Abstract
Inequality in socioeconomic status (SES) is associated with an increased risk for the development of mental health problems. Here, we examined the association between socioeconomic status (SSS) and psychological distress, and measured gene expression signatures in peripheral blood leukocytes responsible for this association, in 129 healthy adults (27 males and 102 females, aged 44.0 ± 13.0 years) working in a private hospital in Japan. Depressive mood was assessed by Zung Self-rating Depression Scale (SDS). A multiple regression analysis adjusted for gender and age showed that subjective SSS was independently and negatively associated with SDS score. We next focused on 9 subjects who exhibited low SSS scores and 11 subjects with high SSS scores. Microarray analysis revealed that levels of 522 mRNAs were differentially expressed in periheral leukocytes between low and high-SSS groups. The differentially expressed genes were preferentially involved in cellular movement or inflammatory responses. Among them, mRNA levels of pro-platelet basic protein (PPBP) and solute carrier family 1 (glutamine transporter), member 7 (SLC1A7) were negatively correlated with SSS scores. Our results re-confirmed the association between negative perception of SES and depressive mood in healthy adults, and suggest a possible involvement of PPBP and SLC1A7 in the association.
Public Interest Statement
This study highlighted the impact of the socioeconomic status on psychological conditions of working adults. Recently, increasing evidence indicates that equality in socioeconomic status is associated with an increased risk for the development of mental health problems. In the current study, the subjective socioeconomic status (SSS) of 129 healthy adults (27 males and 102 females, aged 44.0 ± 13.0 years) working in a hospital in Japan are measured to classify the socioeconomic condition. The results investigate the relationship between SSS and psychological distress and show that perceptions of social disadvantage may be associated with altered inflammatory responses in peripheral blood leucocytes, which may increase the risk for mood disorders.
Competing Interests
The authors declare no competing interest.
1. Introduction
Increasing evidence indicates that socioeconomic status (SES), including variables such as education, occupation, and income, is significantly associated with health problems. In England, the Whitehall II study demonstrated a significant association between socioeconomic position and mortality (Stringhini et al., Citation2010). People with low SES suffer from higher morbidity rates of common diseases, including abdominal obesity (Schumann et al., Citation2011), metabolic syndrome (Manuck, Phillips, Gianaros, Flory, & Muldoon, Citation2010), hypertension (Grotto, Huerta, Grossman, & Sharabi, Citation2007; Singh, Sharma, Rastogi, Niaz, & Singh, Citation1997), diabetes (Evans, Newton, Ruta, MacDonald, & Morris, Citation2000), atherosclerosis (Velásquez et al., Citation2006), and cardiovascular diseases (Muennig, Sohler, & Mahato, Citation2007).
Mental health has also garnered increasing attention over the past decade because the risks of mental disorders are highly prevalent and have an enormous negative impact on daily performance and productivity, which can lead to suicide. An epidemiologic study has shown that persons with higher SES scores develop the fewer psychological health problems (Lorant et al., Citation2003). Indeed, several reports indicate that low SES is a possible risk factor for major depression (Gavin et al., Citation2010; Lemstra et al., Citation2008; Sakurai, Kawakami, Yamaoka, Ishikawa, & Hashimoto, Citation2010). In addition, low-SES groups may have increased chances to encounter stressful life events and chronic problems related to their low SES, such as poor education, unemployment, financial strain, and unhealthy dietary habits. Low SES has been suggested to cause psychological distress, including depressive mood and anxiety (Scott et al., Citation2014). However, epidemiologic studies do not reveal the underlying physiological mechanisms of how low SES affects mental health.
In the current study, we focused on the psychophysiological significance of SES, measured using the subjective socioeconomic status (SSS) questionnaire. SSS is defined as an individual’s perception of his/her own position in the social hierarchy (Jackman & Jackman, Citation1973) and regarded as an index of SES or social class. Since the measurement of SSS estimates an individual’s self-evaluate ranking on the social hierarchy, several studies suggested that SSS reflects negative or positive perception of opportunities in life (Adler, Epel, Castellazzo, & Ickovics, Citation2000; Franzini & Fernandez-Esquer, Citation2006). Therefore, SSS could not perfectly represent the SES, but it is rather used as one of the indicators of subjective situation of social stratification to complement measures of SES. Hoebel, Maske, Zeeb, and Lampert (Citation2017) demonstrates that SSS are significantly correlated with objective SES and show a significant indirect association of SES with depressive symptoms as mediated through SSS. Increasing studies have shown that low SSS is associated with physical health problems, such as the common cold (Cohen et al., Citation2008) and obesity (Goodman et al., Citation2003), as well as higher mortality (Kopp, Skrabski, Réthelyi, Kawachi, & Adler, Citation2004). In addition, it has been indicated that low SSS is associated with poor mental health including depressive symptoms (Collins & Goldman, Citation2008; Demakakos, Nazroo, Breeze and Marmot, Citation2008; Honjo, Iso et al., Citation2014; Singh-Manoux, Marmot, & Adler, Citation2005).
