?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper studies the behavior of a plant–herbivore model including both differential and difference equations. To analyze global behavior of the model, we consider the solution of the system in a certain subinterval which gives to system of difference equations. The boundedness characters, the periodic nature, both local and global stability conditions of the plant–herbivore system are investigated. Numerical studies indicate that the system exhibits Neimark–Sacker bifurcation for different parameter values in a certain regions.
Public Interest Statement
In this study, we consider a plant–herbivore model which consists of ordinary differential equations. Our aim is to build a better understanding of how both discrete and continuous times affect the dynamic behavior of plant–herbivore interactions. Therefore, we add discrete time to this model and obtain a system of differential equations with piecewise constant arguments which gives system of difference equations. The boundedness characters, the periodic nature, both local and global stability conditions of the system are investigated.
1. Introduction
Classical approaches to modeling plant–herbivore interactions are based on predator–prey system (Caughley & Lawton, Citation1981; May, Citation2001). This interaction has been described in much research using discrete and continuous model (Agiza, ELabbasy, EL-Metwally, & Elsadany, Citation2009; Chattopadhayay, Sarkar, Frıtzsche-Hoballah, Turlıngs, & Bersıer, Citation2001; Danca, Codreanu, & Bakó, Citation1997; Das & Sarkar, Citation2001; Edelstein-Keshet, Citation1986; Feng, Qiu, Liu, & DeAngelis, Citation2011; Lebon, Mailleret, Dumont, & Grognard, Citation2014; Li, Citation2011; Liu, Feng, Zhu, & DeAngelis, Citation2008; Mukherjee, Das, & Kesh, Citation2011; Ortega-Cejas, Fort, & Méndez, Citation2004; Owen-Smith, Citation2002; Saha & Bandyopadhyay, Citation2005; Sui, Fan, Loladze, & Kuang, Citation2007; Sun, Chakraborty, Liu, Jin, & Anderson, Citation2014; Zhao, Feng, Zheng, & Cen, Citation2015). The model of Li (Citation2011) is a system of differential equations with Holling type II functional response where the plant toxin’s influence in herbivores is considered. In study, Mukherjee et al. (Citation2011) have used discrete time model with Holling type II functional response for describing the plant–herbivore interaction.
It is well known that discrete time models governed by difference equations are more appropriate than the continuous time models when the populations have non-overlapping generations. So, a significant number of the study on the mathematical models of plant–herbivore interactions are described by the system of difference equations (Agiza et al., Citation2009; Danca et al., Citation1997; Mukherjee et al., Citation2011; Sui et al., Citation2007). In addition, working with difference equations instead of differential equations allows us to some advantages. Discrete dynamical models can bring about easier computational methods for the persistence, periodic solutions, boundedness, local and global properties of the dynamical system.
In plant–herbivore interactions, delay differential equations may widely occur due to herbivore damage and deployment of inducible defenses (Das & Sarkar, Citation2001; Ortega-Cejas et al., Citation2004; Sun et al., Citation2014). From this point of view, Sun et al. (Citation2014) and et all have constructed a reaction-diffusion model with delay governed by system of partial differential equations where the effect of time delay on the herbivore cycles is investigated. In addition, the properties of delay differential equations are very close to differential equation with piecewise constant arguments. In Cooke and Györi study (Citation1994), it was pointed out that these equations can be used to get approximate solutions to delay differential equations that include discrete delays. In such biological situations, dynamics of growth and death of populations can be described by differential equations otherwise, difference equations may reflect the interaction of two populations such as competition or predation phenomena (Gurcan, Kartal, Ozturk, & Bozkurt, Citation2014; Kartal & Gurcan, Citation2015). In the literature, various types of biological model consisting of differential equations with piecewise constant arguments have been analyzed using the method of reduction to discrete equations (Busenberg & Cooke, Citation1982; Gopalsamy & Liu, Citation1998; Gurcan et al., Citation2014; Kartal & Gurcan, Citation2015; Liu & Gopalsamy, Citation1999; Öztürk, Bozkurt, & Gurcan, Citation2012).
In the present paper, our aim is to build a better understanding of how both discrete and continuous times affect the dynamic behavior of plant–herbivore interactions. So we will reconsider the model (see Chattopadhayay et al., Citation2001)(1.1)
(1.1)
as a system of differential equations with piecewise constant arguments such as(1.2)
(1.2)
which include both differential and difference equations. In this model, x(t) and y(t) represent the density of plant and herbivore population, respectively, denotes the integer part of
and all these parameters are positive. The parameter r, K, and
is the intrinsic growth rate, environmental carrying capacity, and specific predation rate of plant species, respectively. s represents the death rate of herbivores and
is the conversion factor of herbivores (Chattopadhayay et al., Citation2001). The logistic term
and the term sy(t) include only a continuous time for the growth of plant and for the death of herbivore, respectively. The predational form
represent the loss of plant population and
is conversion factor of herbivores which include both discrete and continuous time for a each populations. So the plant–herbivore interaction is considered in a certain subinterval and is modeled using a system of differential equations with piecewise constant arguments.
