Abstract
Although previous studies reported a relationship between cognitive dysfunction and depressive symptoms, whether context processing relates to symptoms of depression remains unclear. Hence, the question of whether context processing in depressed individuals is negatively specific or a general deficit also remains unanswered. The purpose of our study was to investigate whether mildly depressed individuals would evince a context processing deficit in response to negative emotional stimuli interference. We employed Emotional AX-CPT (AX version Continuous Performance Task), in which negative distractors were presented in the interval between cue and probe stimuli. ANOVAs revealed that when negative distractors were presented, the depressed group made more BX errors than the non-depressed group, and that the depressed group made more BX errors in response to negative distractors than to neutral distractors. Our results suggest that mildly depressed individuals show a context processing deficit when negatively charged stimuli interfere with retaining contextual information.
Public Interest Statement
Numerous research suggested that depressed individuals had abnormal information processes, such as paying attention to negative things and remembering only unpleasant events. In this article, we investigated the relationships between depressive symptoms and negatively-biased context processing, which involves the adjustment and guide cognitive processes to adaptive behavior with fully using background information. We found that mildly depressed people showed impaired context processing only when negative stimuli interfered in retaining former information. This finding means that when negative events occurred, depressed people would forget things to do later (e.g. preparing a document for an afternoon conference, buying eggs on their way home). Our results would contribute to reveal the cognitive mechanism about the psychopathology of depression and to develop the more effective treatment for depression.
Competing interests
The authors declare no competing interest.
1. Introduction
Recent research has convincingly demonstrated that impaired cognitive control plays a crucial role in maintaining depressive symptoms and/or major depressive disorder relapse (Gotlib & Joormann, Citation2010). Cognitive control involves the maintenance of goal and task sets to guide adaptive behavior (Miller & Cohen, Citation2001). This maintenance system, which includes inhibition, attentional control, switching, working memory, and conflict monitoring, has been extensively examined in terms of its alignment with psychopathology of depression (Hammar & Ardal, Citation2009). For instance, in a sample of depressed individuals, the problem of inhibition-reducing interference from irrelevant information has been observed (Eugène, Joormann, Cooney, Atlas, & Gotlib, Citation2010). Furthermore, regarding attentional control, depression has been linked to the difficulty in disengaging from negative stimuli (Koster, Leyman, De Raedt, & Crombez, Citation2006). Taken together, these findings suggest that depression has complex associations with various functions of cognitive control.
Despite the extensive evidence demonstrating the diversity of functions of cognitive control, cognitive processes underlying the various functions of cognitive control remain unclear. According to several recent studies adhering to the cognitive theory, various cognitive control processes are driven by context processing (Braver, Grayet, & Burgess, Citation2007; Schlaghecken & Martini, Citation2012). Here, context processing refers to a cognitive process that involves any background information required for exhibiting appropriate behavior (Cohen & Barch, Citation1999). Background information frequently employed in cognitive tasks includes, among others, instructions for tasks, temporal cues, and feedback response. In addition, a commonly used tool to measure context processing as a standard procedure is the AX version of Continuous Performance Task (AX-CPT; Braver et al., Citation2001).
Using AX-CPT, context processing has also been investigated in the field of neuroscience. For instance, Lamm, Pine, and Fox (Citation2013) reported that interfering context processing with negative stimuli led to a greater dorsolateral prefrontal cortex (DLPFC) activation. Therefore, the authors suggested that negative emotional interference context processing requires more cognitive resources and prefrontal neural activation. Furthermore, another study showed that cognitive control including context processing is associated with the activation of DLPFC, ventromedial prefrontal cortex (vmPFC), lateral prefrontal cortex (lPFC), and dorsal anterior cingulate cortex (dACC; Chechko et al., Citation2013). For emotional interference, the DLPFC plays an important role in the top-down regulation of emotional processing (Ochsner & Gross, Citation2005). Furthermore, dACC was found to be involved in appraisal and expression of negative emotion (Chechko et al., Citation2013). Taken together, the results of these neuroscientific studies suggest that context processing is intimately related to emotional processing and that both types of processing interact one with another.
