Abstract
Open disposal of battery slag is a major cause of heavy metals (HMs) pollution in soil. The current decontamination option is soil washing with chemicals whose efficiency is limited owing to high cost of reagents. This prompted the need for an environmentally friendly approach to remediate the contaminated soil. Therefore, the potential of Ganoderma lucidum to remediate heavy metals from an abandoned battery slag was investigated in this study. The heavy metals absorbed fungi were immobilised in bricks. The battery slag contaminated and control soil samples were analysed for HM concentrations before and after incubation with G. lucidium for 1–3 months. The harvested rice straw and mycelia were processed and analysed for HMs concentrations. For immobilizing heavy metals absorbed in mycelia, 5 to 30 g of pulverized mycelia were homogenized with a virgin soil, extruded into moulds and fired in a tunnel kiln for making bricks. The leachability of HMs from the bricks was carried out using TLCP. The concentrations (mg/kg) of Pb(4490 ± 14), Zn(147 ± 11), Ni(27.7 ± 0.2), Cu(19.4 ± 0.1) and Cd(2.18 ± 0.06) in dumpsite soil were significantly higher than the corresponding concentrations in the control soil samples. The G. lucidum inoculated on contaminated soil accumulated 138, 29.8, 3.48, 3069 and 1.01 mg/kg of Pb, Zn, Ni, Cu and Cd, respectively. This reveals the strong affinity of G. lucidum for toxic metals. The Pb, Zn, Ni, Cu and Cd immobilised after leaching procedure ranged from 45.3 to 98.10%. Immobilisation of toxic metals hosted by G. lucidum in red bricks can reduce environmental contamination by metals.
PUBLIC INTEREST STATEMENT
Battery slag dumpsite soil is a contamination source of potentially toxic heavy metals particularly lead in the environment. Environmental pollution via leaching of heavy metal is imminent in an area where such hazardous slag is generated in high volumes and indiscriminately deposited as waste. The contaminated soil in such contaminated area requires periodic remediation possibly by phtoextraction method using non-edible muschroom as a remediating agent for toxic metals. The current disposal option for the mushrooms afterwards is to blend it with uncontaminated loamy soil for the manufacture of bricks. Immobilisation of heavy metals particularly lead into bricks remains desirable alternative resource and efficient option to remediate contaminated dumpsite.
Competing interests
The authors declare no conflicting interest among them.
1. Introduction
Solid wastes are generated by industries and the volume is increasing along with increasing urbanisation and population (Ihedioha et al., Citation2017). Their disposal has become a global environmental problem. In the urban areas of many developing countries, industries have designated hectares of land as dumpsites for such wastes (Balkhair & Ashraf, Citation2016). Solid wastes produced by some manufacturing industries are toxic and have potential of contributing significantly to the heavy metal contents of dumpsite surface soils. (Mapanda et al., Citation2005). Batteries, geothermal activities, mining, coal combustion, paint and paper industries are among few sources of such toxic solid wastes (Ayangbenro & Babalola, Citation2017). Major sources of heavy metal contaminations in China (Belliturk et al., Citation2015; Bi et al., Citation2018), Malaysia (Hossain et al., Citation2015) and other countries including Nigeria (Essien et al., Citation2019) have been identified to fall into these categories of manufacturing industries.
Several conventional remediation techniques commonly employed to get rid of heavy metals from contaminated sites include soil flushing, soil washing and stabilisation. These methods exhibit some limitations such as slow metal precipitation and high cost of energy and reagents (Ahalya et al., Citation2003; Aziz et al., Citation2008). The shortcomings with conventional remediation techniques have necessitated the need to adopt an economically feasible and effective method, which has less of these limitations. Bioremediation approach using fungi has been reported to be cost effective and eco-friendly in the cleaning up of toxic metals from contaminated soil.
Mushroom, macro-fungi, remove heavy metals in an efficient way by accumulating them into the fruiting body that consists of a cap with sporophore, strip and mycelium (Adenipekun et al., Citation2013b; Damodaran et al., Citation2014). In this respect, mushrooms have been found relevant for decontamination of the polluted environment. Edible mushrooms have nutritive value as they are a good source of antioxidants and vitamins. Therefore, the use of edible mushrooms to decontaminate polluted environment is not expedient because of their excellent medicinal properties. In pursuit of an alternative for heavy metal adsorbents, non-edible mushrooms such as Ganoderma lucidum can provide a promising mechanism for heavy metal uptake and accumulation. G. lucidium is known to possess excellent tolerance for heavy metals (Gupta et al., Citation2019). The presence of functional groups such as amide, ether, oxalic acid and thiol in G. lucidum have been confirmed and they are responsible for heavy metals biosorption (Akar & Tunali, Citation2005). Therefore, G. lucidum will be a potentially valuable adsorbent for the remediation of heavy metal contaminated soil containing a stack of battery slag. At the smelting step, the pyrometallurgical process step that the used batteries are submitted for the recovery of metallic lead generates huge amount of a by-product called slag. There is much variability in the chemical components of slag, which depends largely on the efficiency of pyrometallurgical process used. The slag contains about 20–25 % of the total furnace-feeding load consisting of metallic lead, sulphuric acid, iron scrape, coke and sodium carbonate.
Open storage of slag was recommended by Coya et al. (Citation2000) during which soluble component could be lost and the slag could become stable. A minimum 6-months of ageing is recommended for the slag to be environmentally safe and they are not to be discarded in a landfills (Coya et al., Citation2000). On the other hand, slag can become hazardous wastes that are toxic, corrosive and whose acid components could be leached into the environment if not managed well. The United State Environmental Protection Agency (US-EPA) has developed a toxicity characteristic leaching procedure (TCLP), which is the regulatory method to determine if a waste has the characteristic of toxicity and is hazardous. The TCLP method was developed to ascertain if a waste is subject to regulation under subtitle C of the Resource Conservative and Regulatory Act promulgated in 1980. The TCLP was designed to simulate landfill leaching under a worst environmental conditions.
