 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Heavy metal pollution and accumulation in aquatic ecosystems present serious threats to sustainability. In the current study, the heavy metal content of water and edible muscles of a piscivorous fish (Protopterus annectens) as well as bioaccumulation of the heavy metals in fish tissues were evaluated. Samples of water (n = 6) and fish (n = 6) were taken from Kirinda bridge and Ruliba station on Nyabarongo river and analyzed by UV spectroscopy and atomic absorption spectrometry, respectively. The heavy metal concentrations in water were: iron (0.63 ± 0.02 and 1.61 ± 0.03 mg/kg), manganese (0.53 ± 0.002 mg/kg at Ruliba station), chromium (0.06 ± 0.002 mg/kg at Kirinda bridge), cadmium (0.106 ± 0.002 mg/kg at Ruliba station) and lead (0.75 ± 0.02 and 0.051 ± 0.01 mg/kg). Edible muscles of Protopterus annectens contained 336.0 ± 0.70, 302.6 ± 1.22, 6.4 ± 0.26, 44.7 ± 0.20, 138.2 ± 0.17 and 302.4 ± 1.50 mg/kg of iron, manganese, copper, zinc, chromium and lead at Kirinda bridge and 272.8 ± 0.36, 292.2 ± 0.25, 8.8 ± 0.36, 135.2 ± 0.15, 148.0 ± 0.21 and 432. 0 ± 0.50 mg/kg of iron, manganese, copper, zinc, chromium and lead, respectively, at Ruliba station. Most of the heavy metal contents were above the recommended levels. Bioaccumulation factors recorded in Protopterus annectens ranged from 403.2 to 15,130 L/kg, implying that consumption of this fish could pose deleterious health risks. The study suggested that P. annectens could be used as a sentinel organism for biomonitoring of aquatic ecosystems.
PUBLIC INTEREST STATEMENT
Heavy metals are serious environmental contaminants which when consumed can have harmful health effects in humans. In the current study, the levels of some selected heavy metals in African lungfish consumed by the locals around Kigali city and Karongi district of Rwanda were determined. We found very high levels of heavy metals both in water and fish, and therefore consumption of this fish could pose serious health problems. The study indicates that interventions are necessary to reduce this contamination, especially by restricting the dumping of industrial and agricultural wastes into Nyabarongo river.
1. Introduction
Deterioration of water quality and heavy metal pollution of aquatic ecosystems due to natural and anthropogenic activities such as industrialization, climate variability, poor waste management, and high population growth rates have evoked concerns from international bodies as well as researchers (Angiro et al., Citation2020; Hossain et al., Citation2018; Meche et al., Citation2010; Oyeleke et al., Citation2018). Global population registered a sharp increase to 7.8 billion in 2020 following the industrial revolution and is expected to reach 9.7 billion by 2050 (Akresh et al., Citation2011). With 13.03 million inhabitants occupying about 26,336 km2 area (495 inhabitants per km2), Rwanda has the second‐highest population density in Africa (Imasiku & Ntagwirumugara, Citation2020; Mukanyandwi et al., Citation2018). In East Africa, it is the most densely populated country (Karamage et al., Citation2016a; UN, Citation2015). Several industries have sprung up that are causing pollution of water bodies due to discharge of untreated industrial wastes as well as sewage into surrounding water bodies (Dhiamba, Citation2016; Imasiku & Ntagwirumugara, Citation2020; Nhapi et al., Citation2011). Thus, conservation and efficient utilization of water to meet the ever-growing requirement by the population and industries is necessary and this can be achieved through constant monitoring of these water resources (Kiptum & Sang, Citation2017).
Environmental studies have reported about the pollution of major Rwandese rivers such as Mpazi, Nyabarongo, Rusine, Muvumba and Nyabugogo (Dusabe et al., Citation2019; Gasana et al., Citation1997; Nhapi et al., Citation2011; Niyonsenga et al., Citation2019; Nkuranga, Citation2007; Nshimiyimana et al., Citation2010; Omara et al., Citation2020; Usanzineza et al., Citation2009, Citation2011). The presence of toxic pollutants such as heavy metals in water and fish poses health threats when they are ingested (Al-Busaidi et al., Citation2011; Castro-González & Méndez-Armenta, Citation2008; Nakiguli et al., Citation2020). High levels of trace metals, including essential ones affect the activities of aquatic organisms as they can interact with key enzymes and cellular components (Copat et al., Citation2013; Qin et al., Citation2015; Rahman et al., Citation2012). Further, trace metals impact the immunity, respiratory, physiological and biochemical functions, as well as embryonic development of aquatic organisms (Feng et al., Citation2020).
Recently, heavy metals (manganese, zinc, copper, iron, nickel, lead and cadmium) were reported in the edible parts of Colocasia esculenta, Amaranthus spinosus, Ipomoea batata and soils from industrially active parts of Kigali, Rwanda (Etale & Drake, Citation2013; Hakizimana et al., Citation2019). In another study (Omara et al., Citation2020), we found that the quality of water and fish, particularly with respect to heavy metals from Nyabarongo and Nyabugogo rivers of Rwanda exceeded compliance guidelines. Health risk assessments further indicated that though consumption and dermal contact with heavy metal contaminated water may not pose obvious health risks, consumption of Protopterus annectens fetched from Nyabarongo river could have deleterious health effects as reflected by the cancer risk values being higher than the safety level of 1 × 10−4 for both adults and children. Nyabarongo river is currently facing pollution from Kigali sewage and industrial effluents, agricultural activities, sand and mineral mining (Dhiamba, Citation2016; Karamage et al., Citation2016b, Citation2016a). Though previous studies reported on water pollution of this river, none reported on the heavy metal content of fish from this river, and how the heavy metals are bioaccumulated in the tissues of fish consumed by natives surrounding the river. As an extension of our previous study (Omara et al., Citation2020), we report on the bioaccumulation of some selected heavy metals in the tissues of P. annectens to guide in environmental monitoring.
2. Materials and methods
2.1. Description of the study area
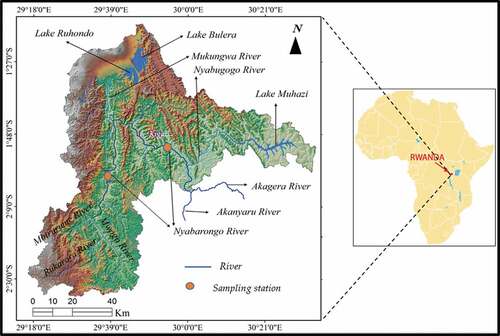
Rwanda is a landlocked East African country covering an expanse of 26,338 km2 on the Eastern shoulder of the Kivu-Tanganyika rift in Africa (Figure ). It lies between 1°4ʹ and 2°51ʹ South latitude and 28°53ʹ and 30°53ʹ East longitude (Karamage et al., Citation2016c). It shares common frontiers with Uganda, Burundi, Tanzania and Democratic Republic of the Congo. The current study was done in Nyabarongo (Nyawarungu) river which form part of the Rwandese drainage system. Nyabarongo river flows over 300 km from its source in Western Rwanda southwards to its outlet into Lake Rweru in South Eastern Rwanda along the border with Burundi. Its main tributary is Akanyaru river that flows from the highlands of Nyungwe National park on the Congo-Nile divide in former Ruhengeri province along the Rwanda-Burundi frontier. The river then flows Eastwards through swampy valleys and small lakes in the lowlands of Bugesera-Gisaka in South-Eastern Rwanda. The river traverses Kigali city through Nyarugenge district due to its numerous tributaries such as the Mwange, Rusine and Marenge rivers on its upstream portion. It is later fed by other rivers from the urbanized parts of Kigali such as Rwanzekuma, Ruganwa, Mpazi and Yanze. Floriculture, sugar processing at Kabuye works sugar industry, sugar cane plantations, agrarian and mining activities along this river are the major sources of anthropomorphic pollution (Nhapi et al., Citation2011).