To examine how SSS is related to psychological conditions, such as anxiety and depressive moods, we analyzed gene expression profiles in peripheral blood leucocytes from healthy subjects working in a private hospital in Japan. We demonstrate that healthy hospital staffs with high depressive mood and a negative perception of SES possessed an altered expression of immune- or inflammatory-related genes in peripheral blood leucocytes.
2. Methods
2.1. Participants
The protocol and informed consent for the present study were approved by the Institutional Review Board of Tokushima University Hospital, Tokushima, Japan. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. We recruited 129 subjects (27 males and 102 females, aged from 19 to 74 years) working in a private hospital in Gifu prefecture, Japan. After the experimental procedures were fully explained, informed consent was obtained from each subject in written format.
2.2. Self-report measurements
Anxiety was assessed using the Japanese version of the Spielberger’s state-trait anxiety inventory (STAI) (Spielberger, Citation1971). The reliability of this Japanese version has been established (Fukuda & Kobayashi, Citation1973). Two parts of STAI serve to assess the degree of anxiety at a particular moment as a situation-dependent state (STAI-state) and the general tendency to be anxious as a personality trait (STAI-trait). Depressive symptoms were assessed using the Japanese version of the Zung self-rating depression scale (SDS) (Zung, Citation1965). We collected these answer sheets and saliva and blood samples on the same day.
2.3. Subjective socioeconomic status
SSS was measured using the self-anchoring striving scale in the form of a 10-rung ladder, which is the most widely used indicator of SSS and has good reliability and validity (Cantril, Citation1950; Giatti et al., Citation2012; Singh-Manoux et al., Citation2005). Participants were given the drawing of a 10-rung ladder and asked to think of this ladder as representing where they stand in society. At the top of the ladder (10) are the people who are best off. At the bottom (1) are the people who are worst off.
2.4. Sampling of blood and saliva
Venous blood (2.5 ml) was taken from each subject between 16:00 and 17:00 and immediately poured into PAXgene blood RNA tubes (Becton Dickinson, Franklin Lakes, NJ). After sufficient mixing, tubes were left standing for 2 h at room temperature, followed by storage at −80°C until analysis. Saliva was collected using Salivette (Sarstedt, Rommelsdorf, Germany) prior to the blood sampling as previously described (Kurokawa et al., Citation2010). Collected saliva in the cotton swab was centrifuged at 2,000 g for 15 min at 4°C and stored at −80°C until analysis. Salivary cortisol levels were measured using a commercial enzyme immunoassay (EIA) kit (Ciron, Tokyo, Japan) following the manufacturer’s instructions (Katsuura et al., Citation2010). Salivary cortisol levels were normalized by total salivary protein concentrations and expressed as pmol/mg protein.
2.5. Gene expression profiling in peripheral blood leukocytes
RNA was isolated using a PAXgene blood RNA kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Contaminated DNA was removed using a DNase-kit (Qiagen). Purified RNA quality was assessed by an Agilent 2100 Bioanalyzer using an RNA 6000 Nano Labchip kit (Agilent Technologies, Santa Clara, CA, USA), and RNA samples with >8.5 RNA integrity number (RIN) were used for further measurements.
The RNA samples were subjected to gene expression analysis using a whole human genome microarray (4 × 44 k, Agilent Technologies) as previously described (Kuwano et al., Citation2011). Microarray data were analyzed by GeneSpring 11.5.1 (Agilent Technologies). The functional pathways related to the set of differentially expressed genes were assessed using the ingenuity pathway analysis (IPA) 9.0 (http://www.ingenuity.com) (Honda et al., Citation2013; Kuwano et al., Citation2011). The probability of a relationship between each biological function and the identified genes was calculated by Fisher’s exact test. The level of significance was set at a p value of 0.05.
2.6. Real-time quantitative PCR (qPCR) validation
Total RNA (400 ng) from each sample was reverse-transcribed using a PrimeScript RT reagent Kit (Takara, Otsu, Japan). Target mRNA levels were measured using a SYBR Green Master Mix (Applied Biosystems) and specific primer sets (Supplementary Table S1). GAPDH mRNA was used as an internal control for normalization. qPCR was performed by an Applied Biosystems 7500 Real-time System (Applied Biosystems).