The above-mentioned prefrontal areas are well known to be related to depression (see Koenigs & Grafman, Citation2009; Rogers et al., Citation2004). For example, as reported by Zhong et al. (Citation2011), hypofrontality including DLPFC in depression was found to result in the dysfunction to inhibit amygdala activity, thereby causing negative moods, such as depressed mood and anxiety. Furthermore, Harvey et al. (Citation2005) reported that, during an effortful cognitive task performance, depressed patients needed a greater activity in DLPFC and dACC. With regard to emotional processing, depressed patients showed hyperactivity in DLPFC when they needed to respond negatively charged stimuli, while the same effect was not observed in healthy controls (Grimm et al., Citation2008). Based on the results of the studies overviewed above, the interaction between emotional and context processing appears to be related to depression. That is, based on abnormal activation of DLPFC, emotional interference would cause impairments in context processing.
With regard to the relationship between depression and context processing, Msetfi, Murphy, Kornbrot, and Simpson (Citation2009) reported that mildly depressed undergraduates showed impaired context processing in retaining contextual information for 10,000 ms. However, as only the difficulty for retaining contextual information in the long term was related to depression; the effects of emotional interference remain largely unknown. While several studies have revealed the effects of negative emotional interference on context processing in undergraduates (Lamm et al., Citation2013), to the best of our knowledge, none of the available studies has investigated the emotional interference with context processing in depressed individuals.
Summing up, impaired context processing in cognitive control has been assumed to worsen depressive symptoms. Indeed, Msetfi et al. (Citation2009) revealed that depressed individuals had generally impaired context processing. Several neuroscientific research suggested that the activation of DLPFC and dACC related to not only cognitive process toward negative stimulus in depressed individuals but also context processing. Furthermore, in psychological studies, negatively biased cognitive processing such as attentional control, were related to depressive symptoms (Koster et al., Citation2006). However, the negative emotional effect on context processing in depressed individuals has been remained still unclear. Investigating the effect of negative emotional processing on context processing in depressed individuals would contribute to reveal psychopathology in depression and to develop the more effective treatment for depression.
2. Aim
The main aim of the present study was to examine whether emotional processing would interfere with context processing in mildly depressed individuals as compared to healthy controls. Based on previous studies, we hypothesized that, as compared to non-depressed participants, mildly depressed participants would show impaired context processing in response to negative distractors, and that this impairment would be more robust in response to negative distractors than to neutral distractors. To measure the degree of impaired context processing, we developed Emotional AX-CPT embedding negative emotional distractors in AX-CPT.
3. Materials and methods
3.1. Participants
Forty-four undergraduate students participated in this study, and some received course credit for their participation. All participants completed Emotional AX-CPT and Beck Depression Inventory II (BDI-II; Beck, Steer, & Brown, Citation1996). In line with Msetfi et al. (Citation2009) study, we used 9 in BDI-II as the cut-off score to identify mildly depressed participants. Using this criterion, 22 participants (17 females: mean age = 20.86, SD = 2.45) were grouped into the mildly depressed group and 22 participants (13 females: mean age = 20.46, SD = 1.51) were grouped into the non-depressed group. All participants provided written informed consent after the protocol of the study was fully explained.
3.2. Measures
3.2.1. Beck Depression Inventory II
BDI-II is a 21-item self-report questionnaire that assesses the severity of depressive symptoms on a scale ranging from 0 to 3 (Beck et al., Citation1996). Participants completed the Japanese version of BDI-II (Kojima, Furukawa, Takahashi, & Kawai, Citation2002). The internal consistency in this study was α = 0.88.
3.2.2. Emotional AX-CPT
The rules and requirements for Emotional AX-CPT resembled those for AX-CPT; the difference was the presentation of negative distractors during the interval between the presentations of the cue and probe distractors in the former. The task required participants to use previously presented cue stimuli to determine whether responses for probe stimuli were appropriate. Specifically, participants had to provide a target response only for the AX trial, in which the probe stimulus (“X”) preceded the cue stimulus (“A”). Participants had to provide a non-target response for conditions in which the probe stimuli and/or cue stimuli were incongruent with the AX trial, as in the BX, AY, and BY trials.