2.2. Apparatus and reagents
The assortment of volumetric ware used were soaked overnight in 10% (v/v) nitric acid solution, rinsed with deionized water and oven dried prior to analysis. Mettler PM200 digital analytical balance (Marshall Scientific, Hampton, NH, USA) was used for all analytical weighing. Hanna 211 digital microprocessor-based benchtop pH/mV/OC meter (Hanna instruments, Italy) calibrated using pH 4.01, 7.01, 10 buffers was used for pH measurements. The analytical reagents used were sourced from Merck (Darmstadt, Germany). Water used as solvent was deionized water.
2.3. Ethical approval
Approval to carry out this study was granted by Department of Chemistry, College of Science and Technology, University of Rwanda, Kigali, Rwanda (Approval No. 213000076).
Figure 1. Map of Rwanda showing the location of Nyabarongo river and the sampled stations. Inset is the location of Rwanda on the African continent. Adapted from.Karamage et al. Citation(2016b)
2.4. Sampling and analysis of water and fish
Water was selected to determine the concentration of heavy metals being released into Nyabarongo river and the deterioration of water quality due to anthropogenic activities whereas fish was chosen because it constitutes the protein diet of many communities both in rural and urban areas. All samples were obtained in triplicate between April 2019 and May 2019 (10:00 am to 11:00 am, Central African Time). Samples were taken from Kirinda bridge in Karongi district, Murambi sector, Shyembe cell at the bridge joint of Ruhango and Karongi districts (20,404” S and 290020ʹ46” E) and Ruliba station in Kigali city, Nyarugenge district, Ruliba cell in Nyabarongo wetland near Nyabarongo bridge at Kigali-Butare road (1058ʹ37” S and 3000ʹ50” E) on Nyabarongo river.
Water samples (n = 1 for each site) were collected in triplicate in 500 ml Teflon plastic bottles. The bottles, previously cleaned by washing in non-ionic detergent were rinsed with tap water, soaked in 10% nitric acid for 72 h and finally rinsed with deionized water before use. Each bottle was rinsed 3 times with river water at each sampling point, filled 20–25 cm below the water surface and capped with airtight stoppers while still under water. The samples were placed in a cooler box with ice and transported to the laboratory where they were stored at 4 until commencement of analysis (Omara et al., Citation2019). The samples were digested by mixing 95 ml of water with 5 ml of 65% nitric acid and heated until around 10 ml of the initial solution was obtained. This concentrate was transferred into a clean 100 ml flask; the digestion bottle was then rinsed three times with volumes of 20 ml of distilled water, which were added to the concentrate, and finally the flask was filled up to the mark with distilled water. Heavy metals: iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), chromium (Cr), cadmium and lead (Pb) in water samples were quantified using HACH DR/2500 spectrophotometer (HACH, USA) following the manufacturer’s instructions (HACH, Citation2004).
West African lungfish samples (n = 3 for each site, 6.2 to 8.1 cm fork length; 703–905 g fresh weight) were caught using hooks fed with earthworms as baits with the help of local fishermen (Omara et al., Citation2018). The samples were taken in ice cool boxes, kept frozen at −20 in the laboratory and eviscerated within two days of collection. Drying of whole edible parts of the fish samples was done in an electric oven at 105°C for 48 hours. The edible muscles of the dried fish samples were separately pulverized using clean porcelain mortars and pestles to obtain fine fish powders. Aliquots (2.15 ± 0.01 g) of the homogenized fish powders were ashed at 550°C for 3 hours and subsequently transferred into 250 ml volumetric flasks. Measured 15 ml of 6 M Nitric acid and 5 ml of 5 M concentrated hydrochloric acid were added to the flasks and the samples were digested for 15 minutes, filtered into 100 ml standard volumetric flasks and made to the marks with sterile double distilled water (Omara et al., Citation2018). Prepared fish samples were analyzed for Fe, Mn, Cu, Zn, Cr, Cd and Pb using Atomic Absorption Spectrophotometer (Varian AA 240). This equipment afforded fast sequential capabilities with four lamps, and elemental analysis was perfected in a rapid sequence from one sample analysis. In addition, it afforded fully automatic wavelength and slit selection operation in the determination of the heavy metal concentrations (Miramar College, Citation2007; Research And Development, Citation2012). The results in mg/L from the instrument were converted to the standard unit for solid foods (mg/kg) for easy comparison with the set international compliance guidelines (Omara et al., Citation2018).
2.5. Assessment of bioaccumulation factors
Metal accumulation and uptake by living organisms can be determined by the use of bioconcentration factor (BCF). This is one of the multipliers that is used to estimate concentrations of chemicals that accumulate in tissues through any route of exposure (US EPA, Citation2000). Thus, the bioconcentration factor of the different metals was calculated as the ratio of the concentration of the respective heavy metals in the whole edible muscles of P. annectens to the concentration of the same metal in water (EquationEquation 1(1)
(1) ) (Ahmed et al., Citation2019; Benson et al., Citation2017; Morrison, Citation2000; Omara et al., Citation2019; Wang et al., Citation2017).
where Cb and Cw are concentrations of the heavy metals in fish and water, respectively.
2.6. Analytical quality assurance and quality control
All reagents used in this investigation were of analytical grade. Quality control was performed with spiked samples analyzed once for every 10 samples. Recovery percentages from the spiked samples ranged from 97.6% to 102.5%. Method detection limits with reagent blanks were calculated and used as a verification tool as well. The detection limits were 1.50, 0.20, 0.60, 2.50, 0.50, 1.14, 1.20 and 0.37 mg/kg for Fe, Mn, Cu, Zn, Cr, Cd and Pb, respectively. Bottle, analytical, equipment and filtration blanks were determined throughout the analyses and blank subtractions were used to correct the metal concentrations obtained. All samples were analyzed at least in triplicate to obtain a relative uncertainty of less than 5%.
2.7. Statistical analysis
Analytical data were presented as means with errors as standard deviations attached. Concentration of the heavy metals in water and fish were compared with their international compliance guidelines. T-tests were used to show the variation of data between the two sampling stations. All statistical analyses were performed at 95% confidence interval using Sigma Plot statistical software (v14, Systat software Inc., USA).
3. Results and discussion
3.1. Heavy metal content of water
The results of heavy metal analysis of water from Nyabarongo river are given in Table . It is known that the ionic composition of natural water depends on factors such as the chemistry of atmospheric precipitation, mineralogy of the rocks encountered along the flow path, residence time of the water, topography and climate (Guler et al., Citation2002; Li & Zhang, Citation2008; Mokhtar et al., Citation2009).
Table 1. Heavy metal content of water from Nyabarongo river, Rwanda
BDL: Below method detection limit. NC: Not of health concern at levels causing acceptability problems in drinking water but may affect acceptability of drinking water. Values in bold indicate exceedance of WHO guidelines.
