?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The scientific evidence that aquatic animal’s model bio-concentrate polycyclic aromatic hydrocarbons (PAHs) in their tissue has been proven beyond reasonable doubt. The worry is how much of these contaminants bio-concentrate in food and fish. This research investigates whether and to what extent residual PAHs bio-concentrate in—atlantic crocker (Micropogonias undulates), tilapia (Oreochromis niloticus), and yellow tail (Seriola lalandi). Twelve samples points were collected covering four kilometers along the Qua Ibeo river. They were quantitatively and qualitatively screened for PAHs concentration in water and in fish samples using gas chromatography tandem mass spectroscopy technique. Bio-concentration factor (BCF) was calculated via finding the ratio of mean PAHs concentration in fish to mean PAHs concentration in water. Results showed variable concentration of individual PAHs in water and in fish samples. Most apprehensively is the elevated concentration of some PAHs beyond permissible limit. And this call for worry or concern for public health and safety. The sixteen priority PAHs listed by the United State Environmental Protection Agency as carcinogenic were detected as: Naphthalene, 2-Methylnaphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benzo (a) anthracene, Triphenylene, Benzo (e) pyrene, Benzo (a) pyrene, Indeno (1,2,3,cd) pyrene, Benzo (g,h,i) perylene, Dibenzo (a,h) anthracene, and 000053-70-3-benzo (e) pyrene, respectively.
Public Interest Statement
There is an increasing public concern about the effects of some environmental contaminants on the endocrine system. Government and private organizations show great interest in public health. Risk analysis is a useful framework for managing potential threat to public health. In the last three decades, the effects of teratogenic and carcinogenic environmental contaminants have become issues in public health. Studies on wildlife have revealed some modifications of reproductive development. A number of abnormalities in the reproductive system of various wildlife species correlate with abnormalities found in human population. Reports from aquatic animal models, human clinical observations, and epidemiological studies implicates polycyclic aromatic hydrocarbons (PAHs) as a significant concern to public health. However, the place of PAHs in the causation of human diseases requires measuring dose matrices of PAHs in selected aquatic animal model. This perception will give insight into PAHs dose uptake during exposure that lead to bio-concentration and bioaccumulation.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are defined as compounds with two or more fused benzene rings and often contain alkyl side groups. Low molecular weight PAHs (LPAHs) are defined as those possessing two or three aromatic rings, and high-molecular-weight PAHs (HPAHs) as those with four or more rings (ATSDR, Citation1995; Dewitt et al., Citation1992). The designation of low and high molecular weight is somewhat arbitrary because the actual difference in molecular weight between 2 and 6-ring compounds (128.2 daltons for naphthalene to indeno [l, 2, 3-c, d] pyrene at 276.3 daltons) (American Society for Testing & Materials, Citation1994). The major difference between PAHs is solubility in water, which can be expressed in terms of hydrophobicity. In general, PAHs are more hydrophobic as molecular weight increases. As hydrophobicity increases, thermodynamic equilibrium tends to favor partitioning of the PAH molecule to more nonpolar environments, such as lipid of an organism or the organic carbon surrounding a sediment particle (Al-Shwafi, Citation2008; Nkpaa, Wegwu, & Essien, Citation2013).
PAHs appear in most urbanized coastal areas of the world. Accumulating in sediments and biota that are unable to efficiently eliminate them. This research focuses specifically on the assessment of residual bioavailability uptake and elimination of PAHs in three different fresh fish species. To determine the extent of accumulation and retention of PAHs in fish in marine ecosystems, these will ultimately explain the factors that control the concentration of PAHs in water and marine organisms. Therefore, in order to understand both the temporal and spatial characteristics of bioaccumulation of environmental PAHs, it is crucial to determine the impact of PAHs on marine populations and health. Thus, to provide a complete assessment of these potential impacts, scientists require knowledge about the distribution of these compounds in different environmental matrices, their uptake and partitioning in different tissues, their rates of elimination, and potential for persistence in certain species.
The combined information on these mechanisms and the environmental factors that control accumulation will help scientists develop predictive models of contaminant accumulation in aquatic animal model for acute events, such as oil spills, and for long-term, chronic exposure as is found in many urban areas in coastal ecosystems. Uptake is an important determinant of body burden that is controlled by factors associated with bioavailability and organismal physiology (Gobas, Wilcockson, Russell, & Haffner, Citation1999). It is speculated that the process of uptake of hydrophobic compounds is passive (vis-a-vis active transport) and controlled by diffusion pressure (fugacity) because of the differential between the environmental matrix and tissue concentration. For uptake of PAHs from water, factors that control the concentration of the free PAH (non-sorbed) are important. Studies on PAHs are largely conducted with specific compounds. For example, many studies that explore the uptake and elimination of PAHs in marine organisms deal with naphthalenes, a major component of petroleum, and carcinogenic PAHs, such as benzo[a]pyrene. Petroleum spills are important acute events; hence, it is important to understand the processes that control the bioaccumulation of naphthalene and associated compounds. Understanding the mechanism of chronic exposure to PAHs from areas is also desirable when assessing the population structure of marine organisms and the possibility of contaminant transfer to humans. Additionally, the carcinogenicity of PAHs is an important concern because some PAHs are biotransformed to reactive metabolites that can interact with DNA.