Several reports abound of environmental problems caused by battery slag discarded by a battery industry in a dumpsite located in Ibadan (Adejumo et al., Citation2011; Ogundele et al., Citation2019; Ogundiran & Osibanjo, Citation2008). The presence of large concentrations of multiple heavy metals including lead (138,000 mg/kg), chromium (12.3 mg/kg), cadmium (41.3 mg/kg), copper (mg/kg) and zinc (1510 mg/kg) had been reported in surface soil of the abandoned battery slag dumpsite situated at Lalupon in Ibadan. The long term dumping of the battery slag had resulted in a significant buildup of heavy metals in the land and the affected community had reported cases of infertile soil, poor vegetation and livestock death. Moreover, the presence of toxic metals in soil has potential of contributing to the contamination of nearby surface groundwater via vertical transportation (Mukherjee & Bhattacharya, Citation2001; Shen et al., Citation2017; Wu et al., Citation2006). The exposure of the populace to toxic metals particularly lead in the abandoned battery slag dumpsite soil requires intervention such as bioremediation of the contaminated soil. The intervention is a welcome development to solid waste management regulators.
Recently, Moringa Oleifera had been used for the remediation of lead-contaminated soil from this battery slag dumpsite. The M. Oleifera is medicinal and an edible plant, which ought not to be employed for the decontamination of polluted soil. The vegetative part of G. lucidium, an alternative, can serve as biosorbent for heavy metals removal from this battery slag contaminated soil. How to immobilised heavy metals adsorbed by G. lucidium, after bioremediation process was an underlying question while designing this research. The application of G. lucidum for remediation becomes effective if such fungi containing the extracted toxic metals could be fixed in a facility such as bricks, which has potential to keep permanently the metals and minimise further contamination. Brick is one of the most useful accommodating stonework units as a construction material owing to its properties (Aeslina & Mohajerani, Citation2012). There have been reports on the incorporation of cigarette butts (Al-Fakih et al., Citation2019; Mohajerani et al., Citation2017), limestone dust (Aukour, Citation2009), wood sawdust (Belhadj et al., Citation2014; Chowdhury et al., Citation2015), processed waste tea (Zhang, Citation2013), fly ash (Rakhimova & Rakhimov, Citation2019; Yao et al., Citation2015), geopolymer (Messina et al., Citation2017) and sludge (Lynn et al., Citation2015; Mashaly et al., Citation2016). Incorporation of these wastes as inert components into building materials is a laudable recycling approach to solving pollution problem. In respect of this dumpsite, the use of bricks for the solidification of G. lucidum which is effective in bioremediating the heavy metals from slag battery dumpsite soil is reported for the first time in this study. Therefore, the objective of this study was to (i) determine the levels of heavy metals in an abandoned battery slag dumpsite soil (ii) remediate the heavy metals in a battery slag dumpsite soil using Ganoderma lucidum (iii) determine the possible levels of heavy metals that can be leached from the fired bricks incorporated with harvested rice straw and mycelia used to bioremediate heavy metals from the dumpsite soil.
2. Materials and methods
2.1. Description of abandoned battery slag dumpsite area and soil sample collection
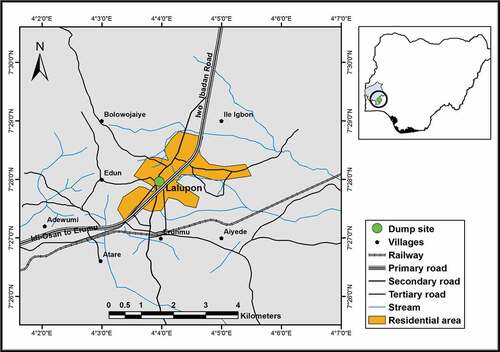
The slag dumpsite area was used by Exide Battery while in operation. It covers about an hectare of land in an old disused granite mine, located at Ajose community in Lalupon village, Lagelu Local Government Area of Oyo State, Nigeria. Lalupon lies between longitude 7° 28′ N and latitude 4° 04′ E as shown in Figure . The dumpsite has a spot for slag deposit, leachate drainage, a leachate pond and a monitoring well.
Figure 1. Selected portion of Ibadan showing the location of abadoned battery slag dumpsite at Lalupon
Sixty five sampling locations within the slag dumpsite area were selected. About 2.0 kg of soil samples were collected at a depth of 10 cm below the surface soil layer at each location. Soil samples collected from these locations were mixed together to make composite sample. Soil samples from the Botanical nursery of the Department of Botany, University of Ibadan served as the control. Red soils from Lanlate of Oyo State were collected for bricks making. The soils were air-dried in the laboratory and sieved with 1 mm (mm) mesh and kept in polyethene bags.
2.2. Preparation of pure isolate spawn
Freshly harvested rice straw was collected from Africa rice farm at the International Institute of Tropical Agriculture (IITA), Ibadan. The rice straw was sun dried to remove moisture content that could cause decomposition. The straw was cut into 5 mm size using a sharp gullotine. Wheat bran was purchased at Kara, Bodija market, Ibadan. The fungus, Ganoderma lucidum was used for this study. The pure spawn of the fungus, prepared according to the modified methods of Jonathan and Fasidi (Jonathan & Fasidi, Citation2001) was collected from the Plant Physiology Laboratory of the Department of Botany, University of Ibadan.