As shown in Table , Fe and Mn have no set regulatory level in drinking water (Frisbie et al., Citation2012; WHO, Citation2017). However, Fe is found in natural fresh water at levels ranging from 0.5 to 50 mg/L (WHO, Citation2004). The levels of Fe reported at the sampled stations differed significantly (p < 0.05) and were lower than reported by previous studies in the same catchment. Usanzineza et al. (Citation2011) reported a mean value of 0.756 ± 0.734 mg/L for Fe at the Lake Muhazi outlet. Nhapi et al. (Citation2011) reported Fe levels of 8.76 ± 8.88 mg/L and 6.85 ± 5.92 mg/L in water samples from Rusine and Marenge rivers. Their study speculated that the pollution could have been due to the geological composition of red soils in the area. It was noted that inflows from Kigali city amplifies the concentration of Fe always recorded in Nyabugogo river, which is a tributary of Nyabarongo river. The levels of Fe recorded in this study are supported by a recent study (Hakizimana et al., Citation2019) which reported that the concentration of iron was as high as 2,896 mg/kg in soils taken from Nyabugogo downstream. Earlier, iron leachate was reported in the range of 1023.1 to 2005.8 mg/kg in clay cooking pots in Rwanda (Nsengimanaa et al., Citation2012). It is reported that iron is an inorganic water quality problem and is mainly transported in groundwater in the reduced ferrous form, Fe2+ (BGS, Citation2001). By comparison, Amadi (Citation2013), Eliku and Leta (Citation2018) reported lower Fe concentrations (0.011 to 2.897 and 1.11 to 4.12 mg/L) in water from Sosiani river (Kenya) and Awash river (Ethiopia) than is reported in Nyabarongo river in this study. Higher levels of Fe in water (12.6 to 15.51 mg/L) comparable to those reported in this study were reported in Mara river of Tanzania by Kihampa and Wenaty (Citation2013). The higher levels of iron reported in this study could be from natural sources or iron, steel and household equipment made of iron that are dumped in the Nyabarongo river. The water samples had dark brown colour especially for samples from Ruliba station. This could be due to the oxidation of iron in the ferrous form to ferric form and the formation of ferric hydroxide colloids and complexes (Chu et al., Citation1994).
On the other hand, Mn levels were higher (p < 0.05) in water from Ruliba station than that from Kirinda bridge. A study conducted in Rwanda in the same catchment recorded 10.28 ± 11.44 mg/L of Mn in water from Rwanzekuma river, 11.58 ± 11.46 mg/L in Ruganwa river, 28.85 ± 23.53 mg/L for Nyabarongo upstream and 25.56 ± 27.91 mg/L on Nyabarongo downstream water samples (Nhapi et al., Citation2011). Nzeyimana (Citation2008) in his study concluded that groundwater in Rwanda contained high levels of Mn and thus the high Mn levels recorded in this study could be due to the surrounding geological formation and disturbance of soils which causes discharge of manganese-rich runoffs (Nhapi et al., Citation2011). Manganese naturally occurs in many surface and groundwater sources, particularly in anaerobic or low oxidation conditions, and this is the most important source for exposure in drinking water. Its introduction into water may also be from other sources such as gasoline additive methylcyclopentadienyl manganese tricarbonyl, an organic derivative of Mn that was introduced into automobile fuel formulae as an octane boosting and antiknock agent to replace or reduce Pb content in petrol in some countries (Rollin & Nogueira, Citation2011). Though Mn is an essential trace metal in animals, excessive intake leads to cognitive, behavioural and neurodevelopmental effects that lower intelligent quotients and academic performance (Arvind, Citation2007; Bouchard et al., Citation2018; Khan et al., Citation2012; Rollin & Nogueira, Citation2011; Wasserman et al., Citation2006).
In this study, both copper and zinc at all the sampled stations were within compliance limits. Ingested at high concentrations, acute symptoms of copper poisoning include vomiting, hematemesis (vomiting blood), and gastrointestinal distress (Arvind, Citation2007). Similarly, zinc at concentrations above 4.0 mg/L imparts an undesirable astringent taste to water, and may cause nausea, vomiting, diarrhea, metallic taste, kidney, and stomach damage (Amiard et al., Citation2006). The occurrence of zinc in concentrations above 0.1 mg/L is usually due to zinc used in older galvanized plumbing materials, zinc compounds in form of zinc-based paints, zinc alloys, dry cells, old and rusty galvanized roofing iron sheets and varnishes that may end up in water courses (Omara et al., Citation2018; WHO, Citation2017). Chromium in water at Kirinda and Cd in samples from Ruliba station also exceeded the maximum permissible guidelines. Since water from Nyabarongo is used for domestic purposes and drinking, this may pose health threats. Cr is usually introduced in aquatic ecosystems in wastes from textile industries (Sanyal et al., Citation2015). In the study area, Usine Textile Du Rwanda (UTEXRWA) industry in Kigali is known to discharge its textile wastes into Nyabarongo river (Muhirwa et al., Citation2010). Cd is typically found at low concentrations in aquatic environments, however, indiscriminate use of phosphate fertilizer, dumping of nickel-cadmium batteries into water, Cd metal incineration and production might increase the Cd concentration level in the aquatic environments (Ahmed et al., Citation2019; Bennet-Chambers et al., Citation1999; Bustueva et al., Citation1994). Ingestion of significant amounts of Cd damages the liver and kidneys, causes high blood pressure, cancerous growths, reduced reproductive capacity and hepatic dysfunction in humans. Cadmium is also implicated in calcium uptake inhibition and impairment of its subsequent retention in bones (El-Moselhy et al., Citation2014; Omara et al., Citation2018).
The concentration of Pb in water from all the sampled stations was higher than the recommended guidelines. Nhapi et al. (Citation2011) reported that the high levels of Pb in Mpazi river (Nyabugogo catchment) of Rwanda could be from Nyabugogo tannery which uses a lot of chemicals and has many car parks in the area. High levels of Pb in Nyabugogo catchment were also reported by Usanzineza et al. (Citation2009) who hinted that the prevalence of Pb in this catchment warranted further investigations. Similar results have been reported in other African rivers; Mvungi et al. (Citation2003) reported 0.213 to 0.544 mg/L of Pb in water from an urban river in Zimbabwe while Okonkwo and Mothiba (Citation2005) reported 0.010 to 0.012 mg/L of Pb from three urban rivers in South Africa. The occurrence of Pb in urban waters has been reported to be due to use of leaded petrol and thoughtless dumping of dead car lead acid accumulators (Omara et al., Citation2018). Lead is a toxic non-essential trace metal even at meagre concentrations. Thus, the concentrations reported in this study is harmful to fish and humans when ingested in high doses (Badr et al., Citation2014). In addition, Pb is an antagonist that interferes with fundamental trace metals of comparable characteristics such as calcium and zinc. It is also associated with human kidney failure and liver degradation (Salem et al., Citation2000). Lead retards interactive, survival, growth, development and metabolic processes in addition to increasing mucus synthesis, inducing neurodevelopmental damage that causes decreased intelligence quotient and behavioural problems, as well as triggering nervous system disorders (Eisler, Citation1988; EPA, Citation2019). It may also cause reproductive problems in both men and women as well as premature birth and reduced foetal growth in pregnant women (EPA, Citation2019).
Generally, physicochemical parameters such as pH, redox potential and salinity affect the mobility of metals in water (Kelderman, Citation2000; Kelderman & Osman, Citation2007; Du Laing et al., Citation2008). The water samples collected from all the stations showed low salinity with mean values between 0.01 and 0.05 mg/L of chloride ions (Omara et al., Citation2020). This could explain the high concentrations of Fe, Pb and Mn recorded in the water samples (Sekomo et al., Citation2011).