2.7. Statistical analysis
Data were analyzed using SPSS statistical software package version 22.0 (Chicago, IL, USA). Sex differences between 27 males and 102 females were analyzed by two-tailed Student’s t-tests with α = 0.05 for questionnaires and salivary cortisol. Univariate correlations between SSS and state anxiety scores, depressive mood, salivary cortisol levels, or mRNA levels were analyzed by Pearson’s correlation coefficients with α = 0.05. Next, multiple regression analyses were employed to examine the association between SSS and STAI-state, STAI-trait, or SDS (independent variable). Age, sex (male = 0, female = 1), and body mass index (BMI) were included in the models as potential confounders. The level of statistical significance for the multiple regression analysis was set at α = 0.05.
3. Results
3.1. Characteristics of participants
We recruited 129 hospital staff members (102 females and 27 males). Table shows the characteristics of participants. Age and BMI of all subjects were 44.0 ± 13.0 (mean years ± SD, ranged from 19 to 74 years) and 21.7 ± 2.9 (ranged from 15.2 to 32.5), respectively. STAI and SDS scores are also shown in Table . Mean scores of STAI-state, trait, and SDS were slightly higher than the respective diagnostic thresholds (<41 for STAI-state for males, <42 for STAI-state for females, <44 for STAI-trait for males, <45 for STAI-trait for females, and <40 for SDS) (Fukuda & Kobayashi, Citation1973; Nakazato & Shimonaka, Citation1989). According to the questionnaires, 10.9% of the subjects presented severe anxious feelings and depressive mood. Taken together, our participants, especially females, seemed to be in more stressful situations than average. Salivary cortisol levels measured in the early evening (17:00–18:00) were not different between males and females (Table ).
Table 1. Characteristics of self-reported questionnaires and salivary cortisol in 129 subjects
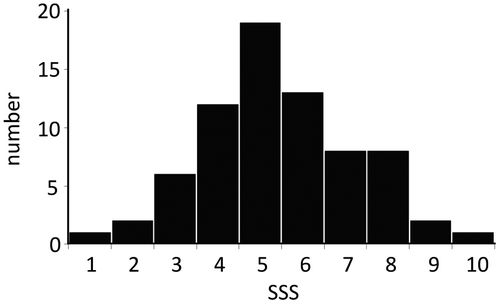
3.2. Distribution of SSS scores
Among these participants, 72 subjects (16 males and 56 females) answered the SSS questionnaire (Table ). The distribution of SSS scores of all subjects is shown in Figure . Their average was calculated to be 5.44 ± 1.80 (mean ± SD). Based on the distribution of SSS scores, we extracted 9 subjects with SSS ≤ 3 as a low-SSS group (41.1 ± 13.5 years old) and 11 subjects as a high-SSS group (SSS ≥ 8, 46.6 ± 9.6 years old) for gene expression analysis. There was no significant difference in age or BMI between the two groups.
Table 2. Scores of SSS
3.3. Correlation between SSS and psychological measures
To examine whether SSS was associated with salivary cortisol levels and mental state (anxiety and depression), the correlation between SSS and STAI or SDS scores was analyzed using multiple regression analysis (Table ). SSS scores significantly and negatively correlated with SDS scores (β = −0.275, p = 0.030), but not with STAI scores or salivary cortisol levels, after adjustment for age. The interaction between SSS scores and BMI was not significant (β = −s0.034, p = 0.778) (Table ).
Table 3. Multiple regression analysis for the associations between SSS and characteristics
3.4. Changes in gene expression in peripheral blood leucocytes from low- and high-SSS groups
SSS was significantly correlated with the score of depressive mood in our subjects. To further investigate this issue, we selected 9 subjects with low SSS scores (low-SSS group; scores 1–3) and 11 subjects with high SSS scores (high-SSS group; scores 8–10), and examined changes in gene expression in peripheral blood leukocytes using a microarray. Microarray analysis showed that 18,861 probes had fluorescence intensities higher than a cut-off value of 20 among all samples. Unpaired t-tests with multiple testing correction and Tukey’s post hoc tests revealed that the expression levels of 1,356 probes were differentially expressed between low- and high-SSS groups (p < 0.05). When the fold-change criterion was set at >1.25-fold in the mean expression level, 670 probes passed the criterion and corresponded to 522 annotated genes.
The differentially expressed genes were then subjected to the biofunctional pathway analysis using IPA. The top 5-scored canonical pathways and biological functions significantly (p < 0.05 by Fisher’s exact test) modified by the 522 genes are shown in Figure (A) and (B), respectively. The top 5-ranked canonical pathways involved according to altered genes were (1) EIF2 signaling (p = 2.90E-15), (2) Leukotriene biosynthesis (p = 4.80E-06), (3) γ-glutamyl cycle (p = 4.80E-06), (4) Regulation of eIF4 and p70S6 K signaling (p = 3.06E-05), and (5) IGF-1 signaling (p = 1.19E-04). The EIF2 signaling-related genes were predominantly up-regulated (red) in the low-SSS group compared with the high-SSS group (Figure (C)). Differentially expressed genes in the EIF2 signaling pathway are listed in Table . The top 5-scored biofunctions were (1) Inflammatory response (p = 1.05E-10), (2) Cellular movement (p = 1.79E-10), (3) Immune cell trafficking (p = 1.79E-10), (4) Infectious diseases (p = 1.82E-9), and (5) Cell death and survival (p = 4.30E-9) (Figure (B)). These results suggest that the perception of SSS may preferentially up-regulate the expression of immune response-related genes in association with increased expression of a group of genes related to protein synthesis machinery.