Sorghum was parboiled in water for 10 min and squeezed through a muslin cloth until no more water oozed out. Wheat bran was added as additive, mixed thoroughly and put in transparent sterile bottles covered with aluminium foil paper. The bottles with the contents were autoclaved at 121 °C for 15 min at 1.05 kg cm−3.(Adenipekun & Fasidi, Citation2005). After cooling, the bottles were all inoculated with vigorously growing spawn of Ganoderma lucidum and then incubated at room temperature for 3 weeks until the substrate was completely ramified. The bottles were kept in a refrigerator at 4 °C pending analysis.
2.3. Inoculation of soil sample with G. lucidum
One kilogram of contaminated soil from the battery slag dumpsite was weighed separately into thirty (30) polyethylene bags for each of the treatment groups. Four treatment groups were set. The polythene bags for the first treatment group contained battery slag contaminated soil inoculated with 60 g of G. lucidum and was designated as BSD+GL. The second group contained control soil sample from nursery section inoculated with the same quantity of G. lucidum and was designated as NSS+GL. The polythene bags for the third and fourth treatment groups contained contaminated soil (BSD) and control soil (NSS) samples separately without inoculation. In each polythene bag, 200 g of previously soaked and moistened clean rice straw was laid on the soil separated with a wire gauze. The neck of each polyethylene bag was tightened using PVC pipe and cotton wool. The polyethylene bags were then autoclaved at 121°C for 15 min and incubated at room temperature for one, two and 3 months. At the end of each month of incubation, the mycelia ramified substrates were carefully separated from the soil layer to ensure that soil particles do not mix with them.
2.4. Chemical analysis of soil samples from treatment groups
Soil samples from the four treatment groups were analysed for physiochemical parameters after the experiments were terminated. pH of the soil sample was carried out by electrometric method (HI-2210-02 benchtop pH meter) (Bates, Citation1954), particle size distribution was conducted using hydrometer method (SA-1, model ASTM 151 H) (Beverwijk, Citation1967) and cation exchange capacity was done using AOAC guide (Poitevin, Citation2016). Organic carbon and Organic matter contents was done using chromic acid titration method (Walkley & Black, Citation1934). Air-dried soil samples were digested with aqua regia solution and the metal concentrations in the digest were determined by flame atomic absorption spectrophotometry (Bulk Scientific Model 200A). A sample blank was also carried out.
2.5. Making of red bricks with rice straw and mycelia
A fixed quantity of red virgin soil was mixed with varying quantity of harvested rice straw and mycelia. The rice straw and mycelia harvested after the third month of incubation was used as part of materials for bricks making. There were five (5) treatment groups of bricks making (A—E) and a control group (F). The harvested mycelia was added to 150 g of virgin red soil in varying proportions. The proportions were 30, 20, 15, 10 and 5 g of air dried and ground harvested mycelia for bricks A, B, C, D and E, respectively. Group F contained only virgin red soil (150 g) without rice straw and mycelia. Water was added to enhance the plasticity of the mixture and increase the porosity of the fired brick. The mixture was homogenized and extruded into a mold of 3.5 cm x 3.0 cm x 3.0 cm to form brick samples. The extruded samples were first dried at 105 °C and fired in a tunnel kiln to a maximum of 900 °C.
2.6. Toxicity-leaching characteristics procedure (TLCP)
Heavy metals leaching from bricks made with contaminated soil and harvested mycelia was conducted using TCLP as specified in the EPA method 1311 (Us Epa, Citation1992). The bricks were crushed with a mortar and pestle. The crushed bricks were separated through 9.5 mm sieve to obtain size-reduced brick samples. A portion (100 g) of size-reduced brick sample was loaded into high-density polyethylene bottle and 1.0 L extraction fluid was added. An appropriate extraction fluid for TCLP test was selected on the basis of the brick sample acidity. In the preliminary pH test, 5.0 g of crushed and sieved brick sample was mixed with 100 mL of distilled water and pH of the solution was measured. Since the pH of the sample was greater than 5.0, TCLP extraction fluid (CH3COOH, pH of 2.88) was employed. The weighed crushed and sieved brick samples from treated groups A to F were rotated at 30 rpm for 18 h in a rotary extractor operated at room temperature. The liquid extracts were separated by filtration through a 0.3 µm glass fibre filter. This extraction was carried out in triplicate. The filtered extract were analysed for heavy metal concentrations by flame atomic absorption spectrophotometry (Bulk Scientific Model 200A). For quality control aspect, sample blank determination was undertaken for each batch of samples run.
2.7. Statistical analysis
Data were analysed using ANOVA (α0.05) and Duncan’s Multiple Range test.
3. Results and discussions
3.1. Physicochemical characteristics of soil inoculated with G. lucidum
Table shows the results of the physicochemical parameters of the soil samples. Parameters such as pH, cation exchange capacity and organic matter are known to play major role in the interactions, dynamics and availability of metals within the soil matrix (Mani & Kumar, Citation2014). The contaminated and control soil pH ranged from 6.73 ± 0.15 to 6.90 ± 0.18 and 6.65 ± 0.19 to 6.90 ± 0.11, respectively. There was a reduction in soil pH when inoculation with G. lucidum was performed over . The pH values of contaminated and control soil samples that were innoculated with G. lucidum slightly reduced over the incubation period (Table ). With G. lucidum colonisation, the soil pH can be reduced due to mycelium interaction with soil particles during which specific enzymes are released into the soil to break down complex molecules that the fruiting body reabsorb. The G. lucidum produces extracellular enzymes including manganese peroxidase and laccase (Nagai et al., Citation2002). The physiological activities of these extracellular enzymes allow root-induced changes in the rhizosphere through the release of hydrogen ion to balance cation uptake at the soil–root interface (Hinsinger et al., Citation2003). This made the soil slightly acidic after biosorption has taken place.