3.2. Heavy metal content of protopterus annectens muscles
Although fish are migratory in an aquatic ecosystem, heavy metal accumulation in fish gives evidence of exposure to polluted aquatic environment (Qadir & Malik, Citation2011) and could be used to evaluate the health condition of the environment from which they are collected. Further, fish are good indicators of heavy metal contamination in aquatic systems because they occupy different trophic levels (Burger et al., Citation2002). The heavy metal content of the edible muscles of P. annectens from Nyabarongo river are shown in Table . Unprecedented levels of heavy metals were recorded in edible muscles of P. annectens from Nyabarongo river. The chemical sequences followed were Fe > Mn > Pb > Cr > Zn > Cu > Cd for samples from Kirinda bridge and Pb > Mn > Fe > Cr > Zn > Cu > Cd for samples from Ruliba station. These observations agreed well with the conclusions of Watanabe et al. (Citation2003) and Masoud et al. (Citation2007) that absorbency of heavy metals in animal tissues varies from one trace metal to another. All the heavy metal concentrations recorded in this study except that of Cu and Cd were above the recommended guidelines in fish recommended by FAO/WHO (Citation1989).
Table 2. Heavy metal concentrations in P. annectens from Nyabarongo river in comparison with other global studies
Published data indicate that fish ingest heavy metals by direct uptake in aqueous solution or by epithelial absorption of heavy metal contaminated water that sluices through their gills, skin, oral cavity and digestive tract (Burger et al., Citation2002). However, chronic intake of heavy metals by fish rest entirely on the metal concentration, volume of the ingested contaminated food or water, the heavy metal uptake speed, exposure duration, uptake route, ecological conditions external to the fish (including availability of water, temperature, pH) and innate factors such as fish age (Bawuro et al., Citation2018; Omara et al., Citation2019; Zeitoun & Mehana, Citation2014), fish nutritional habits as well as the dynamic processes involved in the trace metal metabolism when ingested (Koca et al., Citation2005). Thus, the high levels of heavy metals recorded in P. annectens in this study could be because it is a piscivorous species (Omara et al., Citation2019; Otuogbai et al., Citation2000). Protopterus annectens (popularly known as West African, African or Tana lungfish) feeds on small fish, molluscs, annelids, crustaceans and frogs (Otuogbai et al., Citation2000). Further, the fish samples were taken during the dry season and this is known to influence the concentration of heavy metals recorded in aquatic organisms (Mol et al., Citation2011; Nwani et al., Citation2009).
The results of this study are not in agreement with the report of Mohamed (Citation2014) in which P. annectens was reported to contain the least level of heavy metals (beryllium, boron and molybdenum) in both edible muscles and the head compared to the other species. Further, the levels of heavy metals reported in P. annectens in this study are higher than those reported by preceding authors in other parts of Africa (Table ). Only Alinnor (Citation2014) reported a zinc concentration of 211.33 mg/kg which was higher than the concentration of Zn reported in P. annectens by this study. Overall, the differences in the heavy metal concentrations in the fish samples might be attributed to the differences in the concentrations of the heavy metals in the rivers. Bioavailability of metals may also be influenced by physiological activities of fish, pollutant loads, water chemistry and other environmental factors (Qadir & Malik, Citation2011).
3.3. Bioaccumulation of heavy metals in protopterus annectens
The bioconcentration factor computed for P. annectens in the sampled stations of Nyabarongo river are presented in Table . The concentration of chemicals in aquatic organisms can be calculated by two factors: bioconcentration factor (BCF) and bioaccumulation factor (BAF). Both factors illustrate the partitioning of a chemical between water and aquatic organisms, often fish, at steady-state conditions (Karlsson et al., Citation2002). Bioconcentration factor refers to chemical levels in organisms only due to uptake by the organism from the surrounding water while BAF includes uptake from ingested food. In this study, Fe, Mn, Cr and Pb had BCF more than the recommended limits in edible tissues of freshwater fish especially at Kirinda bridge. Chromium had the highest BCF, about 11 times the recommended limit.
Table 3. Bioconcentration factor of the heavy metals in P. annectens from Nyabarongo river, Rwanda
NA-Not Applicable. Values in bold are higher than the maximum permissible limits.
Bioaccumulation is due to uptake and retention of elements in organisms and the process is usually complex to describe. The uptake of elements depends primarily on environmental conditions whereas the retention is dependent on biological features of the organisms (Ahmed et al., Citation2019; El-Moselhy et al., Citation2014; Karlsson et al., Citation2002; Nwabunike, Citation2016; Ozmen et al., Citation2008). The complexity of these processes may be one of the reasons why the range of the reported values for a given element can be very large in comparison to others (Karlsson et al., Citation2002). Further, the BCF based on dry weight are reported to be 3 to 10 times higher than those based on wet weight, depending on the organism and the sample preparation procedure employed. Ash weight-based values can be up to 100 times higher than those based on wet weight (IAEA, Citation1985).
Alinnor et al. (Citation2016) reported that the BCF of heavy metals in P. annectens were 130, 11, 11 and 27 for Cu, Cr, Cd and Pb, following the sequence Cu > Pb > Cd = Cr. In the current study, heavy metal bioconcentration in P. annectens followed the chemical sequence Mn > Cr > Fe > Zn > Pb > Cu = Cd at Kirinda bridge and Pb > Mn > Fe > Cu > Zn = Cr = Cd at Ruliba station. This observation seems to be in congruence with previous studies which indicated that essential elements tend to be persistent in fish muscles (Ahmed et al., Citation2019; Traina et al., Citation2019). Thus, the bioaccumulation capability of Cd usually takes a long time (Ahmed et al., Citation2019; Zhong et al., Citation2018). Such trace metal accumulation levels in fish as in this concerted study augment published data reported by preceding authors on different species of aquatic organisms (Ahmed et al., Citation2019; Avelar et al., Citation2000; Benson et al., Citation2017; Kwok et al., Citation2005; Omara et al., Citation2019; Zhao et al., Citation2012). Thus, this study suggests that P. annectens could be used as a sentinel organism for biomonitoring of aquatic ecosystems for public health and safety. A bioconcentration factor greater than 1 is indicative that the contaminant is a hydrophobic or lipophilic chemical. It is an indicator of how probable a chemical is able to bioaccumulate, and such chemicals possess high lipid affinities and will thus concentrate in tissues with high lipid content instead of an aqueous environment like the cytosol. Models are used to predict chemical partitioning in the environment which in turn allows the prediction of the biological fate of such lipophilic chemicals (Landis et al., Citation2011). Such models are based on assumptions that chemicals partition between water and aquatic organisms and that chemical equilibrium exists between the organisms and the aquatic environment in which they are found. Overall, chemicals with high BCF values are more lipophilic and at equilibrium, such organisms will have greater concentrations of the chemicals than other phases in the system. Body burden, the total amount of chemical in the body of such an organism becomes greater when dealing with a lipophilic chemical and this may pose health risks if such organisms are eaten by humans (Kalantzi et al., Citation2016; Makedonski et al., Citation2017).
4. Conclusion
This study has shown that Nyabarongo river water and fish are polluted with heavy metals with lead being the major pollutant in concentrations exceeding permissible levels. Nyabarongo river should therefore be protected from anthropogenic activities such as mining and agricultural activities carried out along its banks to mitigate water pollution with heavy metals and agricultural chemicals. Further studies during different seasons of the year should be done to monitor the variation of the heavy metal content of the edible muscles of P. annectens as well as evaluate the trace metal content of its metabolically active organs (gills, liver, kidney). The microbiological quality of water from the studied river is worth assessing.
Abbreviations
BAF: bioaccumulation factor; BCF: bioconcentration factor; FAO: Food and Agriculture Organization of the United Nations; P. annectens: Protopterus annectens; WHO: World Health Organization.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors’ contributions
Both authors designed the study. PN collected and analyzed the samples and performed data analyses. TO performed literature search and wrote the first draft of the manuscript. Both authors revised and approved the final manuscript.