Figure 2. Ingenuity pathway analysis (IPA) of differentially expressed genes between the low- and high-SSS groups. (A) The top 5-scored canonical pathways significantly modified by the 522 differentially expressed genes between the high- and low-SSS groups were analyzed by IPA. The threshold (p < 0.05) is shown by a dotted line. (B) The top 5-scored biological functions significantly modified by these genes were analyzed by IPA. The threshold (p < 0.05) is shown by a dotted line. (C) The network of genes which relate to EIF2 signaling is displayed graphically as nodes (gene) and edges (the biological relationship between genes). The color intensity indicates the genes up-regulated (red) or down-regulated (green) in the low-SSS group compared with the high-SSS group.
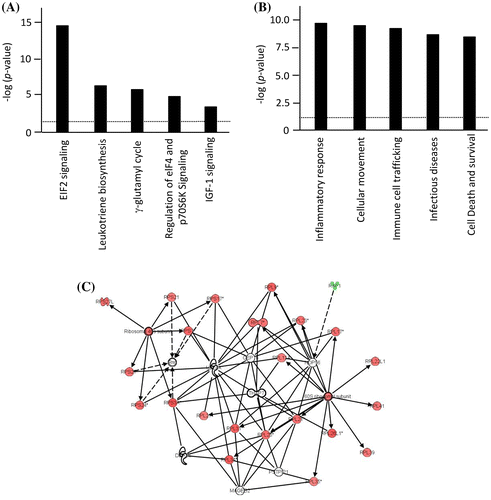
Table 4. Deferentially expressed genes in EIF2 signaling in the low-SSS compared with the high-SSS group
When the fold-change criterion was set at >2.0-fold, five probes passed the criterion and corresponded to four annotated genes (pro-platelet basic protein (PPBP), solute carrier family 1 (glutamine transporter), member 7 (SLC1A7), caspase recruitment domain family member 9 (CARD9), and heterogeneous nuclear ribonucleoprotein U (HNRNPU). These genes were significantly up-regulated in the low-SSS group.
3.5. Validation of differentially expressed genes by qPCR
Next, we confirmed the microarray data using qPCR. The mRNA levels of the four genes (PPBP, SLC1A7, CARD9, and HNRNPU), which were prominently up-regulated in the low-SSS group, were measured in all 72 subjects who answered the SSS questionnaire. Among the four mRNAs, PPBP mRNA levels were significantly increased in the low-SSS group, compared with those in the high-SSS group (Figure (A)). More importantly, multiple regression analysis for the association between SSS and gene expression levels showed that expression levels of both PPBP and SLC1A7 mRNAs were significantly and negatively correlated with SSS scores (β = −0.453, p = 3.26E-05 for PPBP; β = −0.252, p = 0.02 for SLC1A7) (Table , Figure (B)).
Figure 3. Expression of PPBP and SLC1A7 mRNAs in peripheral blood leukocytes from subjects. (A) The mRNA levels for PPBP and SLC1A7 were measured by qPCR and compared between the low- and high-SSS groups. Values are means ± SD. (B) The correlation between SSS scores and PPBP or SLC1A7 mRNA levels in 72 subjects was analyzed by a multiple regression analysis.
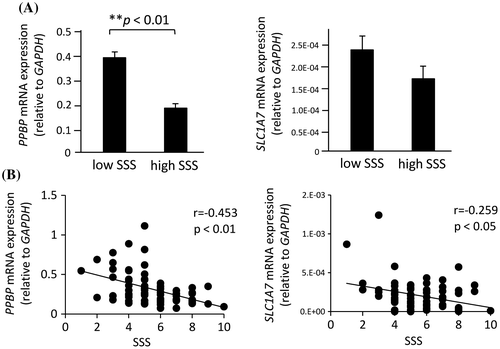
Table 5. Multiple regression analysis for the association between SSS and gene expression levels
4. Discussion
In this study, we demonstrated a significant association between subjective SSS and depressive mood in Japanese medical staffs. The subjects with low SSS scores represented relatively high SDS scores, suggesting that SSS may be one potential risk factor for psychological distress in our cohort. Since individuals with depressed mood may have looked at their SSS more negatively, the utility of the data as a measure of SSS effects may be limited. Further study is needed to examine the direction of causality between development of depression and SSS.