Table 1. The physicochemical parameters of the Nursery Section Soil (NSS) as control sample and Battery Slag Dumpsite (BSD) soil as contaminated samples inoculated with G. lucidum for one, two and three months
The average pH value of negative control soil sample (NSS) reduced from 6.90 ± 0.11 to 6.63 ± 0.26 after the incubation with mycelia of G. lucidum for on month. Similarly, the pH of NSS reduced when incubated with G. lucidium for 2 and 3 months. Also, a reduction in average pH values of positive control sample (BSD) after the incubation (Table ). This is in agreement with similar results obtained for the reduction of pH of cement waste contaminated soil to enzyme activities of Pleurotus ostreatus (Adenipekun et al., Citation2011). This observation is in tandem with the previous reports (Adenipekun et al., Citation2011; Bennett & Dudas, Citation2003). The soil texture was predominantly sandy with compositions of 71.2 ± 0.15% and 73.4 ± 0.11% for battery waste contaminated and control soil samples, respectively. There was no significant change in these compositions for the entire incubation periods. The textural composition of soil was in the order of sand ≫silt > clay.
Contaminated soil cation exchanging capacity (CEC) increased after incubation from 10.9 ± 0.9 to 12.6 ± 0.2 cmol kg−1, 11.7 to 12.6 ± 0.4 cmol kg−1, 12.6 ± 0.2 to 18.1 ± 0.2 cmol kg−1 for first, second and third months of incubation, respectively. Similar increment of 44.4, 27.1 and 59.1% in CEC were obtained for the control soil samples. A range of exchangeable cations occupying the colloidal complex of the soil could have been removed by G. lucidum leaving the vacant complex to be filled with ammonium saturating cation that the soil samples were treated with after incubation. Furthermore, the humification process in incubated soil produces functional that influence the increased oxidation of organic matter, which leads to a rise in CEC. The functional groups responsible for CEC are mainly the carboxyl and phenolic groups (Nadeem et al., Citation2014). It has been revealed that carboxyl and phenolic groups are formed in decayed rice straw that was used as substrate (Chen et al., Citation2018). This could have accounted for high estimate of CEC. Table also shows that there was an improvement on soil organic matter content through filamentous association of G. lucidum. A branch-like association grows on rice straw by filamentous association of this fungus and a small part of the structure is usually degraded and transformed by soil microorganism. The remains of degraded filament could have been responsible for the increase in the constituents of soil organic matter. The organic matter contents of battery waste contaminated soil increased by 3.89, 1.32, and 13.0%, while control soil samples increased by 0.85, 12.7, and 27.3%.
3.2. Accumulation of metals in G. lucidum grown on soil
The concentration of lead in the contaminated soil before incubation was 4490 ± 14 mg/kg, which varied widely from the previous lead levels of 41,500 mg/kg and 134,000 mg/kg reported on the deserted battery slag dumpsite soil at different occasions (Adejumo et al., Citation2011; Ogundiran & Osibanjo, Citation2008). The concentration of Pb, among other metals investigated in this study exceeded the limits prescribed by Canadian soil quality standards for land use such as agriculture (70 mg/kg), residential (140 mg/kg), commercial (260 mg/kg) and industrial (600 mg/kg) (CCME, 1999). However, the lead concentration in the contaminated soil was reduced after it was inoculated with G. lucidum and incubated for one, two and 3 months. Over the first, second and third months, the lead concentration of 4490 ± 14, 4240 ± 11, 3760 ± 16 mg/kg reduced to 4350 ± 21, 4100 ± 25 and 3622 ± 11 mg/kg, respectively, after the incubation (Table ). The reduction in lead concentration contained in the contaminated soil within the incubation periods ranged from 3.12 to 3.7%. The G. lucidum accumulated 19.7–24.4% of Zn, 9.6–38.0% of Ni, 18.6–36.1% of Cu and 34.4–50.0% of Cd contained in battery slag contaminated soil within three months of incubation (Table ). The proportions of metals accumulated from the control soil sample by G. lucidium were much higher as compared to those from the contaminated soil sample. This study indicates that Pb, Zn, Ni, Cu and Cd from a battery contaminated soil can be accumulated to measurable proportions in G. lucidum, revealing it as an accumulator of toxic metals. The findings in this sudy align with other studies of bioaccumulation of heavy metals in white rot fungi (Adenipekun et al., Citation2013a; Isikhuemhen et al., Citation2003). The mycelium of G. lucidum like other fungal species serve as a biological filter that can diffuse a huge area and this characteristic feature make it a potential sorbent for metals uptake from soils (Damodaran et al., Citation2013; Volesky & Holan, Citation1995). Hence, mycelia mimic the plants’ roots in accumulating heavy metals from the contaminated soil. Copper and zinc are trace elements and their presence as notable minerals required by fungi may supplement the rice straw and stimulate the development of G.lucidum (Peksen & Yakupoglu, Citation2009). However, excessive levels of these trace elements may result in undesirable growth of the mycelium of G. lucidum. Other elements including lead, nickel and cadmium are considered to be harmful and their accumulation in the fruiting body can inhibit the growth of G. lucidum.