The ability to predict tissue concentrations in feral organisms is important in the assessment of possible toxic effects (Rocher, Azimi, Moilleron, & Chebbo, Citation2004; Scherer, Frank, Rieder, Meger-Kossion, & Renner, Citation2000). For example, the lethal body burden for nonionic hydrophobic compounds such as parent PAHs compounds, acting by narcosis, is believed to be in the range of 2–6 pmol/g (wet wt) (Tolosa et al., Citation2005). For species with weak or non-existent ability to metabolize PAHs, assessing body burden concentrations in light of this critical body burden may be a useful first approximation of potential deleterious effects. These body burdens could be used to characterize relationships between chemical exposure and ecological effects in marine species.
1.1. Information about research area
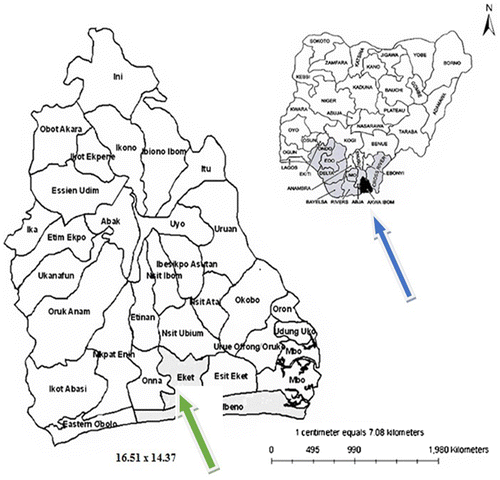
Akwa Ibom State, Nigeria, lies between Latitudes 4° 32″ and 5° 33″ North and Longitudes 7° 35″ and 8° 25″ East, the State is bounded on the East by Rivers State, on the West by Cross River State, on the North by Abia State and on the South by the Gulf of Guinea. Akwa Ibom State have a land area of 7,249 square kilometers. About 13.4% of the 960 km of Nigeria’s Atlantic Ocean coastline runs through the Akwa Ibom State. The land rises steadily northward from the sea-level at Eket in the south to 150 m at Obotme in the north. The whole of Akwa lbom State is underlain by sedimentary formations of Late Tertiary and Holocene ages. Deposits of alluvium and beach ridge sands occur along the coast and the estuaries of Qua lboe river, see Figure .
Figure 1. Map of Nigeria and Akwa Ibom State, showing the map.
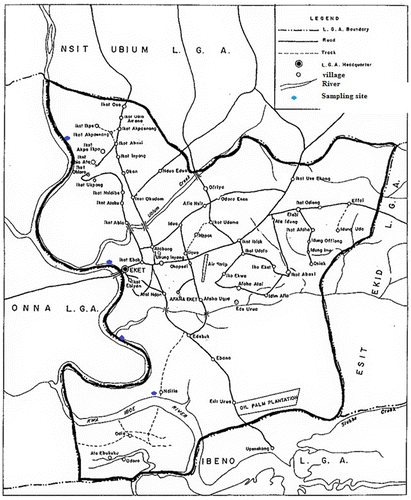
Eket is the second largest city in Akwa Ibom State, Nigeria. The name refers to the indigenous ethnic group of the region and their language. Eket Local Government Area (LGA) occupies the South central portion of Akwa Ibom State. It lies entirely in the tropics with territorial expense, spanning Northward between Latitude 4° 33″ and 4° 45″ and Eastward between Longitude 7° 52″ and 5° 02″. Eket is bounded on the north by Nsit Ubium LGA, on the east by Esit Eket LGA, on the west by Onna LGA and on the south by Ibeno LGA/Bright of Bonny see Figure . Eket has a population of 172,557 (National Population Census, Citation2006). In April 2014, the government of Akwa Ibom State released the projected population growth from 2007 to 2015 by adopting the 2006 population census. Eket LGA, have a projected population of 218, 438 in 2013, and it was estimated that by 2015, the population will increase to 233,544, dominant in 176,000 Km2 (Ministry of Economic Development, Citation2014). Eket is an industrial city that houses various infrastructures. At present, there are activities involving oil exploitation by Shell and Exxon Mobil. A thriving hub of oil and gas business, with more than 250 companies providing support services, such as catering, flights, and exports. An oil refinery is currently under construction at the outskirts of the city, along the Oron road. Qua Iboe river is a hotspot for commercial fishing and has been acclaimed as one of the largest fishing settlements on the Nigerian coastal land. The Qua Iboe river has abundant species of fishes and other aquatic organisms, such as tilapia, crocker, African red snipper, barracuda, yellow tail, and catfish. The human population at risk of contaminants exposure usually occurs via daily intake of fishes and contaminated drinking water. Some other exposure routes are processing food stuffs, swimming, recreation, and occupational exposure. There is currently no intervention on drinking water treatment by government agencies and individuals for their homes usage (Obiakor, Okonkwo, Ezeonyejiaku, & Okonkwo, Citation2014).