2. Local and global stability analysis
System (1.2) can be written an interval as follows:
(2.1)
(2.1)
where
By solving each equations of the system (2.1) and letting , we obtain a system of difference equations
(2.2)
(2.2)
System (2.2) reflects the dynamical behavior of the system of differential equations with piecewise constant arguments. So we will consider the system of difference equation to analyze the global behavior of system (1.2).
The equilibrium points of system (2.2) can be obtained as
We note that the positive equilibrium of the system exists if . Now, we will find Jacobian matrix of the system to investigate the dynamic behavior of the model.
Theorem 2.1.
The equilibrium points and
are saddle point.
Proof
At the equilibrium point , the Jacobian matrix is the form
The matrix has eigenvalues
. Hence
and
and consequently
is saddle point. On the other hand, the Jacobian matrix
at the point
is
which gives eigenvalues . Considering the condition
, we can say that
is saddle point.
On the other hand, the Jacobian matrix at the positive equilibrium point
is
which yields the following characteristic equation
Now, we can apply Schur–Cohn criterion to determine stability conditions of the system with characteristic equation .
Theorem 2.2
The positive equilibrium point of system (2.2) is local asymptotically stable if and only if
Proof
From the Schur–Cohn criterion, is local asymptotically stable if and only if
| (a) | |||||
| (b) | |||||
| (c) | |||||
| (d) | |||||
The condition (a), (b), and (c) gives the inequalities(2.3)
(2.3)
(2.4)
(2.4)
and(2.5)
(2.5)
which always hold under the condition . From (d), we get
which reveal
This completes the proof. □
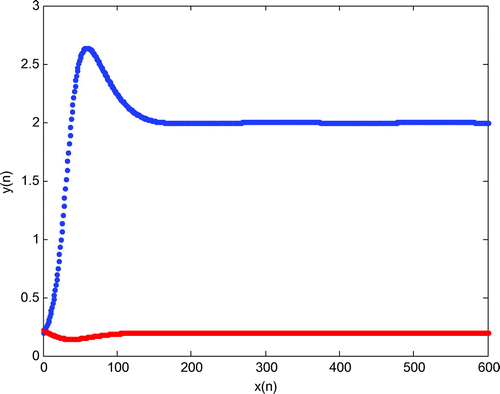
For the parameter values and using initial conditions
, it can be seen that the positive equilibrium point
is local asymptotically stable, where blue and red graphs represent population density of plant and herbivore population, respectively.
Theorem 2.3
Let be a positive solution of system (2.2); then
In addition, if
Proof
It can be easily seen that
Also, it can be shown that under the condition
.
Theorem 2.4
The system has no prime period-two solutions.
Proof
On the contrary, suppose that the system (2.2) has a distinctive prime period-two solutions
where , and
, and
are positive real numbers for
. Then, from system (2.2) one has
Since , we have
and
. From the second and last equation in the system, we have
If is written the above equation, we hold
This equation must satisfy
which is a contradiction and
.
Theorem 2.5
Let and
. Suppose that the conditions of Theorem 2.1 hold and
| (i) | |||||
| (ii) | |||||
| (iii) | |||||
| (iv) | |||||
| (v) | |||||
Then the positive equilibrium point of system (2.2) is global asymptotically stable.
Proof
We define a Lyapunov function as
where is positive equilibrium point of system (2.2).
The change along the solutions of the system is
From the first equation in (2.2), we hold;
By considering (i), (ii), and (iii), we have These imply that
. Additionally, we can show that
which gives
.
3. Bifurcation analysis
In this section, we investigate existence of stationary bifurcation (fold, transcritical, and pitchfork bifurcation), period doubling bifurcation, and Neimark–Sacker bifurcation for the system (2.2). All of these bifurcations can be analyzed under the set of algebraic condition that is called Schur–Cohn criterion. It is well known that the system may undergo stationary bifurcation if and only if ,
,
and
. On the other hand, inequalities
,
and
give the conditions of period doubling bifurcation. But considering (2.3) and (2.4), it is easily seen that these conditions do not hold for the system. Therefore, stationary bifurcation and period doubling bifurcation do not exist for the system.