Table 2. Metal concentrations (mg/kg) in nursery section soil samples (NSS) and battery slag dumpsite soil samples (BSD) inoculated with and without G. lucidum (GL) after first, second and third months
The results of heavy metals analysis of G. lucidum mycelia in contaminated (BSD) and control (NSS) soil samples after incubation periods are shown in Table . The pattern of accumulation of metals in the mycelia inoculated in contaminated soil followed the order of Pb≫Zn>Ni>Cu>Cd for the first and second months of incubation, while the order for third month was Pb≫Zn>Cu>Ni>Cd. Similarly, these trends of accumulation of metals in mycelia inoculated in the control soil samples were obtained. The heavy metals bioremoval particularly for Pb, Ni and Cu increased with increasing duration (first and second months) of the bioremediation (Table ). On the other hand, the concentrations of Zn and Cd decreased within these durations, even though there was variation in the metal-accumulating ability of the fungus during the third months of incubation. This seems to be surprising considering the fact that the enhanced metabolic activity of the fungus to bioremediate was greatly achieved for Pb, Ni and Cu for the first 2 months of incubations. The variation in the uptake of heavy metals by fungi could be attributed to possible soil chemical reactions leading to solution of certain species of metal ions having different mobilities (Brümmer, Citation1986; Violante et al., Citation2010). In soils, solubility equilibria may change significantly within a few period at both horizontal and vertical soil gradient owing to changes in pH, temperature and water conditions (Post et al., Citation2001; Virkutyte et al., Citation2002). Thus, the mobility of heavy metal may often be quite different in certain durations of incubation with fungi. Zinc and cadmium appear to occur in more readily soluble form in soil (Kabała & Singh, Citation2006; Rieuwerts, Citation2007). It has been shown that increasing clay and organic matter contents are capable of holding zinc and cadmium quite strongly (Ayele et al., Citation2014; Levi-Minzi et al., Citation1990). This possible soil constituents interaction could have readily retarded the solubility of zinc and cadmium in soil samples during the second and third months of incubation, thereby reducing its bioremoval from the soil by G. lucidum mycelia. It has been reported that processes involved in heavy metals bioremoval are not yet completely understood (Gavrilescu, Citation2004). However, some generalisation can be made from studies that zinc and cadmium uptake in the fruiting bodies of mushroom can be greatly influenced by soil acidity (Rudawska & Leski, Citation2005). Besides, zinc content seems to be affected by the substrate on which G. lucidum mycelia grow (Peksen & Yakupoglu, Citation2009).
Table 3. Metal concentrations (mg/kg) accumulated in the mycelia of G. lucidum (GL) inoculated in NSS and BSD soil samples
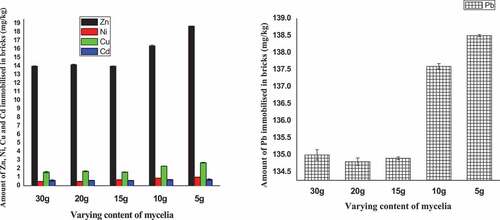
Figure shows the levels of heavy metals immobilised in the bricks containing varying content of mycelia. The bricks showed significant capacity to immobilise lead which has highest concentration in the slag contaminated soil (Figure ). There was no significant change in the level of lead immobilsed in the bricks that contained 30 g, 20 g and 15 g of mycelia. Similar trend was observed for Zn, Ni, Cu and Cd levels when these quantities of mycelium were incorporated into the bricks. The highest concentrations of metals were immobilised with the least content of mycelia (5.0 g) in the bricks (Figure ). As it has been observed in a study, fungi are passive receptors of heavy metals on their tissue (Pardo et al., Citation2003). The heavy metals biosorbed by mycelium can be adsorbed into the soil being utilised in making bricks to form a product that remains within the bricks (Abdel-Gawwad et al., Citation2019). There may be formation of chelating complex when the adsorbed metal mixed with the negatively charged ions such as silicate and functional group at the end of stable humic substances on the soil being used for making bricks (Evans, Citation1989; Hizal & Apak, Citation2006). Humic substances possibly present in the soil have a great affinity for interacting with metal ions. Owing to a particular combination of different groups (maily hydroxyl and sulfhydryl groups), humic substances can form stable complexes with heavy metals when immobilised in the bricks (Senesi et al., Citation1996; Tipping, Citation1981). The metal concentrations in the control brick were negligible compared with other categories of bricks.
4. Conclusion
Soil from the abandoned battery slag dumpsite had high heavy metal concentrations particularly lead. The levels of lead, zinc, nickel, copper and cadmium in battery slag contaminated soil inoculated with Ganoderma lucidum reduced significantly; thus, indicative of its capability to bioaccumulate the metals in the soil. The heavy metals accumulated in the mycelium of Ganoderma lucidum were immobilised successfully in bricks as TCLP results indicated low availability of the studied heavy metals in the leachates. There is a need to further investigate the potential of the bricks to immobilise heavy metals when rice straw is incorporated into the bricks.
Additional information
Funding
Notes on contributors
Ayodele Rotimi Ipeaiyeda
Ayodele Rotimi Ipeaiyeda is a senior lecturer in the field of Analytical and Environmental Chemistry with particular emphasis on the assessment of impacts of pollution from different sources on the environment.
Clementina Oyinkansola Adenipekun
Clementina Oyinkansola Adenipekun Adenipekun is a professor in the field of Mushroom Biotechnology and Plant Physiology.
Oluwatola Oluwole
Oluwatola Oluwole is a graduate research student under the supervision of Prof. Clementina O. Adenipekun and Dr A. R. Ipeaiyeda.
References
- Abdel-Gawwad, H. A., Mohamed, S. A., & Mohammed, M. S. (2019). Recycling of slag and lead-bearing sludge in the cleaner production of alkali activated cement with high performance and microbial resistivity. Journal of Cleaner Production, 220, 568–14. http://sci-hub.tw/10.1016/j.jclepro.2019.02.144
- Adejumo, S. A., Togun, A. O., Adediran, J. A., & Ogundiran, M. B. (2011). Field assessment of progressive remediation of soil contaminated with lead-acid battery waste in response to compost application (Symposium 3.5. 1 Heavy Metal Contaminated Soils,< special issue> International Symposium: Soil Degradation Control, Remediation, and Reclamation, Tokyo Metropolitan University Symposium Series No. 2, 2010). Pedologist, 54, 182–193.