2. Subjects and methods
2.1. Sampling locations
A total of twelve (12) samples were collected from four location points. The sampling locations were established based on ecological settings and human activities. The locations were approximately 1 km away from each other for collection of samples. A total of 3 km was covered from location 1 to location 4.
2.1.1. Location 1
This sampling point was indicated as the upstream locations. It is located at Ikot Ikpe and Ikot Akpoenang at latitude 4° 55.8″ and longitude 7° 40.8″. Activities in this area are mainly fishing and boat making at the river bank. The water is relatively clean at this location by human eyesight evaluation.
2.1.2. Location 2
Location 2 receives effluents from the slaughter house located along the Ikot Aroku and Ikot Naidiba Village Road. Household from residential houses are also discharged into the River. The main activities are mining and loading of sand for commercial purposes. It is about 1 km away from station 1 and located at latitude 4° 22.9″ and longitude 7° 13.8″.
2.1.3. Location 3
This is located along Eket-Etinan Road, within Ebiyan and Ndon directly opposite Onna LGA. Activities at this location, include washing of cars, washing of motor bikes, washing of clothes, and bathing. The vegetation is dominated with bamboo trees. It is about 1 km away from location 2 and located at latitude 4° 23.2″and longitude 7° 40.2″.
2.1.4. Location 4
This is located at Ndilla, opposite villages across the river are Odio and Ale Ebukuku. This descended into Ibeno LGA, see Figure . The human activities include fishing and mining of sand. Vegetation is mainly mangrove. This area is turbid due to the discharge of much wastes, it is also very deep due to the mining activities. It is about 1 km away from location 3 and located at latitude 4° 43.5″ and longitude 7° 53.3″.
2.2. Collection of river water sample
River water samples were collected using 12 amber bottles, each 100 mL at 12 different points in Eket area of Akwa Ibom state on the 2 September 2015 between 10 am to 3 pm along the coast. The samples collected were extracted with n-hexane before concentrating the analytes or target compounds within 30 min.
2.3. Reagents and chemicals
All chemicals used were of analytical grade and were products of British Drug House (BDH) Chemical limited, Poole England.
2.4. Collection of fish sample
The biological sample (fish) were randomly collected to give a fair chance of probability since they are mobile organisms. Some variables that influenced site selection based on field work included proximity to oil well locations, gas flaring from Bonny Bright, high population density, socio-economic activities, particularly fishing within the area, with heavy presence of sewage, indiscriminate disposal of solid waste in and around the shore of Qua-Iboe river.
Locally consumed fresh yellow tail (Seriola lalandi), atlantic crocker (Micropogonias undulates), and tilapia (Oreochromis niloticus) were collected for study by a resident fisherman using set nets. These species of fishes, irrespective of their sex and age were weighed. The fishes were wrapped in hexane-rinsed aluminum foil, labeled and placed inside closed-glass vessel containing ice pack and kept at below −20°C before taken for laboratory analysis.
2.5. Preparation, extraction, and clean-up procedure of fish samples for analysis
Prior to extraction, the fish samples were scale-off using knife and subsequently dissected to obtain the tissues. A quantity, 15 g of the fish tissue was placed in a clean mortar and ground with pestle with 40 g of anhydrous sodium sulfate until completely dried and homogenized. The sample extraction was carried out using dichloromethane (DCM). A quantity, 10 g of the homogenized sample was placed in 50 mL extraction bottle and 1 mL of 60 ng/mL of 1-chloro-octadecane surrogate standard was added in the extraction bottle. The content was agitated or vortexed for five hrs and allowed to settle for one hour. The sample was carefully filtered through a funnel fitted with cotton wool, silica gel, and sodium sulfate Na2SO4 in a clean volumetric flask. The residue was washed and made up volume using the extracting solvent. The sample was concentrated to 2 mL for PAHs analysis using a gas chromatography tandem mass spectroscopy (GC-MS).
2.6. Gas chromatography-mass spectrometric analyses with purge and trap method (P&T)
This GC-MS method is also known as the dynamic headspace. This function by separating volatile compounds from the sample matrix (fish or water) by passing an inert gas such as helium or nitrogen through the matrix (purging). The target, volatile compounds will desorbed from the aqueous phase to the gas phase (purged) and are then separated from the stream of gas (trapped) by adsorbent filters (Lee, Wang, Hsieh, & Tien, Citation2005). The adsorbent material was then heated in a stream of GC carrier gas (pure helium). This released the trapped substances into the carrier gas, the target analytes were introduced to GC, and analyzed. Typical trapping (adsorbent) materials are porous polymer beads, activated charcoal, silica gel, other GC column packing materials, or combinations of such materials. A quantity 1μL of the extracted sample was analyzed with Agilent US EPA 8270 GC-MS. See Section 2.6 for operational conditions: The chromatograms were calibrated with internal standards. The calibration standard used for the PAHs consists of the following components. (1) Naphthalene, (2) Acenaphthylene, (3) Fluorene, (4) Acenaphthene, (5) Phenanthrene, (6) Anthracene, (7) Pyrene, (8) Benzo(a) antracene, (9) Chrysene, (10) Benzo(b)floranthene, (11) Benzo(k)floranthene, (12) Benzo(a)pyrene, (13) Indeno(1,2,3-cd)pyrene, (14) Fluoranthene, (15) Dibenzo(a,h)anthracene and (16) Benzo(ghi)perylene.