Supplemental Material
Download MS Excel (12.1 KB)Acknowledgements
The authors are grateful to the World Bank and the Inter-University Council of East Africa (IUCEA) for the scholarship awarded to them through the Africa Centre of Excellence II in Phytochemicals, Textiles and Renewable Energy (ACE II PTRE) at Moi University, Kenya that made this communication possible. The support of Department of Chemistry, University of Rwanda is greatly acknowledged for the analytical success of this research.
Supplementary materials
Supplementary data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Timothy Omara
Papias Nteziyaremye is a research fellow at Moi University, Eldoret, Kenya. He holds a MSc (Analytical Chemistry) from Moi University, Kenya and a BSc (Environmental Chemistry) from University of Rwanda, Kigali, Rwanda. Timothy Omara is a researcher with Moi University and a Quality Assurance Specialist with the Product Development Directory of AgroWays Uganda Limited, Jinja, Uganda. He holds a MSc (Analytical Chemistry) from Moi University, Kenya and a BSc (Chemistry) from Kyambogo University, Kampala, Uganda. The authors are both currently engaged in research at the Africa Center of Excellence in Phytochemicals, Textiles and Renewable Energy (ACEII-PTRE), Moi University, Kenya.
References
- Ahmed, A. S. S., Sultana, S., Habib, A., Ullah, H., Musa, N., Hossain, M. B., Sarker, M. S. I., & Rahman, M. M. (2019). Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PloS One, 14(10), e0219336. https://doi.org/10.1371/journal.pone.0219336
- Akresh, R., Verwimp, P., & Bundervoet, T. (2011). Civil war, crop failure, and child stunting in Rwanda. Economic Development and Cultural Change, 59(4), 777–16. https://doi.org/10.1086/660003
- Al-Busaidi, M., Yesudhason, P., Al-Mughairi, S., Al-Rahbi, W. A., Al-Harthy, K. S., Al-Mazrooei, N. A., & Al-Habsi, S. H. (2011). Toxic metals in commercial marine fish in Oman with reference to national and international standards. Chemosphere, 85(1), 67–73. https://doi.org/10.1016/j.chemosphere.2011.05.057
- Alinnor, I. J. (2014). Trace metal distribution in fish, sediment and water samples from Nkisa river, Nigeria. British Journal of Applied Science and Technology, 4(20), 2901–2913. https://doi.org/10.9734/BJAST/2014/8199
- Alinnor, I. J., Ayuk, A. A., & Igbomezie, M. C. (2016). Distribution of elemental contaminants in water and fish samples from Oguta Lake, Nigeria. International Journal of Fish and Aquatic Studies, 4(3), 659–664. http://www.fisheriesjournal.com/archives/?year=2016&vol=4&issue=3&part=I&ArticleId=806
- Amadi, E. K. (2013). Nutrient loads and heavy metals assessment along Sosiani River, Kenya. Chemical Materials Research, 3(12), 14–20. https://iiste.org/Journals/index.php/CMR/article/view/8654
- Amiard, J., Amiard-Triquet, C., Barka, S., Pellerin, J., & Rainbow, P. S. (2006). Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquatic Toxicology, 76(2), 160–202. https://doi.org/10.1016/j.aquatox.2005.08.015
- Angiro, C., Abila, P. P., & Omara, T. (2020). Effects of industrial effluents on the quality of water in Namanve stream, Kampala Industrial and Business Park, Uganda. BMC Research Notes, 13(1), 220. https://doi.org/10.1186/s13104-020-05061-x
- Arvind, K. (2007). Ecobiology of Polluted Waters. Daya Publishing House, India, 1–291.
- Atuanya, E. I., Edefetah, M. A., & Nwogu, N. A. (2011). Microbiological qualities and some heavy metals (Mercury and Cadmium) levels of fresh and dry fish species sold in Benin City, Edo State, Nigeria. Bulletin of Environment, Pharmacology & Life Sciences, 1(1), 10–14. http://www.bepls.com/dec2011/3.pdf
- Avelar, W. E. P., Mantelatto, F. L. M., Tomazelli, A. C., Silva, D. M. L., Shuhama, T., & Lopes, J. L. C. (2000). The marine mussel Perna perna (Mollusca, Bivalvia, Mytilidae) as an indicator of contamination by heavy metals in the Ubatuba Bay, Sao Paulo, Brazil. Water, Air, and Soil Pollution, 118(1/2), 65–72. https://doi.org/10.1023/A:1005109801683
- Badr, A. M., Mahana, N. A., & Eissa, A. (2014). Assessment of heavy metal levels in water and their toxicity in some tissues of nile tilapia (Oreochromis niloticus) in river nile basin at Greater Cairo, Egypt. Global Veterinaria, 13(4), 432–443. https://doi.org/10.5829/idosi.gv.2014.13.04.8561
- Bawuro, A. A., Voegborlo, R. B., & Adimado, A. A. (2018). Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa State, Nigeria. Journal of Environmental and Public Health, 2018, 1–7. https://doi.org/10.1155/2018/1854892
- Bennet-Chambers, M., Davies, P., & Knott, B. (1999). Cadmium in aquatic ecosystems in Western Australia: A legacy of nutrient-deficient soils. Journal of Environmental Management, 57(4), 283–295. https://doi.org/10.1006/jema.1999.0304
- Benson, N. U., Anake, W. U., Essien, J. P., Enyong, P., & Olajire, A. A. (2017). Distribution and risk assessment of trace metals in Leptodius exarata, surface water and sediments from Douglas Creek in the Qua Iboe Estuary. Journal of Taibah University for Science, 11(3), 434–449. https://doi.org/10.1016/j.jtusci.2016.08.004
- BGS. (2001). Groundwater quality: Uganda, London, British Geological Survey.
- Bouchard, M. F., Surette, C., Cormier, P., & Foucher, D. (2018). Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology, 64, 110‐117. https://doi.org/10.1016/j.neuro.2017.07.024
- Burger, J., Gaines, K. F., Boring, C. S., Stephens, W. L., Snodgrass, J., Dixon, C., McMahon, M., Shukla, S., Shukla, T., & Gochfeld, M. (2002). Metal levels in fish from the Savannah river: Potential hazards to fish and other receptors. Environmental Research, 89(1), 85–97. https://doi.org/10.1006/enrs.2002.4330
- Bustueva, K. A., Revich, B. A., & Bezpalko, L. E. (1994). Cadmium in the environment of three Russian cities and in human hair and urine. Archives of Environmental Health: An International Journal, 49(4), 284–288. https://doi.org/10.1080/00039896.1994.9937481
- Castro-González, M. I., & Méndez-Armenta, M. (2008). Heavy metals: Implications associated to fish consumption. Environmental Toxicology and Pharmacology, 26(3), 263–271. https://doi.org/10.1016/j.etap.2008.06.001
- Chu, L. M., Cheung, K. C., & Wong, M. H. (1994). Variations in the chemical properties of landfill leachate. Environmental Management, 18(1), 105–117. https://doi.org/10.1007/BF02393753
- Copat, C., Arena, G., Fiore, M., Ledda, C., Fallico, R., Sciacca, S., & Ferrante, M. (2013). Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food and Chemical Toxicology, 53, 33–37. https://doi.org/10.1016/j.fct.2012.11.038
- Dhiamba, D. (2016). Who will save rwanda’s lifeline-the 300 Km Nyabarongo river ecosystem? https://medium.com/@dhimbara/who-will-save-rwanda-s-lifeline-the-300-km-nyabarongo-river-ecosystem-5d4d1278c9e7.