- Adenipekun, C., Ayanleye, O., & Oyetunji, O. (2013a). Bioremediation of soil contaminated by spent diesel oil using Pleurotus pulmonarius Fries (Quelet) and its effects on the growth of Corchorus olitorius (L). Journal of Applied Biosciences, 68, 5366–5373.
- Adenipekun, C., & Fasidi, I. (2005). Bioremediation of oil-polluted soil by Lentinus subnudus, a Nigerian white-rot fungus. African journal of Biotechnology, 4, 796–798.
- Adenipekun, C., Olanrewaju, O., & Ogunjobi, A. (2011). Bioaccumulation of heavy metals and nutrient content supplementation by two white rot fungi in crude oil polluted soils. Researcher, 3, 13–20.
- Adenipekun, C. O., Ipeaiyeda, A., & Olayonwa, A. (2013b). Bioremediation of soil contaminated with spent and fresh cutting fluids by Pleurotus pulmonarius (Fries) Quelet. African Journal of Biotechnology, 12.
- Aeslina, A., & Mohajerani, A. (2012). Leachability of heavy metals from fired clay bricks incorporated with cigarette butts. 2012 IEEE Symposium on Business, Engineering and Industrial Applications (pp. 872–877). IEEE.
- Ahalya, N., Ramachandra, T., & Kanamadi, R. (2003). Biosorption of heavy metals. The Research Journal of Chemistry and Environment, 7, 71–79.
- Akar, T., & Tunali, S. (2005). Biosorption performance of Botrytis cinerea fungal by-products for removal of Cd(II) and Cu(II) ions from aqueous solutions. Minerals Engineering, 18(11), 1099–1109. http://sci-hub.tw/10.1016/j.mineng.2005.03.002
- Al-Fakih, A., Mohammed, B. S., Liew, M. S., & Nikbakht, E. (2019). Incorporation of waste materials in the manufacture of masonry bricks: An update review. Journal of Building Engineering, 21, 37–54. http://sci-hub.tw/10.1016/j.jobe.2018.09.023
- Aukour, F. J. (2009). Incorporation of marble sludge in industrial building eco-blocks or cement bricks formulation. Jordan Journal of Civil Engineering, 3, 58–65.
- Ayangbenro, A., & Babalola, O. (2017). A new strategy for heavy metal polluted environments: A review of microbial biosorbents. International Journal of Environmental Research and Public Health, 14(1), 94. http://sci-hub.tw/10.3390/ijerph14010094
- Ayele, T., Ayana, M., Tanto, T., & Asefa, D. (2014). Evaluating the status of micronutrients under irrigated and rainfed agricultural soils in Abaya Chamo Lake Basin, South-west Ethiopia. Journal of Scientific Research and Reviews, 3, 018–027.
- Aziz, H. A., Adlan, M. N., & Ariffin, K. S. (2008). Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresource Technology, 99(6), 1578–1583. http://sci-hub.tw/10.1016/j.biortech.2007.04.007
- Balkhair, K. S., & Ashraf, M. A. (2016). Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi Journal of Biological Sciences, 23(1), S32–S44. http://sci-hub.tw/10.1016/j.sjbs.2015.09.023
- Bates, R. G. (1954). Electronic pH determinations; theory and practice.
- Belhadj, B., Bederina, M., Montrelay, N., Houessou, J., & Quéneudec, M. (2014). Effect of substitution of wood shavings by barley straws on the physico-mechanical properties of lightweight sand concrete. Construction and Building Materials, 66, 247–258. http://sci-hub.tw/10.1016/j.conbuildmat.2014.05.090
- Belliturk, K., Shrestha, P., & Görres, J. H. (2015). The importance of phytoremediation of heavy metal contaminated soil using vermicompost for sustainable agriculture. Rice Research: Open Access.
- Bennett, B., & Dudas, M. J. (2003). Release of arsenic and molybdenum by reductive dissolution of iron oxides in a soil with enriched levels of native arsenic. Journal of Environmental Engineering and Science, 2(4), 265–272. http://sci-hub.tw/10.1139/s03-028
- Beverwijk, A. (1967). Particle size analysis of soils by means of the hydrometer method. Sedimentary Geology, 1, 403–406. http://sci-hub.tw/10.1016/0037-0738(67)90070-X
- Bi, C., Zhou, Y., Chen, Z., Jia, J., & Bao, X. (2018). Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Science of the Total Environment, 619, 1349–1357. http://sci-hub.tw/10.1016/j.scitotenv.2017.11.177
- Brümmer, G. (1986). Heavy metal species, mobility and availability in soils. In The importance of chemical “speciation” in environmental processes (pp. 169–192). Berlin, Heidelberg: Springer.