2.7. Operational conditions of the GC-MS
The methodology used for GC-MS has the following conditions. GCMS-QP2010 Agilent Plus, ion source temperature: 200.00°C, interface temperature: 250.00°C, solvent cut time: 2.50 min, detector gain mode: MS, detector gain: 0.00 kV, threshold: 2,000, column oven initial temperature: 70.0°C, injection final temperature: 250.00°C, injection Mode: Split, flow control mode: linear velocity, pressure: 116.9 kPa, total flow: 40.8 mL/min, column flow: 1.80 mL/min, linear velocity: 49.2 cm/s, trap and purge flow: 3.0 mL/min, Split Ratio: 20.0, high pressure injection: OFF, Carrier Gas: Helium and Splitter hold: OFF. While oven rating was as follows: Oven Temp. Program Rate Temperature (°C) Hold Time (min) Initial: 0.00 70.0 0.00 Final: 10.0 280 5.00.
2.8. Extraction of fish and water samples for PAH determination
Liquid–liquid extraction procedure was used in this analysis. One liter of sample was extracted in a 2 L glass separator funnel fitted with a glass stopper using 30 mL hexane as extract.
The separating funnel was vigorously shaken in 3 min and the organic layer was allowed to separate clearly from the aqueous phase for a minimum of 5 min, after which, the organic layer was collected into a separate glass bottle. The extraction was repeated thrice for each sample. Water residues were expelled from the organic layer by passing extracts through funnels containing anhydrous sodium sulfate. Extracts were concentrated using rotary evaporators with water bath preset at 85°C. Concentrated extracts were transferred to a pre-weighed sample bottle and evaporated to dryness.
2.9. Calculation of bio concentration factor
The bio-concentration factor (BCF) was carried using the (Mccarty, Citation1986) method. The BCF is the ratio of the tissue concentration of a particular chemical to its water concentration see Equation (1). We took notice that BCF is relevant only for accumulation from water; wherein to compare among BCFs it is important to establish that water is the only route of uptake. Contrary to the bioaccumulation factor (BAF) which is generally computed as the ratio between the contaminant concentrations in tissue and multiple external sources (e.g. sediment, water, and diet) and is useful in determining the tendency of hydrophobic compounds to accumulate in tissue. A seldom used term, dietary accumulation, is used to determine the ratio between the concentration of a contaminant in an organism and its food.(1)
(1)
BCF, (bio-concentration factor with free PAH in water) = [Tissue]/[Water free]
BCF is BCF predicted. For example the equation from (National Population Census, Citation2006) = 0.046 KOw.
2.10. Statistical analysis
All investigations were carried out in triplicate and data obtained were presented as mean ± standard deviation using descriptive statistics. One-way analysis was used to compare mean variance among samples. Significance was accepted at p < 0.05 level.
3. Results
Table shows the mean concentration of individual PAHs in river water samples, mean bio-concentration of individual PAHs in fish species, and BCF of individual PAHs in atlantic crocker (M. undulates) fish species.
Table 1. Residual toxicants bio-concentration of atlantic crocker (M. undulates)
Table shows the mean concentration of individual PAHs in river water samples, mean bio-concentration of individual PAHs in fish species, and BCF of individual PAHs in tilapia (O. niloticus) fish species.
Table 2. Residual toxicants bio-concentration tilapia (O. niloticus)
Table shows mean concentration of individual PAHs in river water samples, mean bio-concentration of individual PAHs in fish species, and bio-concentration factor of individual PAHs in yellow tail (S. lalandi) fish specie.
Table 3. Residual toxicants bio-concentration of yellow tail (S. lalandi)
4. Discussion
Many studies have shown that marine organisms can accumulate PAHs from the environment. It is not so much a question of “if an animal will accumulate PAHs, rather how much it will accumulate”? This and some other question were our concern for the assessment of human health risk, since human live by consuming this contaminated resources. The environmental concentrations, controlling factors of bioavailability, time scale, and organism physiology are factors for bio-concentration to occur in aquatic organism. Bio-concentration patterns of PAHs in marine organisms may differ based on many factors including, but not limited to, exposure medium, uptake rate, metabolic capability, lipid content, and feeding strategy (Adams, Citation1987; Meador, Casillas, Sloan, & Varanasi, Citation1995; Roesijadi, Anderson, & Blaylock, Citation1978; Schrap & Opperhuizen, Citation1990; Varanasi, Reichert, Stein, Brown, & Sanborn, Citation1985). These factors have to be considered whenever accumulations of PAHs are considered and compared. Because biotransformation is one of the important processes, evaluating metabolic capacity and disposition of parent compounds are included in any study of PAHs bioaccumulation to accurately assess total uptake.
In this investigation, three different fish species that are customarily consumed by the residents of Eket community in Akwa Ibom State Nigeria, were randomly selected for the screening of PAHs levels. This was to establish residue accumulation of PAHs in aquatic animal model. Upon investigation, the total mean concentration of individual toxicant in water and in fish was determined and reported in triplicate. The BCF of the fishes was extrapolated by adapting the method of (Mccarty, Citation1986) see Equation (1).