Unlike in the original version of AX-CPT, emotional stimuli (neutral and negative pictures) taken from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, Citation2008) were presented in the interval between the cue and probe stimuli as distractors. A total of 300 pictures, comprising 150 negative pictures and 150 neutral pictures, were used in Emotional AX-CPT. As shown in Figure , in Emotional AX-CPT each trial started with a presented fixation of 300 ms. A cue then appeared for 500 ms, followed by a distractor for 4000 ms. A probe then appeared and remained on the screen until the participant responded or until 3000 ms had elapsed. The inter-trial interval was 500 ms long. The cue and probe letters were Arial 240 points in size, and the emotional stimuli were adjusted to the same size (326 × 326 pixels).
Figure 1. Construction of AX condition in emotional AX-CPT.
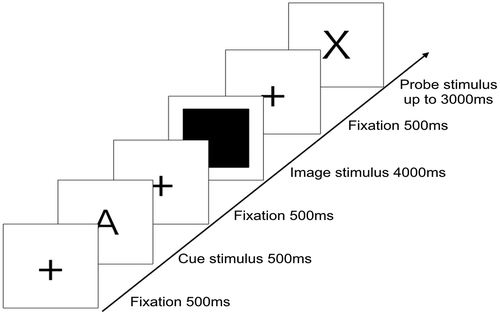
The task comprised 150 negative trials and 150 neutral trials that were randomly presented to the participants on the screen. In each negative and neutral trial, the target trial (AX condition) occurred 70% of the time (105 trials), and each non-target trial (AY, BX, and BY conditions) occurred 10% of the time (15 trials each).
3.3. Procedure
After providing informed consent, participants were asked to complete BDI-II. They were then seated approximately 30 cm from a computer screen, and Emotional AX-CPT was administered. When participants finished half of Emotional AX-CPT, they were given the opportunity to rest for approximately five minutes. To counterbalance the order of the procedure, half of the participants completed BDI-II after Emotional AX-CPT. Emotional AX-CPT was run on PsychoPy (version 2.7.2; Peirce, Citation2009) and displayed on a 15″ laptop.
3.4. Data analysis
For statistical analysis in each emotional and trial condition, the reaction times (RTs) were log transformed, and the accuracy was z-transformed. For the transformation in accuracy, according to previous studies (Cohen & Barch, Citation1999; Servan-Schreiber, Cohen, & Steingard, Citation1996), a correction was applied in case of proportions 1.0 and 0.0 in error rate. 1.0 was replaced with (1−1/3n), while 0.0 was replaced with 1/3n, where n was the number trials. As an additional index, d′-context reflecting sensitivity to contextual information was computed by z(AX hit rate) −z(BX false alarm). In statistical analysis, mixed three-way ANOVAs with trial type (AX, AY, BX, and BY) and emotion (negative, neutral) as within-subject factors, and with group (depressed, non-depressed) as a between-subject factor, were conducted on RTs and error rate as dependent variables. Greenhouse-Geisser corrections were applied where appropriate in ANOVAs.
4. Results
Table shows the descriptive statistics. Depressed group and non-depressed group showed significantly higher score in the BDI-II, whereas did not in age (t(42) = 0.438, p = 0.66) and sex ratio (χ(1) = 1.676, p = 0.20).
Table 1. Descriptive statistics
4.1. Reaction times
For RTs, as shown in Table , the three-way ANOVA showed significant main effect of trial type (p < 0.05), non-significant three-way interactions between trial type, emotion, and group, any two-way interactions, and main effects of emotion and group (all at p > 0.05). Contrast analysis showed that RTs in BX trial were significantly slower that those in AX, AY, BY trial (p < 0.05). These results suggested that the RTs of Emotional AX-CPT were not affected by depression, emotional valence of distractors, though each group showed the difficulty to faster response toward BX trial.