- Du Laing, G., De Vos, R., Vandecasteele, B., Lesage, E., Tack, F. M. G., & Verlooa, M. G. (2008). Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuarine, Coastal and Shelf Science, 77(4), 589–602. https://doi.org/10.1016/j.ecss.2007.10.017
- Dusabe, M., Wronski, T., Gomes-Silva, G., Plath, M., Albrecht, C., & Apio, A. (2019). Biological water quality assessment in the degraded Mutara rangelands, Northeastern Rwanda. Environmental Monitoring and Assessment, 191(3), 139. https://doi.org/10.1007/s10661-019-7226-5
- Eisler, R. (1988). Lead hazards to fish, wildlife and invertebrates: A synoptic review. In; Contaminant Hazard Reviews, Report 14; Biological Report 85(1.14). U.S. Department of the Interior, Fish and Wildlife Service, 1–14.
- Eliku, T., & Leta, S. (2018). Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Applied Water Science, 8(6), 177. https://doi.org/10.1007/s13201-018-0803-x
- El-Moselhy, K. M., Othman, A. I., Abd El-Azem, H., & El-Metwally, M. E. A. (2014). Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian Journal of Basic and Applied Sciences, 1(2), 97–105. https://doi.org/10.1016/j.ejbas.2014.06.001
- EPA. (2019). Basic Information about Lead in Drinking Water. Environmental Protection Agency. https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water.
- Etale, A., & Drake, D. C. (2013). Industrial pollution and food safety in Kigali, Rwanda. International Journal of Environmental Research, 7, 403–406. https://doi.org/10.22059/IJER.2013.619
- FAO/WHO. (1989). WHO technical report series No 505, Evaluation of certain food additives and the contaminants, mercury, lead and cadmium for environment monitory report No 52 center for environment. Tech. Rep., Fisheries And Aquaculture Science Lowest Tofit UK.
- Feng, W., Wang, Z., Xu, H., Chen, L., & Zheng, F. (2020). Trace metal concentrations in commercial fish, crabs, and bivalves from three lagoons in the South China Sea and implications for human health. Environmental Science and Pollution Research, 27(14), 16393–16403. https://doi.org/10.1007/s11356-019-06712-8
- Frisbie, S. H., Mitchell, E. J., Dustin, H., Maynard, D. M., & Sarkar, B. (2012). World Health Organization discontinues its drinking-water guideline for manganese. Environmental Health Perspectives, 120(6), 775‐778. https://doi.org/10.1289/ehp.1104693
- Gasana, J., Twagilimana, L., Hallenbeck, W., & Brenniman, G. (1997). Industrial discharges of metals in kigali, rwanda, and the impact on drinking water quality. Bulletin of Environmental Contamination and Toxicology, 58(4), 523–526. https://doi.org/10.1007/s001289900366
- Guler, C., Thyne, G. D., McCray, J. E., & Turner, K. A. (2002). Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology Journal, 10(4), 455–474. https://doi.org/10.1007/s10040-002-0196-6
- HACH. (2004). Model DR/2500 Laboratory Spectrophotometer. https://www.hach.com/asset-get.download.jsa?id=7639984433.
- Hakizimana, P., Maniragaba, A., & Nshimiyimana, F. X. (2019). Assessment of heavy metals in amaranthus Spinosus, Kigali, Rwanda. International Journal of Advanced Research and Publications, 3(10), 7–12. http://www.ijarp.org/paper-details.php?ref_number=RP0619-2461
- Hossain, M. B., Ahmed, A. S. S., & Sarker, M. S. I. H. (2018). Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environmental Science and Pollution Research, 25(31), 31727–31736. https://doi.org/10.1007/s11356-018-3158-9
- IAEA. (1985). Sediment Kds and concentration factors for radionuclides in the marine environment. IAEA, Austria, Vienna. Technical Reports Series No. 247.
- Ikape, S. I., Solomon, S. G., & Ed-Idoko, J. (2018). Proximate and Macro Element Composition of Four Fish Species from Lower River Benue Makurdi Benue State Nigeria. Journal of Nutritional Health & Food Science, 6(3), 1–6. https://doi.org/10.15226/jnhfs.2018.001127
- Imasiku, K., & Ntagwirumugara, E. (2020). An impact analysis of population growth on energy-water-food-land nexus for ecological sustainable development in Rwanda. Food and Energy Security, 9(1), e185. https://doi.org/10.1002/fes3.185
- Kalantzi, I., Pergantis, S. A., Black, K. D., Shimmield, T. M., Papageorgiou, N., Tsapakis, M., & Karakassis, I. (2016). Metals in tissues of seabass and seabream reared in sites with oxic and anoxic substrata and risk assessment for consumers. Food Chemistry, 194, 659–670. https://doi.org/10.1016/j.foodchem.2015.08.072
- Karamage, F., Zhang, C., Kayiranga, A., Shao, H., Fang, X., Ndayisaba, F., Nahayo, L., Mupenzi, C., & Tian, G. (2016b). USLE-based assessment of soil erosion by water in the Nyabarongo River Catchment, Rwanda. International Journal of Environmental Research and Public Health, 13(8), 835. https://doi.org/10.3390/ijerph13080835
- Karamage, F., Zhang, C., Ndayisaba, F., Nahayo, L., Kayiranga, A., Omifolaji, J. K., Shao, H., Umuhoza, A., Nsengiyumva, J. B., & Liu, T. (2016c). The need for awareness of drinking water loss reduction for sustainable water resource management in Rwanda. Journal of Geoscience and Environment Protection, 4(10), 74–87. https://doi.org/10.4236/gep.2016.410005
- Karamage, F., Zhang, C., Ndayisaba, F., Shao, H., Kayiranga, A., Fang, X., Nahayo, L., Muhire Nyesheja, E., & Tian, G. (2016a). Extent of cropland and related soil erosion risk in Rwanda. Sustainability, 8(7), 609. https://doi.org/10.3390/su8070609
- Karlsson, S., Meili, M., Eco, U. B. S., & Safety, A. (2002). Bioaccumulation factors in aquatic ecosystems: A critical review. July 2002.
- Kelderman, P. (2000). Pollution assessment of the canal sediments in the city of Delft (The Netherlands). Water Research, 34(3), 936–944. https://doi.org/10.1016/S0043-1354(99)00218-3
- Kelderman, P., & Osman, A. A. (2007). Effect of redox potential on heavy metal binding forms in polluted canal sediments in Delft (The Netherlands). Water Research, 41(18), 4251–4261. https://doi.org/10.1016/j.watres.2007.05.058
- Khan, K., Wasserman, G. A., Liu, X., Ahmed, E., Parvez, F., Slavkovich, V., Levy, D., Mey, J., van Geen, A., Graziano, J. H., & Factor-Litvak, P. (2012). Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology, 33(1), 91–97. https://doi.org/10.1016/j.neuro.2011.12.002
- Kihampa, C., & Wenaty, A. (2013). Impact of mining and farming activities on water and sediment quality of the Mara river basin, Tanzania. Research Journal of Chemical Sciences, 3(7), 15–24. http://www.isca.in/rjcs/Archives/v3/i7/3.ISCA-RJCS-2013-060.php
- Kiptum, A. R., & Sang, C. C. (2017). Determinants of groundwater retention in wells: A case of Keiyo North district, Elgeyo Marakwet County, Kenya. Journal of Water Security, 3(1), jws2017007. https://doi.org/10.15544/jws.2017.007
- Koca, Y. B., Koca, S. Y., Gürcü, S., Osanç, B., Tunçbaş, E., Aksoy, G., & Aksoy, G. (2005). Investigation of histopathological & cytogenetic effects on Lepomis gibbosus (Pisces: Perciformes) in the Cine stream (Aydin/Turkey) with determination of water pollution. Environmental Toxicology, 20(6), 560–571. https://doi.org/10.1002/tox.20145
- Kwok, C. K., Liang, Y., Wang, H., Dong, Y. H., Leung, S. Y., & Wong, M. H. (2005). Bioaccumulation of heavy metals in fish and Ardeid at Pearl River Estuary, China. Ecotoxicology and Environmental Safety, 106, 62–67. https://doi.org/10.1016/j.ecoenv.2014.04.016
- Landis, W. G., Sofield, R. M., & Yu, M. H. (2011). Introduction to environmental toxicology: Molecular structures to ecological landscapes (4th ed.) CRC Press.