The BCF is the ratio of the tissue concentration of a particular chemical to its water concentration. It was kept in perspective that the BCF is relevant only to the extent of accumulation from water; to compare among BCFs, it is important to establish that water is the only route of uptake. At equilibrium, the BCF generally increases with increasing chemical hydrophobicity because of the increased fugacity or tendency of the chemical to partition into the animal’s lipid rather than stay in solution (Bruner, Fisher, & Landrum, Citation1994; de Mora, Fowler, Wyse, & Azemard, Citation2004). The results obtained as mean concentration for toxicants body residues of atlantic crocker (M. undulates) showed naphthalene have 10.370 ± 0.302 ppm, 2-methylnaphthalene 10.160 ± 0.112 ppm, acenaphthylene 10.170 ± 0.151 ppm, acenaphthene 10.403 ± 0.431 ppm, fluorene 12.707 ± 4.593 ppm, phenanthrene 10.360 ± 0.355 ppm, anthracene 9.443 ± 0.551 ppm, fluoranthene 16.416 ± 3.787 ppm, pyrene 13.873 ± 3.639 ppm, benzo (a) anthracene 10.240 ± 0.832 ppm, triphenylene 10.216 ± 0.120 ppm, Benzo (e) pyrene 15.616 ± 4.779 ppm, benzo (a) pyrene 16.200 ± 5.232 ppm, indeno (1,2,3,cd) pyrene 17.670 ± 3.836 ppm, benzo (g,h,i) perylene 14.126 ± 4.564 ppm, dibenzo (a,h) anthracene 16.076 ± 5.168 ppm and 000053-70-3-benzo (e) pyrene 13.590 ± 5.477 ppm, respectively (see Table ). Several researchers have noticed that PAH concentrations in marine organisms appear to show seasonal variation, which may be caused by a number of factors (Atuanya & Nwogu, Citation2013). As customary with organic contaminants, PAHs accumulate in certain tissues with the highest proportions found in the liver of vertebrates or the hepato-pancreas of invertebrates (Nyarko, Botwe, & Klubi, Citation2001). In general, lipid-rich tissues preferentially accumulate parent PAHs because of their strong hydrophobic nature (Umeh, Citation2009). Meanwhile, the total mean concentration of the individual contaminants or toxicants in water column was determined for atlantic crocker (M. undulates). Results 23.302 ± 0.114 ppm for naphthalene, 28.225 ± 0.231 ppm 2-methylnaphthalene, 16.564 ± 0.220 ppm acenaphthylene, 27.585 ± 1.210 ppm acenaphthene, 27.239 ± 0.123 ppm fluorene, 28.421 ± 2.100 ppm phenanthrene, 28.256 ± 0.221 ppm anthracene, 27.481 ± 0.401 ppm fluoranthene, 28.201 ± 1.091 ppm pyrene, 26.264 ± 0.220 ppm benzo (a) anthracene, 29.077 ± 0.329 ppm triphenylene, 27.594 ± 0.211 ppm benzo (e) pyrene, 13.873 ± 0.111 ppm Benzo (a) pyrene, 13.774 ± 0.213 ppm indeno (1,2,3,cd) pyrene, 28.383 ± 0.210 ppm benzo (g,h,i) perylene, 28.302 ± 0.101 ppm, dibenzo (a,h) anthracene and 27.828 ± 0.171 ppm for 000053-70-3-benzo (e) pyrene differently. Concentration may be a function of PAHs hydrophobicity, but time of exposure and metabolic capacity must be considered. Several studies have shown rapid attainment of steady-state BCFs in marine organisms exposed to PAHs in water. For example, similar values (BCFs ≅ 50) were calculated for Mytilus edulis after 4 h of exposure to labeled naphthalene and after 4 weeks exposure to unlabeled naphthalene, indicating rapid equilibration with exposure concentrations (Widdows, Moore, Clarke, & Donkin, Citation1983).
Determination of BCFs in field-collected subjects is uncommon, because it is difficult to assure water-only exposure and accurately determine temporally variable concentrations. In this study, the bio-concentration for atlantic crocker (M. undulates). Results showed variable bio-concentration of toxicants in fish as shown in Tables –.
Naphthalene 0.4450 ± 2.649, 2-methylnaphthalene 0.3599 ± 0.485, acenaphthylene 0.6139 ± 0.686, acenaphthene 0.3771 ± 0.356, fluorene 0.4665 ± 37.34, phenanthrene 0.3666 ± 0.169, anthracene 0.3341 ± 2.493, fluoranthene 0.5974 ± 9.444, pyrene 0.4919 ± 3.335, benzo (a) anthracene 0.3899 ± 3.782, triphenylene 0.3513 ± 0.365, benzo (e) pyrene 0.5659 ± 22.65, benzo (a) pyrene 1.1677 ± 47.13, indeno (1,2,3,cd) pyrene 1.2829 ± 18.01, benzo (g,h,i) perylene 0.9769 ± 21.73 dibenzo (a,h) anthracene 0.5681 ± 51.17 and 000053-70-3-benzo (e) pyrene 0.4884 ± 32.03 separately.