- Chen, X., Hu, Y., Feng, S., Rui, Y., Zhang, Z., He, H., He, X., Ge, T., Wu, J., & Su, Y. (2018). Lignin and cellulose dynamics with straw incorporation in two contrasting cropping soils. Scientific Reports, 8(1), 1–10. doi:10.1038/s41598-018-20134-5
- Chowdhury, S., Mishra, M., & Suganya, O. (2015). The incorporation of wood waste ash as a partial cement replacement material for making structural grade concrete: An overview. Ain Shams Engineering Journal, 6(2), 429–437. http://sci-hub.tw/10.1016/j.asej.2014.11.005
- Coya, B., Marañon, E., & Sastre, H. (2000). Ecotoxicity assessment of slag generated in the process of recycling lead from waste batteries. Resources, Conservation and Recycling, 29(4), 291–300. http://sci-hub.tw/10.1016/S0921-3449(00)00054-9
- Damodaran, D., Shetty, K. V., & Mohan, B. R. (2013). Effect of chelaters on bioaccumulation of Cd (II), Cu (II), Cr (VI), Pb (II) and Zn (II) in Galerina vittiformis from soil. International Biodeterioration & Biodegradation, 85, 182–188. http://sci-hub.tw/10.1016/j.ibiod.2013.05.031
- Damodaran, D., Shetty, K. V., & Mohan, B. R. (2014). Uptake of certain heavy metals from contaminated soil by mushroom—Galerina vittiformis. Ecotoxicology and Environmental Safety, 104, 414–422. http://sci-hub.tw/10.1016/j.ecoenv.2013.10.033
- Essien, J. P., Inam, E. D., Ikpe, D. I., Udofia, G. E., & Benson, N. U. (2019). Ecotoxicological status and risk assessment of heavy metals in municipal solid wastes dumpsite impacted soil in Nigeria. Environmental Nanotechnology, Monitoring & Management, 11, 100215. http://sci-hub.tw/10.1016/j.enmm.2019.100215
- Evans, L. (1989). Chemistry of metal retention by soils. Environmental Science & Technology, 23(9), 1046–1056. http://sci-hub.tw/10.1021/es00067a001
- Gavrilescu, M. (2004). Removal of heavy metals from the environment by biosorption. Engineering in Life Sciences, 4(3), 219–232. http://sci-hub.tw/10.1002/elsc.200420026
- Gupta, C., Balakrishnan, R. M., Priyanka, U., & Pugazhendhi, A. (2019). Mycosensing of soil contaminants by Ganoderma lucidum and Omphalotus subilludens including the insights on growth media requirements. Biocatalysis and Agricultural Biotechnology, 20, 101239. http://sci-hub.tw/10.1016/j.bcab.2019.101239
- Hinsinger, P., Plassard, C., Tang, C., & Jaillard, B. (2003). Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant and Soil, 248(1/2), 43–59. http://sci-hub.tw/10.1023/A:1022371130939
- Hizal, J., & Apak, R. (2006). Modeling of copper(II) and lead(II) adsorption on kaolinite-based clay minerals individually and in the presence of humic acid. Journal of Colloid and Interface Science, 295(1), 1–13. http://sci-hub.tw/10.1016/j.jcis.2005.08.005
- Hossain, M. A., Ali, N. M., Islam, M. S., & Hossain, H. Z. (2015). Spatial distribution and source apportionment of heavy metals in soils of Gebeng industrial city, Malaysia. Environmental Earth Sciences, 73(1), 115–126. http://sci-hub.tw/10.1007/s12665-014-3398-z
- Ihedioha, J., Ukoha, P., & Ekere, N. (2017). Ecological and human health risk assessment of heavy metal contamination in soil of a municipal solid waste dump in Uyo, Nigeria. Environmental Geochemistry and Health, 39(3), 497–515. http://sci-hub.tw/10.1007/s10653-016-9830-4
- Isikhuemhen, O. S., Anoliefo, G. O., & Oghale, O. I. (2003). Bioremediation of crude oil polluted soil by the white rot fungus, Pleurotus tuberregium (Fr.) Sing. Environmental Science and Pollution Research, 10(2), 108–112. http://sci-hub.tw/10.1065/espr2002.04.114
- Jonathan, S., & Fasidi, I. (2001). Effect of carbon, nitrogen and mineral sources on growth of Psathyerella atroumbonata (Pegler), a Nigerian edible mushroom. Food Chemistry, 72(4), 479–483. http://sci-hub.tw/10.1016/S0308-8146(00)00265-X
- Kabała, C., & Singh, B. (2006). Distribution and forms of cadmium in soils near a copper smelter. Polish Journal of Environmental Studies, 15, 90–97.
- Levi-Minzi, R., Riffaldi, R., & Saviozzi, A. (1990). Carbon mineralization in soil amended with different organic materials. Agriculture, Ecosystems & Environment, 31(4), 325–335. http://sci-hub.tw/10.1016/0167-8809(90)90231-2
- Lynn, C. J., Dhir, R. K., Ghataora, G. S., & West, R. P. (2015). Sewage sludge ash characteristics and potential for use in concrete. Construction and Building Materials, 98, 767–779. http://sci-hub.tw/10.1016/j.conbuildmat.2015.08.122
- Mani, D., & Kumar, C. (2014). Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. International Journal of Environmental Science and Technology, 11(3), 843–872. http://sci-hub.tw/10.1007/s13762-013-0299-8
- Mapanda, F., Mangwayana, E., Nyamangara, J., & Giller, K. (2005). The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agriculture, Ecosystems & Environment, 107(2–3), 151–165. http://sci-hub.tw/10.1016/j.agee.2004.11.005
- Mashaly, A. O., El-Kaliouby, B. A., Shalaby, B. N., El–Gohary, A. M., & Rashwan, M. A. (2016). Effects of marble sludge incorporation on the properties of cement composites and concrete paving blocks. Journal of Cleaner Production, 112, 731–741. http://sci-hub.tw/10.1016/j.jclepro.2015.07.023
- Messina, F., Ferone, C., Molino, A., Roviello, G., Colangelo, F., Molino, B., & Cioffi, R. (2017). Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Construction and Building Materials, 133, 14–26. http://sci-hub.tw/10.1016/j.conbuildmat.2016.12.039
- Mohajerani, A., Tanriverdi, Y., Nguyen, B. T., Wong, K. K., Dissanayake, H. N., Johnson, L., Whitfield, D., Thomson, G., Alqattan, E., & Rezaei, A. (2017). Physico-mechanical properties of asphalt concrete incorporated with encapsulated cigarette butts. Construction and Building Materials, 153, 69–80. http://sci-hub.tw/10.1016/j.conbuildmat.2017.07.091
- Mukherjee, A. B., & Bhattacharya, P. (2001). Arsenic in groundwater in the Bengal Delta Plain: Slow poisoning in Bangladesh. Environmental Reviews, 9(3), 189–220. http://sci-hub.tw/10.1139/a01-007
- Nadeem, A., Baig, S., Iqbal, K., & Sheikh, N. (2014). Impact of laccase enzyme inducers on solid waste compost maturity and stability. Environmental Technology, 35(24), 3130–3138. http://sci-hub.tw/10.1080/09593330.2014.932439
- Nagai, M., Sato, T., Watanabe, H., Saito, K., Kawata, M., & Enei, H. (2002). Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Applied Microbiology and Biotechnology, 60(3), 327–335. http://sci-hub.tw/10.1007/s00253-002-1109-2
- Ogundele, L. T., Adejoro, I. A., & Ayeku, P. O. (2019). Health risk assessment of heavy metals in soil samples from an abandoned industrial waste dumpsite in Ibadan, Nigeria. Environmental Monitoring and Assessment, 191(5), 290. http://sci-hub.tw/10.1007/s10661-019-7454-8
- Ogundiran, M. B., & Osibanjo, O. (2008). Heavy metal concentrations in soils and accumulation in plants growing in a deserted slag dumpsite in Nigeria. African Journal of Biotechnology, 7(17).