Table 2. Statistics of three-way ANOVAs
4.2. Error rate
For error rate, as shown in Table , the three-way ANOVA revealed a significant three-way interaction between trial type, emotion, and group (p < 0.05). To further examine the interaction, planned contrasts were conducted.
First, a mixed two-way ANOVA (emotion, group) was conducted for each trial type. In BX error, there were significant main effects of emotion and a significant interaction (F(1, 43) = 8.080, p < 0.05; F(1, 38) = 6.900, p < 0.05, respectively). The contrast analysis in the BX error showed that the depressed group made more errors than the non-depressed group did in the negative BX condition, and also made more errors in the negative interference BX trial than in the neutral interference BX trial (p < 0.05). However, no main effect and interaction were observed in the AX, AY, and BY errors (all at p > 0.05).
Next, a two-way ANOVA (trial type, emotion) was conducted for each group. This ANOVA revealed significant main effects and two-way interaction (trial type: F(1.808, 33.743) = 30.995, p < 0.05, ηp2 = 0.57; emotional valence: F(1, 30.995) = 17.701, p < 0.05, ηp2 = 0.44; interaction: F(1.467, 33.743) = 5.664, p < 0.05, ηp2 = 0.20). The contrast analysis revealed that the depressed group made significantly more BX errors than AX, AY, and BY errors in negative condition (p < 0.05). However, the ANOVA for the non-depressed group revealed non-significant main effects and interaction (trial type: F(1.918, 50.529) = 2.38, p = 0.11, ηp2 = 0.10; emotional valence: F(1, 50.529) = 0.240, p = 0.63, ηp2 = 0.01; interaction: F(2.406, 50.529) = 0.198, p = 0.86, ηp2 = 0.01). These results indicated, as we predicted, that the depressed group showed greater impairment in context processing when negative distractors appeared than the non-depressed group did, and that the depressed group made more BX errors when negative stimuli, as compared to neutral stimuli, interfered with retaining contextual information.
4.3. d′-context
For d′-context, the mixed two-way ANOVA (emotion, group) revealed significant interaction (F(1, 42) = 4.880, p < 0.05, ηp2 = 0.10), main effect of emotion interaction (F(1, 42) = 14.857, p < 0.001, ηp2 = 0.26), and non-significant main effect of group (F(1, 42) = 0.046, p > 0.05). The contrast analysis showed that the d′-context of negative valence in the depressed group was significantly lower than that of the neutral valence (p < 0.05). This indicated that the sensitivity in the depressed group reduced when a negative distractor appeared after a cue stimulus.
5. Discussion
In our study, we examined whether individuals with depressed symptoms had impaired context processing when a negative distractor interfered with retaining context information. We found that participants in the mildly depressed group made more errors in the negative BX trial as compared to the neutral BX trial, and more errors than participants in the non-depressed group in the negative BX trial. These results indicated that depression was related to impaired context processing in the case of negative distractors interfering with the retention of context information.
A previous study (Msetfi et al., Citation2009) found evidence of impaired context processing in mild to moderately depressed students by setting cue–probe interval of 10,000 ms in AX-CPT. Despite the fact that Emotional AX-CPT had a cue–probe interval of 5000 ms in our study, we observed impairment similar to Msetfi et al.’s (Citation2009) findings by inserting negative distractors in the cue–probe interval. This indicated that when mildly depressed individuals were exposed to negative distractors, while they were required to retain contextual information for the long term, the dysfunction occurred in the context of holding cue information. Msetfi et al. (Citation2009) also found no significant difference between the depressed and non-depressed students when the cue–probe interval was set at 1000 ms in AX-CPT. Our results revealed no significant difference between individuals with and without depressive symptoms in the neutral condition though the cue–probe interval was set at 5000 ms. This meant that both depressed undergraduates and non-depressed undergraduates could appropriately retain contextual information—if no negative distractor appeared—for up to 5000 ms. Taken together, it is possible that depression-related impaired context processing occurred either when negative distractors interfered with context processing or when there was a relatively long period of having to retain contextual information. In this study, sensitivity to contextual information based on d′-context was also investigated. The statistical analysis revealed a significant difference between neutral and negative emotional valence in the depressed group and no significant difference between the depressed and non-depressed groups. These results indicated that the depressed group represented attenuated context sensitivity when there was negative distractor interference until a probe stimulus appeared.