One study reported variable BCFs in the range of 7 × 103 to 3 × l04 in an urban area and 1 × 101 to 5 × 101 in an oil refinery area for mussels (M. edulis planulatus) from southeast Australia (Murray, Richardson, & Gibbs, Citation1991). Interestingly, the BCFs for benzo [b] fluoranthene, benzo [k] fluoranthene, and benzo [a] pyrene were 10–44 times higher in individuals collected from the oil refinery sites versus those in animals collected from the urban sites. BCFs for fish are very difficult to calculate because extensive biotransformation results in the rapid disappearance of parent PAHs from tissues. Measurement of tissue burdens of parent compounds normalized to water concentrations will severely underestimate the BCF. As described earlier, alternate measurements of PAH accumulation may be more useful in assessing exposure. In the same vein, results in Tables and showed the same trend. As mean bio-concentration of toxicants varies in fish tissues based on environmental and associated factors.
The mean bio-concentration of toxicants body residues for tilapia (O. niloticus) was calculated, results revealed naphthalene 0.5825 ± 36.263, 2-methylnaphthalene 0.4655 ± 22.342, acenaphthylene 0.8311 ± 3.9215, acenaphthene 0.4976 ± 3.9215, fluorene 0.6865 ± 4.6504, Phenanthrene 0.5837 ± 2.4229, anthracene 0.5881 ± 22.434, fluoranthene 0.5083 ± 11.481, pyrene 0.4761 ± 4.7580, benzo (a) anthracene 0.5056 ± 23.072, triphenylene 0.4749 ± 15.428, benzo (e) pyrene 0.5876 ± 24.597, benzo (a) pyrene 1.1586 ± 45.667, indeno (1,2,3,cd) pyrene 0.9072 ± 23.967, benzo (g,h,i) perylene 0.4747 ± 22.892, dibenzo (a,h) anthracene 0.4717 ± 49.594 and 000053-70-3-benzo (e) pyrene 0.4766 ± 30.673, respectively (see Table ).
Correlations between PAHs in the environmental matrix and tissue can be useful in assessment of environmental exposure (Dhananjayan & Muralidharan, Citation2012). One study found a strong gradient of PAH concentration both in sediment and in mussel (M. edulis and Modiolus modiolus) tissue up to several kilometers away from a ferro-alloy smelter (Boese, Lee Ii, Specht, Randall, & Winsor, Citation1990). Both oysters (Crassostrea virginica) and clams (Rangia cuneata) sampled in the fall contained about 2–3 times more aromatic hydrocarbons than those sampled at the same sites during the spring season (Bender, DeFur, & Huggett, Citation1986), leading to the hypothesis that differences resulted from the spawning cycle. Bio-concentration and bioaccumulation factors (BCFs and BAFs) are useful ratios that can indicate steady-state exposure and expected tissue burdens based on environmental concentrations. Table present the toxicants body residues of yellow tail fish (S. lalandi). So far, the elucidation of BCF showed that naphthalene 0.8022 ± 5.114, 2-Methylnaphthalene 0.5714 ± 22.64, acenaphthylene 0.9726 ± 23.97, acenaphthene 0.5999 ± 4.603, fluorene 0.7068 ± 3.349, phenanthrene, 0.6820 ± 0.189, anthracene 0.6566 ± 6.131, fluoranthene 0.6874 ± 1.983, pyrene 0.5700 ± 4.784, benzo (a) anthracene 0.6117 ± 23.65, triphenylene 0.6556 ± 3.191, benzo (e) pyrene 0.6922 ± 0.369, benzo (a) pyrene 1.4344 ± 0.909, indeno (1,2,3,cd) pyrene 1.3883 ± 2.929, benzo (g,h,i) perylene 0.6902 ± 9.277, dibenzo (a,h) anthracene 0.7138 ± 9.277 and 000053-70-3-benzo (e) pyrene 0.6754 ± 3.339.
Some researchers have noticed a pattern of differential accumulation which differ over major PAH groups (for example, compounds containing 2 through 6 aromatic rings). Mccarty (Citation1986), reported that 2, 3, and 5-ring PAHs were poorly taken up by amphipods (Eohaustorius washingtonianus and Rhepoxynius abronius) and a clam (Macoma nasuta) compared to 4-ring compounds (Rose, Ken, Kehinde, & Babajide, Citation2012). This pattern may have resulted from the volatility of the 2 and 3-ring compounds, which may be released directly without metabolism from the organism, slower uptake kinetics of the more hydrophobic PAHs, and the reduced uptake of the 5 and 6-ring compounds, which are suspected of being more tightly bound to organic carbon and hence less available to organisms as exemplify in various individual toxicant.
