo GAD. 2018. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends Parasitol. 34(1):23–40.
- Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, Lafferty KD, Kuris AM, Rickards C, De Leo GA. 2016. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis. 10(7):e0004794–12. doi:10.1371/journal.pntd.0004794
- Southgate VRR. 1997. Schistosomiasis in the Senegal river basin: before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J Helminthol. 71(2):125–132. doi:10.1017/s0022149x00015790
- Southgate VR, Tchuem Tchuenté LA, Sène M, De Clercq D, Théron A, Jourdane J, Webster BL, Rollinson D, Gryseels B, Vercruysse J. 2001. Studies on the biology of schistosomiasis with emphasis on the Senegal river basin. Mem Inst Oswaldo Cruz. 96(suppl):75–78. doi:10.1590/S0074-02762001000900010
- Sow S, De Vlas SJ, Polman K, Gryseels B. 2004. Hygiene practices and contamination risks of surface waters by schistosomae eggs: the case of an infested village in Northern Senegal. Bull Soc Pathol Exot. 97(1):12–14.
- Sow S, de Vlas SJ, Stelma F, Vereecken K, Gryseels B, Polman K. 2011. The contribution of water contact behavior to the high Schistosoma mansoni infection rates observed in the Senegal River Basin. BMC Infect Dis. 11(1):1–11. doi:10.1186/1471-2334-11-198
- Stauffer JR, Jr, Madsen H, McKaye K, Konings A, Bloch P, Ferreri CP, Likongwe J, Makaula P. 2006. Schistosomiasis in Lake Malawi: relationship of fish and intermediate host density to prevalence of human infection. EcoHealth. 3(1):22–27. doi:10.1007/s10393-005-0007-3
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 6(7):411–425. doi:10.1016/S1473-3099(06)70521-7
- Swartz SJ, De Leo GA, Wood CL, Sokolow SH. 2015. Infection with schistosome parasites in snails leads to increased predation by prawns: implications for human schistosomiasis control. J Exp Biol. 218(24):3962–3967. doi:10.1242/jeb.129221
- Swartz S, Rickards C. 2015. The history of schistosomiasis in Brazil. Stanford (CA). Upstream Alliance at Stanford University.
- Swiss T P H. 2020. Global neglected tropical diseases database. Basel (Switzerland). Swiss Tropical and Public Health Institute; [accessed 2020 May 25]. https://gntd.org/.
- Tayamen M, Brown JH. 1999. A condition index for evaluating larval quality of Macrobrachium rosenbergii (de Man 1879). Aquac Res.30(11–12):917–922. doi:10.1046/j.1365-2109.1999.00411.x
- Teugels GG, Lévêque C, Paugy D, Troaré K. 1988. Annotated checklist of the freshwater fishes of the coastal basins of Ivory Coast and western Ghana. Rev D’Hydrobiol Trop. 21:221–237.
- The SCI Foundation. 2020. Our work. London (UK). Schistosomiasis Control Initiative Foundation; [accessed 2020 June 6]. https://schistosomiasiscontrolinitiative.org/work.
- Tian-Bi Y-NT, Ouattara M, Knopp S, Coulibaly JT, Hürlimann E, Webster B, Allan F, Rollinson D, Meïté A, Diakité NR, et al. 2018. Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d’Ivoire: a SCORE study protocol. BMC Public Health. 18(1):1–12. doi:10.1186/s12889-018-5044-2
- United Nations Children’s Fund (UNICEF). 2018. Cote d’Ivoire multiple indicator cluster survey 2016. New York (NY): United Nations Children’s Fund; [accessed 2020 July 20]. http://ghdx.healthdata.org/record/cote-divoire-multiple-indicator-cluster-survey-2016.
- United Nations Development Program. 2020. Ending poverty. New York (NY): United Nations Development Program; [accessed 2020 Sept 7]. https://www.undp.org/sustainable-development-goals.
- United Nations Development Program (UNDP). 2021. Sustainable development goals. New York (NY). United Nations Development Program; [accessed 2020 Sept 7]. https://www.undp.org/sustainable-development-goals.
- Utzinger J, Müller I, Vounatsou P, Singer BH, N’Goran EK, Tanner M. 2003. Random spatial distribution of Schistosoma mansoni and hookworm infections among school children within a single village. J Parasitol. 89(4):686–692.
- Utzinger J, N’Goran EK, Caffrey CR, Keiser J. 2011. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 120:S121–S137. doi:10.1016/j.actatropica.2010.08.020
- Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, Singer BH, N’Goran EK. 2009. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 136(13):1859–1874. doi:10.1017/S0031182009991600
- van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. 2003. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 86(2–3):125–139.
- Walakira J, Molnar J. 2014. Culturing the African lungfish in Uganda: effects of exogenous fish feed on growth performance in tanks. Uganda J Agric Sci. 15:137–155.
- Walakira J, Reading B, Borski R, Terhune J, Boyd C, Molnar JJ. 2016. Development of captive breeding, larval rearing technologies and management practices for African lungish (Propopterus aethiopicus). Climate change adaptation: indigenous species development. Corvallis (OR): Oregon State University. Report No: Experiment/16IND03AU.
- Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature. 416(6879):389–396.
- Welander A, Lyttkens CH, Nilsson T. 2015. Globalization, democracy, and child health in developing countries. Soc Sci Med. 136–137:52–63.
- Willführ-Nast J, Rosenthal H, Udo PJ, Nast F. 1993. Laboratory cultivation and experimental studies of salinity effects on larval development in the African river prawn Macrobrachium vollenhovenii (Decapoda, Palaemonidae). Aquat Living Resour. 6(2):115–137. doi:10.1051/alr:1993012
- Wohlin C. 2014. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Shepperd M, editor. Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering (EASE) 2014, May 13–14; London (UK): Karlskrona (Sweden): Blekinge Institute of Technology, 38; p. 1–10. 10.1145/2601248.2601268.
- Woolhouse MEJ, Chandiwana SK. 1990. Population biology of the freshwater snail Bulinus globosus in the Zimbabwe highveld. J Appl Ecol. 27(1):41–59. doi:10.2307/2403567
- World Bank. 2019a. Overview. The World Bank in Côte d’Ivoire. Abidjan (Côte d’Ivoire): World Bank; [accessed 2020 June 23]. https://www.worldbank.org/en/country/cotedivoire/overview#1.
- World Bank. 2019b. Poverty & equity brief: Cote d’Ivoire. Abidjan (Côte d’Ivoire): The World Bank Group; [accessed 2020 June 6]. https://povertydata.worldbank.org/poverty/country/CIV.
- World Bank. 2019c. Sub-Saharan Africa. Regional Economic Data. Abidjan (Côte d’Ivoire): The World Bank Group; [accessed 2020 June 6]. https://data.worldbank.org/region/sub-saharan-africa.
- World Bank. 2020. Cote d’Ivoire: recent developments. MPO. Abidjan (Côte d’Ivoire): World Bank Group; [accessed 2020 June 6]. https://www.worldbank.org/en/country/cotedivoire/overview#1.
- World Food Programme. 2021. Cote d’Ivoire Country Strategic Plan. 2019-2023. [Accessed 18 April 2021]. https://www.wfp.org/operations/ci02-cote-divoire-country-strategic-plan-2019-2023.
- World Health Organization. 2006. Neglected tropical diseases: preventive chemotherapy and transmission control: soil-transmitted helminthiasis, onchocerciasis, lymphatic filariasis, schistosomiasis, Guinea-worm disease. Geneva (Switzerland): World Health Organization.
- World Health Organization. 2013. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. Geneva (Switzerland): World Health Organization; [accessed 2020 April 12]. https://apps.who.int/iris/handle/10665/78074.
- World Health Organization (WHO). 2015. Investing to overcome the global impact of neglected tropical diseases. In: Holmes P, editor. WHO report on neglected tropical diseases. Geneva (Switzerland): World Health Organization. Report No: WHO/HTM/NTD/2015.1.
- World Health Organization. 2017. Field use of molluscicides in schistosomiasis control programmes: an operational manual for program managers. Paris (France): World Health Organization.
- World Port Source. 2020. Map of ports in Côte d’Ivoire. Waters, M: Port Index; [accessed 2020 April 26]. http://www.worldportsource.com.
- Wrable M, Kulinkina AV, Liss A, Koch M, Cruz MS, Biritwum NK, Ofosu A, Gute DM, Kosinski KC, Naumova EN. 2019. The use of remotely sensed environmental parameters for spatial and temporal schistosomiasis prediction across climate zones in Ghana. Environ Monit Assess. 191(S2):1–18. doi:10.1007/s10661-019-7411-6
- Xia J, Harmon JL, Connolly KG, Donnelly R, Anderson MR, Howard HA. 2015. Who publishes in “predatory” journals? J Assn Inf Sci Tec. 66(7):1406–1417. doi:10.1002/asi.23265
- Xu J, Yu Q, Tchuenté LAT, Bergquist R, Sacko M, Utzinger J, Lin DD, Yang K, Zhang LJ, Wang Q, et al. 2016. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 16(3):376–383.
- Yao AH, Koumi AR, Atsé BC, Kouamelan EP. 2016. Contribution des femmes a la production piscicole en Côte D’Ivoire. Eur Sci J. 12:325–325.
- Yao HA, Koumi RA, Boua Atsé C, Kouamelan PE, Kouamé PL. 2017. Côte d’Ivoire aquaculture systems perception: characteristics and influence on national fish production. Int J Fish Aquacul. 9:108–118.
- Yapo ALM, Diawara A, Yoroba F, Kouassi BK, Sylla MB, Kouadio K, Odoulami RC, Tiémoko DT. 2019. Twenty-first century projected changes in extreme temperature over Côte d’Ivoire (West Africa). Int J Geophys. 2019:1–19. doi:10.1155/2019/5610328
- Zimmerman S, Nair CM, New MB. 2010. Freshwater prawns: biology and farming. West Sussex (UK): Wiley Blackwell.