Previous studies in the UK and USA suggested that SSS, an incorporated perception of SES, was associated with psychological distress among men and women (Adler, Koschorreck, & Rechenberg, Citation2008; Demakakos, Nazroo, Breeze and Marmot, Citation2008; Singh-Manoux et al., Citation2005). Moreover, these studies showed that SSS was correlated with mental health independent of SES indicators such as education and income. Correlations between education or income and mental health have been reported in Japan (Honjo et al., Citation2006; Honjo, Kawakami et al., Citation2014b; Kagamimori, Gaina, & Nasermoaddeli, Citation2009). Using a multiple logistic regression analysis of 574 men and 621 women, Sakurai et al. (Citation2010) demonstrated that SSS was a stronger predictor of psychological distress than traditional measures of SES in the Japanese community.
Although genetic factors are involved in the development of mental disorders, environmental stressors also have a significant impact on psychological distress (Kessler, Citation1997). Levels of stress have increased with growing social and economic disparity in recent decades, resulting in a rapid elevation in the prevalence of mental disorders (Kessler et al., Citation2003). Increasing evidence shows the relationship between immune dysfunction and psychological distress. For example, chronic psychological stress changes the levels of inflammatory cytokines and influences host defense (Evans et al., Citation2005; Kamezaki, Katsuura, Kuwano, Tanahashi, & Rokutan, Citation2012; Koo & Duman, Citation2008). Elevated levels of several cytokines including interleukin (IL)-1β, IL-6, and tumor necrosis factor α were reported in depressed patients and influenced the development of depressive symptoms (Dowlati et al., Citation2010; Hannestad, DellaGioia, & Bloch, Citation2011; Howren, Lamkin, & Suls, Citation2009). Lower SSS also affected IL-6 responses to a laboratory stressor (Derry et al., Citation2013). Recent studies have indicated that gene expression profiling of peripheral blood can be used as a biomarker for psychological disorders such as mood disorders (Le-Niculescu et al., Citation2009) and psychosis (Kurian et al., Citation2011). The dysregulation of gene expression in response to psychological stress could be at least partly detectable in peripheral blood leukocytes (Honda et al., Citation2013; Kawai et al., Citation2007; Kurokawa et al., Citation2010). The present microarray analysis showed that 522 genes were differentially expressed in peripheral blood leucocytes between the low- and high-SSS groups. The biofunctional analysis using IPA revealed that the 522 differentially expressed genes preferentially included immune response-related genes, suggesting that stressful situations likely associated with the negative perception of SSS may have a significant impact on immune/inflammatory responses.
PPBP mRNA levels were significantly increased in the low-SSS group, and PPBP and SLC1A7 mRNA levels were significantly and negatively correlated with SSS scores. PPBP encodes pro-platelet basic protein/Nap-2, which is a chemoattractant that guides leukocytes to sites of vascular injury (Ghasemzadeh et al., Citation2013). Experiments using mice showed that exposure to stress for longer periods of time increases levels of Ppbp (Stankiewicz et al., Citation2014). SLC1A7 encodes a high affinity cationic amino acid transporter catalase-1 (CAT-1). At present, there is no report showing a relationship between catalase-1 and SSS. However, a recent study with CAT-1 transgenic mice has shown that catalase-1 functions as an oxidative stress-dependent pressor response to stressful stimuli such as aversive stressors (Rajapakse et al., Citation2014). Psychological stress is involved in the pathogenesis of hypertension. It is possible to speculate that SLC1A7 mRNA levels may be altered by social factors that also influence SSS.
In conclusion, we re-confirmed that SSS is negatively correlated with scores of depressive mood in working adults in Japan, similar to other studies in Western countries. Moreover, we demonstrated that psychological distress alters expression of a group of genes related to immune/inflammatory responses in peripheral blood leukocytes. Our results further suggest that perceptions of social disadvantage may be associated with altered inflammatory responses, which may increase the risk for mood disorders. In addition, we identified the expression levels of PPBP and SLC1A7 in peripheral blood leukocytes as possible biomarkers which correlated with SSS-related mental distress.
Funding
A part of this work was funded by the Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science [grant number 26293169 and 26713027].
Supp_Table.docx
Download MS Word (17.2 KB)Additional information
Notes on contributors
Kinuyo Fujita
Kinuyo Fujita is a PhD student in Department of Pathophysiology at Institute of Biomedical Sciences, Tokushima University graduate school, Japan. In her research project, she examines the relationship between socioeconomic status and psychological distress. Yuki Kuwano, phD, and Tatsuya Nishikawa, MD, PhD, are assistant professors and Kensei Nishida, MD, PhD is an associate professor in Department of Pathophysiology at Institute of Biomedical Sciences, Tokushima University graduate school.