Now, we can investigate the existence of Neimark–Sacker bifurcation for the plant–herbivore model (Hone, Irle, & Thurura, Citation2010). The algebraic condition of Neimark–Sacker bifurcation can be obtained from the analysis of inequalities ,
,
and
. In local stability analysis, we have already shown that the inequalities
,
,
are always exist. Therefore, we will only analyse the equation
to determine Neimark–Sacker bifurcation
Theorem 3.1
System (2.2) undergoes Neimark–Sacker bifurcation if and only if
Proof
This result comes from the analysis of
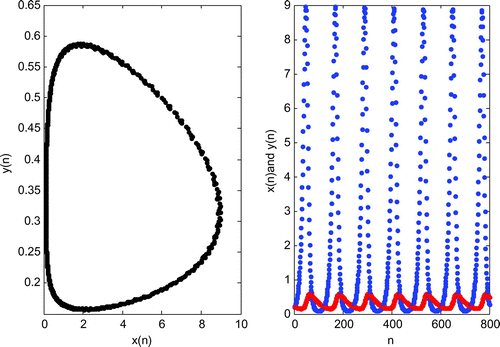
Using the condition of Theorem 3.1 with the parameters given in Figure , we have the Neimark–Sacker bifurcation point as (Figure ).
4. Result and discussion
In this paper, dynamics of a discrete-continuous time plant–herbivore model has been investigated. Local and global stability properties of the positive equilibrium point are analyzed. It is interesting to note that when conversion factor of herbivores becomes low then the system converges to a stable situation. On the other hand, we investigate possible bifurcation types for the system and observe that the system exhibits Neimark–Sacker bifurcation. This type of bifurcation has been observed many plant–herbivore models (Liu et al., Citation2008; Saha & Bandyopadhyay, Citation2005; Zhao et al., Citation2015) and shows that periodic or quasi-periodic solutions occurs as a result of limit cycle.
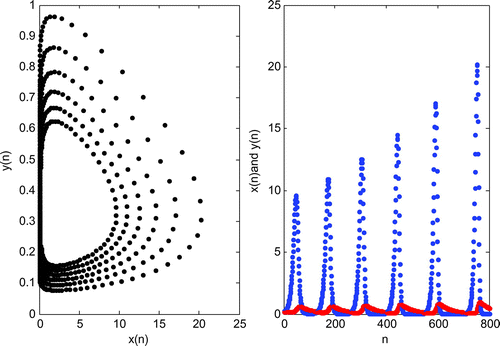
In our manuscript, the parameter K (environmental carrying capacity of plant species) is determined as a bifurcation parameter. When the environmental carrying capacity of plant species reaches to , the system enters a Neimark–Sacker bifurcation as a result of stable limit cycle (Figure ). If K exceeds the
, the system continues oscillatory behavior with growing amplitude (Figure ). So we can say that the parameter K has a strong effect on the stability of the system so as to control two populations.
Additional information
Funding
Notes on contributors
S. Kartal
Senol Kartal is an associate professor in Nevsehir Haci Bektas Veli University, Turkey. His PhD thesis is about the population dynamics (Modeling of Tumor Immune System Dynamics Using System of Difference Equations and Its Stability Analysis). His research interests are related to population dynamics, discrete and continuous dynamical system, and bifurcation theory. He has published his research contributions in some internationally renowned journals whose publishers are Elsevier, Taylor & Francis, Wiley, and other journals.
References
- Agiza, H. N., ELabbasy, E. M., EL-Metwally, H., & Elsadany, A. A. (2009). Chaotic dynamics of a discrete prey-predator model with Holling type II. Nonlinear Analysis Real World Applications, 10, 116–129.10.1016/j.nonrwa.2007.08.029
- Busenberg, S., & Cooke, K. L. (1982). Models of vertically transmitted diseases with sequential continuous dynamics. Nonlinear phenomena in mathematical sciences. New York, NY: Academic Press.
- Caughley, G., & Lawton, J. H. (1981). Plant-herbivore systems. In R. M. May (Ed.), Theoretical ecology (pp. 132–166). Sunderland: Sinauer Associates.
- Chattopadhayay, J., Sarkar, R., Frıtzsche-Hoballah, M. E. F., Turlıngs, T. C. J., & Bersıer, L. F. (2001). Parasitoids may determine plant fitness—A mathematical model based on experimental data. Journal of Theoretical Biology, 212, 295–302.10.1006/jtbi.2001.2374
- Cooke, K. L., & Györi, I. (1994). Numerical approximation of the solutions of delay-differential equations on an infinite interval using piecewise constant argument. Computers & Mathematics with Applications, 28, 81–92.