5. Conclusion
The observed levels of PAHs in fish species indicate that Qua Iboe river in Eket community of Akwa Ibom state is contaminated with PAHs. Therefore, Eket community may be at risk of contracting diseases associated with PAHs effects as a result of their persistence exposure to/and consumption of the fishes. It is believed that other fish species do bio-concentrate PAHs and possibly, beyond permissible limit. Therefore, periodic monitoring of the aquatic environment will give insight into the dose matrices of PAHs in the water body. Since bio-concentration of PAHs know no boundary, critical studies of major aquatic environment may be beneficial to health safety. Future work on the subjects of uptake efficiency, the role of qualitative and quantitative differences in organic carbon in determining bioavailability, assumed environmental equilibrium, trophic movement of parent compounds and metabolites, predictable concentration factors, and the variability in toxicokinetic parameters as a function of chemical hydrophobicity, environmental changes, and physiological factors will help to enhance understanding of the mechanisms of PAH concentration and elimination in marine organisms. These will ultimately give empirical evidence for impact and risk assessment of both aquatic population and public health and safety.
Funding
The authors received no direct funding for this research.
Acknowledgments
We would like to thank Mr Paul Nwachukwu of the international energy center and research development, Port Harcourt, River state for an excellent knowledge for running the GC/MS analysis. We also thank Dr J. Parkar Elijah for his generosity in running the statistical analysis and Mr Christopher Akaka for supplying the ice park and vessels used to convey the water and fish samples to the analytical laboratory.
Additional information
Notes on contributors
Victor Eshu Okpashi
Victor Eshu Okpashi, is a PhD candidate in the Department of Biochemistry, University of Nigeria, Nsukka. His specialty is Molecular Biochemistry and Environmental Toxicology. He did the investigation both at field and laboratory.
Victor Nwadiogbu Ogugua
Victor Nwadiogbu Ogugua is a senior lecturer, he supervised the investigation.
Samuel Chibuike Ubani
Samuel Chibuike Ubani is a senior lecturer, an expert in Industrial Biochemistry and Biotechnology, did the design of the investigation.
Innocent Izuchukwu Ujah
Innocent Izuchukwu Ujah is a PhD graduate of University of Nigeria. He did the literature gathering.
Juliet Nwamaka Ozioko
Juliet Nwamaka Ozioko is an MSc graduate of same department. She did the laboratory analysis.
References
- Adams, W. J. (1987). Bioavailability of neutral lipophilic organic chemicals contained on sediments: A review. In K. L. Dickson, A. W. Maki, & W. A. Brungs (Eds.), Fate and Effects of Sediment-Bound Chemicals in Aquatic Systems (pp. 219–244). New York, NY: Pergamon Press.10.1016/B978-0-08-034866-7.50023-0
- Al-Shwafi, N. A. A. (2008). Total petroleum hydrocarbon carcinogens in commercial fish in the red sea and Gulf of Aden-Yemen. JKAU: Marine Science, 2008, 15–28.
- American Society for Testing and Materials. (1994). Annual book of standards (Vol. 11). Philadelphia, PA: Author.
- ATSDR. (1995). Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). Atlanta, GA: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
- Atuanya, E. I., & Nwogu, N. A. (2013). Evaluation of bacteriological and mercury level in cod (Gadus morhua) and saithe (Pollachius virens) stockfish sold in Benin City, Edo State. Nigeria. International Journal of Advanced Research, 1, 211–214.
- Bender, M. E., DeFur, P. O., & Huggett, R. J. (1986). Polynuclear aromatic hydrocarbon monitoring in estuaries utilizing: Oysters, brackish water clams and sediments. Oceans ‘86, Conference Record, Monitoring Strategies Symposium (Vol. 3, pp 791–796). Institute of Electrical and Electronics Engineers, Piscataway, NJ and Marine Technology Society, Washington, DC.
- Boese, B. L., Lee Ii, H. I. I., Specht, D. T., Randall, R. C., & Winsor, M. H. (1990). Comparison of aqueous and solid-phase uptake for hexachlorobenzene in the tellinid clam Macoma nasuta (conrad): A mass balance approach. Environmental Toxicology and Chemistry, 9, 221–231.10.1002/etc.v9:2
- Bruner, K. A., Fisher, S. W., & Landrum, P. F. (1994). The role of the zebra mussel (Dreissena polymorpha) in contaminant cycling: 11. Zebra mussel contaminant accumulation from algae and suspended particles, and transfer to the benthic invertebrate Gammarus fasciatus. Journal of Great Lakes Research, 20, 735–750.10.1016/S0380-1330(94)71191-6
- de Mora, S., Fowler, S. W., Wyse, E., & Azemard, S. (2004). Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and Gulf of Oman. Marine Pollution Bulletin, 49, 410–424.10.1016/j.marpolbul.2004.02.029
- Dewitt, T. H., Jones, J. K. P., Ozretich, R. J., Swartz, R. C., Lamberson, J. O., Schults, D. W., … Ditsworth, G. R. (1992). The influence of organic matter quality on the toxicity and partitioning of sediment-associated fluoranthene. Environmental Toxicology and Chemistry, 11, 197–208.10.1002/etc.v11:2
- Dhananjayan, V., & Muralidharan, S. (2012). Polycyclic aromatic hydrocarbons in various species of fishes from Mumbai harbor, India and their dietary intake concentration to Human. International Journal of Oceanography. doi:10.1155/2012/645178.