- Pardo, R., Herguedas, M., Barrado, E., & Vega, M. (2003). Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Analytical and Bioanalytical Chemistry, 376(1), 26–32. http://sci-hub.tw/10.1007/s00216-003-1843-z
- Peksen, A., & Yakupoglu, G. (2009). Tea waste as a supplement for the cultivation of Ganoderma lucidum. World Journal of Microbiology & Biotechnology, 25(4), 611–618. http://sci-hub.tw/10.1007/s11274-008-9931-z
- Poitevin, E. (2016). Official methods for the determination of minerals and trace elements in infant formula and milk products: A review. Journal of AOAC International, 99(1), 42–52. http://sci-hub.tw/10.5740/jaoacint.15-0246
- Post, W. M., Izaurralde, R. C., Mann, L. K., & Bliss, N. (2001). Monitoring and verifying changes of organic carbon in soil. In Storing carbon in agricultural soils: A multi-purpose environmental strategy (pp. 73–99). Dordrecht: Springer.
- Rakhimova, N. R., & Rakhimov, R. Z. (2019). Toward clean cement technologies: A review on alkali-activated fly-ash cements incorporated with supplementary materials. Journal of Non-crystalline Solids, 509, 31–41. http://sci-hub.tw/10.1016/j.jnoncrysol.2019.01.025
- Rieuwerts, J. S. (2007). The mobility and bioavailability of trace metals in tropical soils: A review. Chemical Speciation & Bioavailability, 19(2), 75–85. http://sci-hub.tw/10.3184/095422907X211918
- Rudawska, M., & Leski, T. (2005). Macro-and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chemistry, 92(3), 499–506. http://sci-hub.tw/10.1016/j.foodchem.2004.08.017
- Senesi, N., Miano, T., & Brunetti, G. (1996). Humic-like substances in organic amendments and effects on native soil humic substances. In Humic substances in terrestrial ecosystems (pp. 531–593). Elsevier.
- Shen, F., Liao, R., Ali, A., Mahar, A., Guo, D., Li, R., Xining, S., Awasthi, M. K., Wang, Q., & Zhang, Z. (2017). Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County, China. Ecotoxicology and Environmental Safety, 139, 254–262. http://sci-hub.tw/10.1016/j.ecoenv.2017.01.044
- Tipping, E. (1981). The adsorption of aquatic humic substances by iron oxides. Geochimica et cosmochimica acta, 45(2), 191–199. http://sci-hub.tw/10.1016/0016-7037(81)90162-9
- Us Epa, E. (1992). Method 1311. Toxicity characteristic leaching procedure. EPA SW-846: test methods for evaluating solid waste, physical/chemical methods.
- Violante, A., Cozzolino, V., Perelomov, L., Caporale, A., & Pigna, M. (2010). Mobility and bioavailability of heavy metals and metalloids in soil environments. Journal of Soil Science and Plant Nutrition, 10(3), 268–292. http://sci-hub.tw/10.4067/S0718-95162010000100005
- Virkutyte, J., Sillanpää, M., & Latostenmaa, P. (2002). Electrokinetic soil remediation—critical overview. Science of the Total Environment, 289(1–3), 97–121. http://sci-hub.tw/10.1016/S0048-9697(01)01027-0
- Volesky, B., & Holan, Z. (1995). Biosorption of heavy metals. Biotechnology Progress, 11(3), 235–250. http://sci-hub.tw/10.1021/bp00033a001
- Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38. http://sci-hub.tw/10.1097/00010694-193401000-00003
- Wu, W.-M., Carley, J., Fienen, M., Mehlhorn, T., Lowe, K., Nyman, J., Luo, J., Gentile, M. E., Rajan, R., & Wagner, D. (2006). Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. Conditioning of a treatment zone. Environmental Science & Technology, 40(12), 3978–3985. http://sci-hub.tw/10.1021/es051954y
- Yao, Z., Ji, X., Sarker, P., Tang, J., Ge, L., Xia, M., & Xi, Y. (2015). A comprehensive review on the applications of coal fly ash. Earth-Science Reviews, 141, 105–121. doi:10.1016/j.earscirev.2014.11.016
- Zhang, L. (2013). Production of bricks from waste materials–A review. Construction and Building Materials, 47, 643–655. http://sci-hub.tw/10.1016/j.conbuildmat.2013.05.043