- Li, S., & Zhang, Q. (2008). Geochemistry of the upper Han River basin, China, 1: Spatial distribution of major ion compositions and their controlling factors. Applied Geochemistry, 23(12), 3535–3544. https://doi.org/10.1016/j.apgeochem.2008.08.012
- Makedonski, L., Peycheva, K., & Stancheva, M. (2017). Determination of some heavy metal of selected black sea fish species. Food Control, 72, 313–318. https://doi.org/10.1016/j.foodcont.2015.08.024
- Masoud, M. S., El-Samra, M. I., & El-Sadawy, M. M. (2007). Heavy-metal distribution and risk assessment of sediment and fish from El-Mex Bay, Alexandria, Egypt. Chemical Ecology, 23(3), 201–216. https://doi.org/10.1080/02757540701339760
- Meche, A., Martins, M. C., Lofrano, B. E. S. N., Hardawaya, C. J., Merchanta, M., & Verdadeb, L. (2010). Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Microchemical Journal, 94(2), 171–174. https://doi.org/10.1016/j.microc.2009.10.018
- Miramar College. (2007). Using the AA240 atomic absorption spectroscopy. AAS Spectrometer Setup Instructions. http://faculty.sdmiramar.edu/fgarces/LabMatters/Instruments/AA/AAS_Operation/AATutorial.pdf?products/spectr/aa/s140240/s140240&cid=ILKLJLQHFQ.
- Mohamed, H. E. A. (2014). Bioaccumulation of heavy metals in muscle tissues and head of some commercial nile fish in sudan. International Journal of Aquaculture, 4(20), 118–122. https://doi.org/10.5376/ija.2014.04.0020
- Mokhtar, M., Aris, A. Z., Abdullah, M. H., Yusoff, M. K., Abdullah, M. P., Idris, A. R., & Uzir, R. I. R. (2009). A pristine environment and water quality in perspective: Maliau Basin, Borneo’s mysterious world. Water and Environment Journal, 23(3), 219–228. https://doi.org/10.1111/j.1747-6593.2008.00139.x
- Mol, J. H., Ramlal, J. S., Lietar, C., & Verloo, M. (2011). Mercury contamination in freshwater, estuarine, and marine fishes in relation to small-scale gold mining in Suriname, South America. Environmental Research, 86(2), 183–197. https://doi.org/10.1006/enrs.2001.4256
- Morrison, H. A. (2000). Biococentration and biomagnification in the aquatic environment. In R. S. Boethling & D. Mackay (Eds.), Handbook of property estimation methods for chemicals: environmental and health sciences (pp. 189–231). Lewis.
- Muhirwa, D., Nhapi, I., Wali, U. G., Banadda, N., Kashaigili, J. J., & Kimwaga, R. (2010). Characterisation of wastewater from the Nyabugogo Abattoir, Rwanda and the impact on downstream water quality. International Journal of Ecological Development, 16(10), 30–46. http://repository.udsm.ac.tz:8080/xmlui/bitstream/handle/20.500.11810/3409/Characterization%20of%20wastewater%20from%20an%20Abattoir%20in%20Rwanda%20and%20the%20impact%20on%20downstream%20water%20quality.pdf?sequence=1&isAllowed=y
- Mukanyandwi, V., Nahayo, L., Hakorimana, E., Gasirabo, A., & Otgon, S. (2018). Review on water resources management and key threats in Rwanda, East Africa. Journal of Water Security, 4, jws2018003. https://doi.org/10.15544/jws.2018.003
- Mvungi, A., Hranova, R. K., & Love, D. (2003). Impact of home industries on water quality in a tributary of the Marimba River, Harare: Implications for urban water management. Physics and Chemistry of the Earth, 28(20–27), 1131–1137. https://doi.org/10.1016/j.pce.2003.08.034
- Nakiguli, C. K., Ojok, W., Omara, T., Wasswa, J., & Ntambi, E. (2020). Mobility of chromium, copper and arsenic in amended chromated copper arsenate contaminated soils. BMC Research Notes, 13(1), (in press).
- Nhapi, I., Wali, U. G., Uwonkunda, B. K., Nsengimana, H., Banadda, N., & Kimwaga, R. (2011). Assessment of water pollution levels in the Nyabugogo catchment Rwanda. The Open Environmental Engineering Journal, 4(1), 40–53. https://doi.org/10.2174/1874829501104010040
- Niyonsenga, J. D., Birame, C. S., & Maniragaba, A. (2019). Rainy seasonal analysis of Physico-chemical parameters of Mukungwa River at NGARU point. International Journal of Environmental & Agriculture Research, 5(8), 21–28. https://doi.org/10.5281/zenodo.3383019
- Nkuranga, E. (2007). Heavy metal removal and accumulation by an Urban Natural Wetland: The Nyabugogo Swamp, Rwanda. MSc Thesis, UNESCO-IHE Institute for Water Education.
- Nsengimanaa, H., Munyentwalia, A., Muhayimanaa, P., & Muhizia, T. (2012). Assessment of heavy metals leachability from traditional clay pots “inkono”and “ibibindi” used as food contact materials. Rwanda Journal, 25(1), 52–65. https://doi.org/10.4314/rj.v25i1.4
- Nshimiyimana, F., Nhapi, I., Wali, U. G., Nsengimana, H., Banadda, N., Nansubuga, I., & Kansiime, F. (2010). Assessment of heavy metal pollution in a trans-boundary river: The case of the Akagera River. International Journal of Mathematics and Computation, 9(10), D10.
- Nwabunike, M. O. (2016). The Effects of bioaccumulation of heavy metals on fish fin over two years. Journal of Fisheries & Livestock Production, 4, 170. https://doi.org/10.4172/2332-2608.1000170
- Nwani, C. D., Nwoye, V. C., Afiukwa, J. N., & Eyo, J. E. (2009). Assessment of heavy metals concentrations in the tissues (gills and muscles) of six commercially important fresh water fish species of Anambra River South-East Nigeria. Asian Journal of Microbiology, Biotechnology and Environmental Sciences, 11(1), 7–12. http://www.envirobiotechjournals.com/article_abstract.php?aid=4232&iid=150&jid=1
- Nzeyimana, V. (2008). Assessment of groundwater quality in nyagatare and gatsibo Districts, Rwanda Unpublished MSc Thesis, National University of Rwanda.