- Gobas, F. A. P. C., Wilcockson, J. B., Russell, R. W., & Haffner, G. D. (1999). Mechanism of biomagnification in fish under laboratory and field conditions. Environmental and Scientific Technology, 33, 133–141.10.1021/es980681m
- Lee, C. C., Wang, T., Hsieh, C. Y., & Tien, C. J. (2005). Organotin contamination in fishes with different living patterns and its implications for human health risk in Taiwan. Environmental Pollution, 137, 198–208. doi:10.1016/j.envpol.2005.02.011
- Mccarty, L. S. (1986). The relationship between aquatic toxicity QSARs and bioconcentration for some organic chemicals. Environmental Toxicology and Chemistry, 5, 1071–1080.10.1002/etc.v5:12
- Meador, J. P., Casillas, E., Sloan, C. A., & Varanasi, U. (1995). Comparative bioaccumulation of polycyclic aromatic hydrocarbons from sediment by two in faunal organisms. Marine Ecology Progress Series, 123, 107–124.10.3354/meps123107
- Ministry of Economic Development. (2014). Statistical year book of Akwa-Ibom state of Nigeria (MECOD). edn. Retrieved from https://wwwnigerianstat.gov.ng/pages/download/232
- Murray, A. P., Richardson, B. J., & Gibbs, C. F. (1991). Bio-concentration factors for petroleumhydrocarbons, PAHs, LABS, and biogenic hydrocarbons in the blue mussel. Marine Pollution Bulletin, 22, 595–603.10.1016/0025-326X(91)90247-P
- National Population Census. (2006). Federal Republic of Nigeria official gazzete no. 2 Abuja. 2nd February, 2009 Vol. 96 B. 25. Federal Government Printer.
- Nkpaa, K. W., Wegwu, M. O., & Essien, E. B. (2013). Assessment of polycyclic aromatic hydrocarbons (PAHs) levels in two commercially important fish species from crude oil polluted waters of Ogoniland and their carcinogenic health risk. Journal of Environment and Earth Science, 3, 128–137.
- Nyarko, E., Botwe, B. O., & Klubi, E. (2001). Polycyclic aromatic hydrocarbon (PAHs) levels in two commercially important fish species from the Coastal Waters of Ghana and their carcinogenic health risks. West African Journal of Applied Ecology, 19, 53–66.
- Obiakor, M. O., Okonkwo, J. C., Ezeonyejiaku, C. D., & Okonkwo, C. N. (2014). Polycyclic aromatic hydrocarbons (PAHs) in freshwater media: Fatoriel effects and Human Dietary exposure risk assessment. Resources and Environment, 4, 247–259.
- Rocher, V., Azimi, S., Moilleron, R., & Chebbo, G. (2004). Hydrocarbons and heavy metals in the different sewer deposits in the “Le Marais” catchment (Paris, France): Stocks, distributions and origins. Science of the Total Environment, 323, 107–122.10.1016/j.scitotenv.2003.10.010
- Roesijadi, G., Anderson, J. W., & Blaylock, J. W. (1978). Uptake of hydrocarbons from marine sediments contaminated with prudhoe bay crude oil: Influence of feeding type of test species and availability of polycyclic aromatic hydrocarbons. Journal of the Fisheries Research Board of Canada, 35, 608–614.10.1139/f78-107
- Rose, A., Ken, D., Kehinde, O., & Babajide, A. (2012). Bioaccumulation of PAHs in fish and invertebrates of Lagos lagoon, Nigeria. Journal of Emerging Trends in Engineering and Applied Sciences, 3, 287–296.
- Scherer, G., Frank, S., Rieder, K., Meger-Kossion, I., & Renner, I. (2000). Biomonitoring of exposure to polycyclic aromatic hydrocarbons of non-occupationally exposed persons. Cancer Epidemiology Biomarkers and Prevention, 9, 373–380.
- Schrap, S. M., & Opperhuizen, A. (1990). Relationship between bioavailability and hydrophobicity: Reduction of the uptake of organic chemicals by fish due to the sorption on particles. Environmental Toxicology and Chemistry, 9, 715–724.
- Tolosa, I., de Mora, S. J., Fowler, S. W., Villeneuve, J. P., Bartocci, J., & Cattini, C. (2005). Aliphatic and aromatic hydrocarbons in marine biota and coastal sediments from the Gulf and the Gulf of Oman. Marine Pollution Bulletin, 50, 1619–1633.10.1016/j.marpolbul.2005.06.029
- Umeh, G. I. (2009). Impacts of petroleum hydrocarbons on fish communities of river Areba, Niger Delta, Southern Nigeria. Tropical Freshwater Biology, 18(1), 79–91.
- Varanasi, U., Reichert, W. L., Stein, J. E., Brown, D. W., & Sanborn, H. R. (1985). Bioavailability and biotransformation of aromatic hydrocarbons in benthic organisms exposed to sediment from an urban estuary. Environmental Science and Technology, 19, 836–841.10.1021/es00139a012
- Widdows, J., Moore, S. L., Clarke, K. R., & Donkin, P. (1983). Uptake, tissue distribution and elimination of [1-14C]napthalene in the mussel Mytilus edulis. Marine Biology, 76, 109–114.10.1007/BF00392727