The department of Pathophysiology, established and directed by Kazuhito Rokutan, PhD, focuses on the physiological responses associated with chronic or acute stress and stress-related diseases including depression. The current project is to find out the biological markers to gain insight into responses to a psychosocial stressor. Especially, the lab has worked on the comprehensive analysis of gene expression in human peripheral leukocytes to obtain a better understanding of stress responses.
References
- Adler, N. E., Epel, E. S., Castellazzo, G., & Ickovics, J. R. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychology, 19, 586–592.10.1037/0278-6133.19.6.586
- Adler, N. E., Koschorreck, J., & Rechenberg, B. (2008). Environmental impact assessment and control of pharmaceuticals: the role of environmental agencies. Water Science & Technology, 57, 91–97. doi:10.2166/wst.2008.816
- Cantril, H. (1950). An inquiry concerning the characteristics of man. The Journal of Abnormal and Social Psychology, 45, 490–503. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1542816710.1037/h0059485
- Cohen, S., Alper, C. M., Doyle, W. J., Adler, N., Treanor, J. J., & Turner, R. B. (2008). Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychology, 27, 268–274. doi:10.1037/0278-6133.27.2.268
- Collins, A. L., & Goldman, N. (2008). Perceived social position and health in older adults in Taiwan. Social Science & Medicine, 66, 536–544. doi:10.1016/j.socscimed.2007.10.004
- Demakakos, P., Nazroo, J., Breeze, E., & Marmot, M. (2008). Socioeconomic status and health: The role of subjective social status. Social Science & Medicine, 67, 330–340. doi:10.1016/j.socscimed.2008.03.038
- Derry, H. M., Fagundes, C. P., Andridge, R., Glaser, R., Malarkey, W. B., & Kiecolt-Glaser, J. K. (2013). Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology, 38, 2676–2685. doi:10.1016/j.psyneuen.2013.06.026
- Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., & Lanctôt, K. L. (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67, 446–457. doi:10.1016/j.biopsych.2009.09.033
- Evans, D. L., Charney, D. S., Lewis, L., Golden, R. N., Gorman, J. M., Krishnan, K. R., … Valvo, W. J. (2005). Mood disorders in the medically Ill: Scientific review and recommendations. Biological Psychiatry, 58, 175–189. doi:10.1016/j.biopsych.2005.05.001
- Evans, J. M., Newton, R. W., Ruta, D. A., MacDonald, T. M., & Morris, A. D. (2000). Socio-economic status, obesity and prevalence of Type 1 and Type 2 diabetes mellitus. Diabetic Medicine, 17, 478–480. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1097521810.1046/j.1464-5491.2000.00309.x
- Franzini, L., & Fernandez-Esquer, M. E. (2006). The association of subjective social status and health in low-income Mexican-origin individuals in Texas. Social Science & Medicine, 63, 788–804. doi:10.1016/j.socscimed.2006.01.009
- Fukuda, K., & Kobayashi, S. (1973). [A study on a self-rating depression scale (author’s transl)]. Seishin Shinkeigaku Zasshi, 75, 673–679. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4798819
- Gavin, A. R., Walton, E., Chae, D. H., Alegria, M., Jackson, J. S., & Takeuchi, D. (2010). The associations between socio-economic status and major depressive disorder among Blacks, Latinos, Asians and non-Hispanic Whites: Findings from the Collaborative Psychiatric Epidemiology Studies. Psychological Medicine, 40, 51–61. doi:10.1017/S0033291709006023
- Ghasemzadeh, M., Kaplan, Z. S., Alwis, I., Schoenwaelder, S. M., Ashworth, K. J., Westein, E., … Jackson, S. P. (2013). The CXCR1/2 ligand NAP-2 promotes directed intravascular leukocyte migration through platelet thrombi. Blood, 121, 4555–4566. doi:10.1182/blood-2012-09-459636
- Giatti, L., Camelo, L., & do, V., Rodrigues, J. F., de, C., & Barreto, S. M. (2012). Reliability of the MacArthur scale of subjective social status - Brazilian longitudinal study of adult health (ELSA-Brasil). BMC Public Health, 12, 1387–1102. doi:10.1186/1471-2458-12-1096
- Goodman, E., Adler, N. E., Daniels, S. R., Morrison, J. A., Slap, G. B., & Dolan, L. M. (2003). Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obesity Research, 11, 1018–1026. doi:10.1038/oby.2003.140
- Grotto, I., Huerta, M., Grossman, E., & Sharabi, Y. (2007). Relative impact of socioeconomic status on blood pressure lessons from a large-scale survey of young adults. American Journal of Hypertension, 20, 1140–1145. doi:10.1016/j.amjhyper.2007.06.004