- Okonkwo, J. O., & Mothiba, M. (2005). Physico-chemical characteristics and pollution levels of heavy metals in the rivers in Thohoyandou, South Africa. Journal of Hydrology, 308(1–4), 122–127. https://doi.org/10.1016/j.jhydrol.2004.10.025
- Omara, T., Karungi, S., Kalukusu, R., Nakabuye, B. V., Kagoya, S., & Musau, B. (2019). Mercuric pollution of surface water, superficial sediments, Nile Tilapia (Oreochromis nilotica Linnaeus 1758 [Cichlidae]) and yams (Dioscorea alata) in auriferous areas of Namukombe stream, Syanyonja, Busia, Uganda. PeerJ, 7(10), e7919. https://doi.org/10.7717/peerj.7919
- Omara, T., Nteziyaremye, P., Akaganyira, S., Opio, D. W., Karanja, L. N., Nyangena, D. M., Kiptui, B. J., Ogwang, R., Epiaka, S. M., Jepchirchir, A., & Maiyo, A. (2020). Physicochemical quality of water and health risks associated with consumption of African lung fish (Protopterus annectens) from Nyabarongo and Nyabugogo rivers, Rwanda. BMC Research Notes, 13(1), 66. https://doi.org/10.1186/s13104-020-4939-z
- Omara, T., Ogwang, R., Ndyamuhaki, S., Kagoya, S., Kigenyi, E., Musau, B., & Adupa, E. (2018). Spectroscopic analysis of selected priority trace metals in the Extant East African Gilled Lungfish (Protopterus amphibius) in Lira Municipal Lagoon and Its Edibility Health Risk. Science Journal of Analytical Chemistry, 6(5), 38–45. https://doi.org/10.11648/j.sjac.20180605.11
- Otuogbai, T. M., Ekhenoba, A., & Elakhame, L. (2000). Food and feeding habits of the African lungfish, Protopterus annectens (Owens) (Pisces: Sarcopterygii) in the flood plains of River Niger in Etsako east of Edo State, Nigeria. African Journal of Tropical Hydrobiology and Fisheries, 10(1), 14–26. https://doi.org/10.4314/ajthf.v10i1.1397
- Oyeleke, P. O., Okparaocha, F. J., & Abiodun, O. A. (2018). Human health risk assessment of heavy metals (Lead, Cadmium and Copper) in fresh water tilapia fish (Oreochromis niloticus) from Eleyele River, Ibadan, Southwestern Nigeria. Chemistry Research Journal, 3(4), 134–142. http://chemrj.org/download/vol-3-iss-4-2018/chemrj-2018-03-04-134-142.pdf
- Ozmen, M., Ayas, Z., Gungordu, A., Ekmekci, G. F., & Yerli, S. (2008). Ecotoxicological assessment of water pollution in Sariyar Dam Lake, Turkey. Ecotoxicology and Environmental Safety, 70(1), 163–173. https://doi.org/10.1016/j.ecoenv.2007.05.011
- Qadir, A., & Malik, R. N. (2011). Heavy metals in eight edible fish species from two polluted Tributaries (Aik and Palkhu) of the River Chenab, Pakistan. Biological Trace Element Research, 143(3), 1524–1540. https://doi.org/10.1007/s12011-011-9011-3
- Qin, D., Jiang, H., Bai, S., Tang, S., & Mou, Z. (2015). Determination of 28 trace elements in three farmed cyprinid fish species from Northeast China. Food Control, 50, 1–8. https://doi.org/10.1016/j.foodcont.2014.08.016
- Rahman, M. S., Molla, A. H., Saha, N., & Rahman, A. (2012). Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chemistry, 134(4), 1847–1854. https://doi.org/10.1016/j.foodchem.2012.03.099
- Research And Development. (2012). Atomic absorption spectrometer. https://www.iitk.ac.in/dordold/index.php?option=com_content&view=featured&Itemid=158.
- Rollin, H. B., & Nogueira, C. M. C. A. (2011). Manganese: Environmental Pollution and Health Effects. In J. O. Nriagu (Ed.), Encyclopedia of Environmental Health (pp. 617–629). Elsevier.
- Salem, H. M., Eweida, E. A., & Farag, A. (2000). Heavy metals in drinking water and their environmental impact on human health. In The International Conference for Environmental Hazard Mitigation ICEHM 2000. 9–12 September 2000. Cairo University.
- Sanyal, T., Kaviraj, A., & Saha, S. (2015). Deposition of chromium in aquatic ecosystem from effluents of handloom textile industries in Ranaghat-Fulia region of West Bengal, India. Journal of Advanced Research, 6(6), 995–1002. https://doi.org/10.1016/j.jare.2014.12.002
- Sekomo, C. B., Nkuranga, E., Rousseau, D. P. L., & Lens, P. N. L. (2011). Fate of heavy metals in an urban natural wetland: The Nyabugogo swamp (Rwanda). Water, Air, & Soil Pollution, 214(1–4), 321–333. https://doi.org/10.1007/s11270-010-0426-9
- Traina, A., Bono, G., Bonsignore, M., Falco, F., Giuga, M., Quinci, E. M., Vitale, S., & Sprovieri, M. (2019). Heavy metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): Potential human health risk estimation. Ecotoxicology and Environmental Safety, 168, 466–478. https://doi.org/10.1016/j.ecoenv.2018.10.056
- UN. (2015). World population prospects: The 2015 revision, population division, Desa. United Nations. http://esa.un.org/unpd/wpp/
- US EPA. (2000). Bioaccumulation testing and interpretation for the purpose of sediment quality assessment. U.S. Environmental Protection Agency, EPA-823-R-00-001.
- Usanzineza, D., Nhapi, I., Wali, U. G., Kashaigili, J. J., & Banadda, N. (2009). Distribution of heavy metals in Lake Muhazi, Rwanda. 10th WaterNet/WARFSA/GWP Symposium, IWRM: Environmental Sustainability, Climate Change and Livelihoods. October.
- Usanzineza, D., Nhapi, I., Wali, U. G., Kashaigili, J. J., & Banadda, N. (2011). Nutrients Inflow and levels in Lakes: A case study of Lake Muhazi, Rwanda. International Journal of Ecology and Development, 19(2), 53–62. http://www.ceser.in/ceserp/index.php/ijed/article/view/1722
- Wang, Q., Chen, M., Shan, G., Chen, P., Cui, S., Yi, S., & Zhu, L. (2017). Bioaccumulation and biomagnification of emerging bisphenol analogues in aquatic organisms from Taihu Lake, China. Science of the Total Environment, 598, 814‐820. https://doi.org/10.1016/j.scitotenv.2017.04.167
- Wasserman, G. A., Liu, X., Parvez, F., Ahsan, H., Levy, D., Factor-Litvak, P., Kline, J., van Geen, A., Slavkovich, V., LoIacono, N. J., Cheng, Z., Zheng, Y., & Graziano, J. H. (2006). Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environmental Health Perspectives, 114(1), 124–129. https://doi.org/10.1289/ehp.8030
- Watanabe, K. H., Desimone, F. W., Thiyagarajah, A., Hartley, W. R., & Hindrichs, A. E. (2003). Fish tissue quality in the lower Mississippi River and health risks from fish consumption. Science of Total Environment, 302(1–3), 109–126. https://doi.org/10.1016/S0048-9697(02)00396-0
- WHO. (2004). Guidelines for drinking-water quality, Third edition, incorporating first addendum (third edition). World Health Organization. Retrieved from http://www.who.int/water_sanitation_health
- WHO. (2017). Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. World Health Organization.
- Zeitoun, M. M., & Mehana, E. (2014). Impact of water pollution with heavy metals on fish health: Overview and updates. Global Veterinaria, 12(2), 219–231. doi: 10.5829/idosi.gv.2014.12.02.82219
- Zhao, S., Feng, C., Quan, W., Chen, X., Niu, J., & Shen, Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Marine Pollution Bulletin, 64(6), 1163–1171. https://doi.org/10.1016/j.marpolbul.2012.03.023
- Zhong, W., Zhang, Y., Wu, Z., Yang, R., Chen, X., Yang, J., & Zhu, L. (2018). Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicology and Environmental Safety, 157, 343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048