- Danca, M., Codreanu, S., & Bakó, B. (1997). Detailed analysis of a nonlinear prey-predator model. Journal of Biological Physics, 23, 11–20.10.1023/A:1004918920121
- Das, K., & Sarkar, A. K. (2001). Stability and oscillations of an autotroph-herbivore model with time delay. International Journal of Systems Science, 32, 585–590.10.1080/00207720117706
- Edelstein-Keshet, L. E. (1986). Mathematical theory for plant-herbivore systems. Journal of Mathematical Biology, 24, 25–58.10.1007/BF00275719
- Feng, Z., Qiu, Z., Liu, R., & DeAngelis, D. L. (2011). Dynamics of a plant–herbivore–predator system with plant-toxicity. Mathematical Biosciences, 229, 190–204.10.1016/j.mbs.2010.12.005
- Gopalsamy, K., & Liu, P. (1998). Persistence and global stability in a population model. Journal of Mathematical Analysis and Applications, 224, 59–80.10.1006/jmaa.1998.5984
- Gurcan, F., Kartal, S., Ozturk, I., & Bozkurt, F. (2014). Stability and bifurcation analysis of a mathematical model for tumor-immune interaction with piecewise constant arguments of delay. Chaos Solitons & Fractals, 68, 169–179.
- Hone, A. N. W., Irle, M. V., & Thurura, G. W. (2010). On the Neimark–Sacker bifurcation in a discrete predator-prey system. Journal of Biological Dynamics, 4, 594–606.10.1080/17513750903528192
- Kartal, S., & Gurcan, F. (2015). Stability and bifurcations analysis of a competition model with piecewise constant arguments. Mathematical Methods in the Applied Sciences, 38, 1855–1866.10.1002/mma.v38.9
- Lebon, A., Mailleret, L., Dumont, Y., & Grognard, F. (2014). Direct and apparent compensation in plant-herbivore interactions. Ecological Modelling, 290, 192–203.10.1016/j.ecolmodel.2014.02.020
- Li, Y. (2011). Toxicity impact on a plant-herbivore model with disease in herbivores. Computers Mathematics with Applications, 62, 2671–2680.10.1016/j.camwa.2011.08.012
- Liu, P., & Gopalsamy, K. (1999). Global stability and chaos in a population model with piecewise constant arguments. Applied Mathematics and Computation, 101, 63–88.10.1016/S0096-3003(98)00037-X
- Liu, R., Feng, Z., Zhu, H., & DeAngelis, D. L. (2008). Bifurcation analysis of a plant–herbivore model with toxin-determined functional response. Journal of Differential Equations, 245, 442–467.10.1016/j.jde.2007.10.034
- May, R. M. (2001). Stability and complexity in model ecosystems. Princeton, NJ: Princeton University Press.
- Mukherjee, D., Das, P., & Kesh, D. (2011). Dynamics of a plant-herbivore model with Holling type II functional response. Computational and Mathematical Biology, 2, 1–9.
- Ortega-Cejas, V. O., Fort, J., & Méndez, V. (2004). The role of the delay time in the modeling of biological range expansions. Ecology, 85, 258–264.10.1890/02-0606
- Owen-Smith, N. O. (2002). A metaphysiological modelling approach to stability in herbivore–vegetation systems. Ecological Modelling, 149, 153–178.10.1016/S0304-3800(01)00521-X
- Öztürk, I., Bozkurt, F., & Gurcan, F. (2012). Stability analysis of a mathematical model in a microcosm with piecewise constant arguments. Mathematical Biosciences, 240, 85–91.10.1016/j.mbs.2012.08.003
- Saha, T., & Bandyopadhyay (2005). M. Dynamical analysis of a plant-herbivore model: Bifurcation and global stability. Journal of Applied Mathematics & Computing, 19, 327–344.
- Sui, G., Fan, M., Loladze, I., & Kuang, Y. (2007). The dynamics of a stoichiometric plant-herbivore model and its discrete analog. Mathematical Biosciences Engineering, 4, 29–46.
- Sun, G. Q., Chakraborty, A., Liu, Q. X., Jin, Z., & Anderson, K. E. (2014). Influence of time delay and nonlinear diffusion on herbivore outbreak. Communications in Nonlinear Science and Numerical Simulation, 19, 1507–1518.10.1016/j.cnsns.2013.09.016
- Zhao, Y., Feng, Z., Zheng, Y., & Cen, X. (2015). Existence of limit cycles and homoclinic bifurcation in a plant-herbivore model with toxin-determined functional response. Journal of Differential Equations, 258, 2847–2872.10.1016/j.jde.2014.12.029