- Hannestad, J., DellaGioia, N., & Bloch, M. (2011). The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology, 36, 2452–2459. doi:10.1038/npp.2011.132
- Hoebel, J., Maske, U. E., Zeeb, H., & Lampert, T. (2017). Social inequalities and depressive symptoms in Adults: The role of objective and subjective socioeconomic status. PLOS ONE, 12, e0169764. doi:10.1371/journal.pone.0169764
- Honda, M., Kuwano, Y., Katsuura-Kamano, S., Kamezaki, Y., Fujita, K., Akaike, Y., … Rokutan, K. (2013). Chronic academic stress increases a group of microRNAs in peripheral blood. PLoS ONE, 8, e75960. doi:10.1371/journal.pone.0075960
- Honjo, K., Kawakami, N., Takeshima, T., Tachimori, H., Ono, Y., Uda, H., … Kikkawa, T. (2006). Social class inequalities in self-rated health and their gender and age group differences in Japan. Journal of Epidemiology, 16, 223–232. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1708587210.2188/jea.16.223
- Honjo, K., Iso, H., Fukuda, Y., Nishi, N., Nakaya, T., Fujino, Y., … JACC Study Group. (2014). Influence of municipal- and individual-level socioeconomic conditions on mortality in Japan. International Journal of Behavioral Medicine, 21, 737–749. doi:10.1007/s12529-013-9337-7
- Honjo, K., Kawakami, N., Tsuchiya, M., Sakurai, K., & WMH-J 2002–2006 Survey Group. (2014). Association of subjective and objective socioeconomic status with subjective mental health and mental disorders among Japanese men and women. International Journal of Behavioral Medicine, 21, 421–429. doi:10.1007/s12529-013-9309-y
- Howren, M. B., Lamkin, D. M., & Suls, J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine, 71, 171–186. doi:10.1097/PSY.0b013e3181907c1b
- Jackman, M. R., & Jackman, R. W. (1973). An interpretation of the relation between objective and subjective social status. American Sociological Review, 38, 569–582. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/474563010.2307/2094408
- Kagamimori, S., Gaina, A., & Nasermoaddeli, A. (2009). Socioeconomic status and health in the Japanese population. Social Science & Medicine, 68, 2152–2160. doi:10.1016/j.socscimed.2009.03.030
- Kamezaki, Y., Katsuura, S., Kuwano, Y., Tanahashi, T., & Rokutan, K. (2012). Circulating cytokine signatures in healthy medical students exposed to academic examination stress. Psychophysiology, 49, 991–997. doi:10.1111/j.1469-8986.2012.01371.x
- Katsuura, S., Kamezaki, Y., Tominaga, K., Masuda, K., Nishida, K., Yamamoto, Y., … Rokutan, K. (2010). High-throughput screening of brief naturalistic stress-responsive cytokines in university students taking examinations. International Journal of Psychophysiology, 77, 135–140. doi:10.1016/j.ijpsycho.2010.05.004
- Kawai, T., Morita, K., Masuda, K., Nishida, K., Shikishima, M., Ohta, M., … Rokutan, K. (2007). Gene expression signature in peripheral blood cells from medical students exposed to chronic psychological stress. Biological Psychology, 76, 147–155. doi:10.1016/j.biopsycho.2007.07.008
- Kessler, R. C. (1997). The effects of stressful life events on depression. Annual Review of Psychology, 48, 191–214. doi:10.1146/annurev.psych.48.1.191
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D., Merikangas, K. R., … Replication, N. C. S. (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289, 3095–3105. doi:10.1001/jama.289.23.3095
- Koo, J. W., & Duman, R. S. (2008). IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences, 105, 751–756. doi:10.1073/pnas.0708092105
- Kopp, M., Skrabski, Á., Réthelyi, J., Kawachi, I., & Adler, N. E. (2004). Self-rated health, subjective social status, and middle-aged mortality in a changing society. Behavioral Medicine, 30, 65–72. doi:10.3200/BMED.30.2.65-72
- Kurian, S. M., Le-Niculescu, H., Patel, S. D., Bertram, D., Davis, J., Dike, C., … Niculescu, A. B. (2011). Identification of blood biomarkers for psychosis using convergent functional genomics. Molecular Psychiatry, 16, 37–58. doi:10.1038/mp.2009.117
- Kurokawa, K., Kuwano, Y., Tominaga, K., Kawai, T., Katsuura, S., Yamagishi, N., … Rokutan, K. (2010). Brief naturalistic stress induces an alternative splice variant of SMG-1 lacking exon 63 in peripheral leukocytes. Neuroscience Letters, 484, 128–132. doi:10.1016/j.neulet.2010.08.031
- Kuwano, Y., Kamio, Y., Kawai, T., Katsuura, S., Inada, N., Takaki, A., & Rokutan, K. (2011). Autism-Associated Gene Expression in Peripheral Leucocytes Commonly Observed between Subjects with Autism and Healthy Women Having Autistic Children. PLoS ONE, 6, e24723. doi:10.1371/journal.pone.0024723
- Lemstra, M., Bennett, N. R., Neudorf, C., Kunst, A., Nannapaneni, U., Warren, L. M., … Scott, C. R. (2008). A meta-analysis of marijuana and alcohol use by socio-economic status in adolescents aged 10-15 years. Canadian Journal of Public Health, 99, 172–177. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18615935
- Le-Niculescu, H., Kurian, S. M., Yehyawi, N., Dike, C., Patel, S. D., Edenberg, H. J., … Niculescu, A. B. (2009). Identifying blood biomarkers for mood disorders using convergent functional genomics. Molecular Psychiatry, 14, 156–174. doi:10.1038/mp.2008.11
- Lorant, V., Deliège, D., Eaton, W., Robert, A., Philippot, P., & Ansseau, M. (2003). Socioeconomic inequalities in depression: A meta-analysis. American Journal of Epidemiology, 157, 98–112. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1252201710.1093/aje/kwf182
- Manuck, S. B., Phillips, J. E., Gianaros, P. J., Flory, J. D., & Muldoon, M. F. (2010). Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosomatic Medicine, 72, 35–45. doi:10.1097/PSY.0b013e3181c484dc
- Muennig, P., Sohler, N., & Mahato, B. (2007). Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: Evidence from NHANES. Preventive Medicine, 45, 35–40. doi:10.1016/j.ypmed.2007.04.005
- Nakazato, K., & Shimonaka, Y. (1989). The Japanese state-trait anxiety inventory: age and sex differences. Perceptual and Motor Skills, 69, 611–617. doi:10.2466/pms.1989.69.2.611
- Rajapakse, N. W., Konstantinidis, G., Evans, R. G., Nguyen-Huu, T.-P., Kaye, D. M., & Head, G. A. (2014). Endothelial cationic amino acid transporter-1 overexpression blunts the effects of oxidative stress on pressor responses to behavioural stress in mice. Clinical and Experimental Pharmacology and Physiology, 41, 1031–1037. doi:10.1111/1440-1681.12279
- Sakurai, K., Kawakami, N., Yamaoka, K., Ishikawa, H., & Hashimoto, H. (2010). The impact of subjective and objective social status on psychological distress among men and women in Japan. Social Science & Medicine, 70, 1832–1839. doi:10.1016/j.socscimed.2010.01.019
- Schumann, B., Kluttig, A., Tiller, D., Werdan, K., Haerting, J., & Greiser, K. H. (2011). Association of childhood and adult socioeconomic indicators with cardiovascular risk factors and its modification by age: the CARLA study 2002-2006. BMC Public Health, 11, 5–298. doi:10.1186/1471-2458-11-289
- Scott, K. M., Al-Hamzawi, A. O., Andrade, L. H., Borges, G., Caldas-de-Almeida, J. M., Fiestas, F., … Kessler, R. C. (2014). Associations between subjective social status and DSM-IV mental disorders. JAMA Psychiatry, 71, 1400–1408. doi:10.1001/jamapsychiatry.2014.1337
- Singh, R. B., Sharma, J. P., Rastogi, V., Niaz, M. A., & Singh, N. K. (1997). Prevalence and determinants of hypertension in the Indian social class and heart survey. Journal of Human Hypertension, 11, 51–56. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/940244710.1038/sj.jhh.1000384
- Singh-Manoux, A., Marmot, M. G., & Adler, N. E. (2005). Does subjective social status predict health and change in health status better than objective status? Psychosomatic Medicine, 67, 855–861. doi:10.1097/01.psy.0000188434.52941.a0
- Spielberger, C. D. (1971). Notes and comments trait-state anxiety and motor behavior. Journal of Motor Behavior, 3, 265–279. doi:10.1080/00222895.1971.10734907
- Stankiewicz, A. M., Goscik, J., Swiergiel, A. H., Majewska, A., Wieczorek, M., Juszczak, G. R., & Lisowski, P. (2014). Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neuroscience, 15, 143–145. doi:10.1186/s12868-014-0130-6
- Stringhini, S., Sabia, S., Shipley, M., Brunner, E., Nabi, H., Kivimaki, M., & Singh-Manoux, A. (2010). Association of socioeconomic position with health behaviors and mortality. JAMA, 303, 1159–1166. doi:10.1001/jama.2010.297
- Velásquez, E., Adela Barón, M., Solano, L., Páez, M., Llovera, D., & Portillo, Z. (2006). Lipid profile in Venezuelan preschoolers by socioeconomic status. Archivos Latinoamericanos de Nutrición, 56, 22–28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16786730
- Zung, W. W. (1965). A self-rating depression scale. Archives of General Psychiatry, 12, 63–70. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1422169210.1001/archpsyc.1965.01720310065008