Our results showed that depressed individuals had negatively biased context processing. This finding was congruent to previous studies (Goeleven, De Raedt, Baert, & Koster, Citation2006; Gupta & Kar, Citation2012) in that depressive symptoms were related only to cognitive dysfunction toward negative materials. For instance, Goeleven et al. (Citation2006) showed that depressed patients had difficulty for inhibiting negative materials, suggesting that depression had been characterized by the negative mood-congruent impairments in cognitive function. However, De Lissnyder et al. (Citation2012) suggested that depression was related not only to cognitive dysfunction toward negative materials but also toward neutral them. In many cognitive functions, whether impairment in the cognitive process would be negative-specific or general remains unclear. Combined our results and those of Msetfi et al. (Citation2009), in context processing, it would be indicated that depressed individuals show negative-specific impaired context processing in the relatively short-term cognitive process, while they show generally impaired context processing in long-term cognitive process.
Recent clinical psychological studies have reported that computerized interventions using cognitive tasks decrease psychopathological symptoms. This intervention has been called cognitive bias modification (CBM) or cognitive control training. Several studies revealed the efficacy of CBM for anxiety and depression (Hallion & Ruscio, Citation2011; Holmes, Lang, & Shah, Citation2009), suggesting that attentional control and interpret bias could be suitable for the target of CBM. For context processing of schizophrenia, but not of depression, Edwards, Barch, & Braver (Citation2010) demonstrated that 2 weeks of computerized intervention using AX-CPT decreased schizophrenic symptoms. Further study, which investigates cognitive intervention for depression with targeting impaired context processing, would be needed.
In conclusion, although the results of this study indicate a relatively slight link between depression and context information, owing to the small sample size and minimal cut-off point in BDI-II, we found that impaired retention of contextual information was shown when negative emotional interference occurred in mildly depressed individuals. This impairment might have been the result of attenuated sensitivity to contextual information.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Akihiro Masuyama
Akihiro Masuyama is a PhD student at the Tsukuba University, cognitive psychology. His main fields of interest comprise the relationships between depressive symptoms and impaired/biased information processing such as cognitive control, working memory, executive functions. In current research, he mainly investigated depression-related impaired cognitive control with highlighting context processing.
Yuriko Kaise
Yuriko Kaise is a graduate student in Health Science University in Hokkaido. Her main interest is the relationships between cognitive function and psychopathological symptoms such as addiction, anxiety, depression.
Yuji Sakano
Yuji Sakano is a professor in Health Science University in Hokkaido. He also works in hospital as a clinical psychologist, and is specialist of cognitive behavior therapy.
Satoshi Mochizuki
Satoshi Mochizuki is a professor in Tsukuba University. His main research interest is executive function and cognitive disorder.
References
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation.
- Braver, T. S., Barch, D. M., Keys, B. A., Carter, C. S., Cohen, J. D., Kaye, J. A., … Reed, B. R. (2001). Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: Genaral, 130, 746–763. doi:10.1037/0096-3445.130.4.746
- Braver, T. S., Grayet, J. R., & Burgess, G. C. (2007). Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In A. Conway, C. Jarrold, M. Kane, A. Miyake, & J. Towse (Eds.), Variation in working memory (pp. 76–106). NewYork, NY: Oxford University Press.
- Chechko, N., Augustin, M., Zvyagintsev, M., Schneider, F., Habel, U., & Kellermann, T. (2013). Brain circuitries involved in emotional interference task in major depression disorder. Journal of Affective Disorders, 149, 136–145. doi:10.1016/j.jad.2013.01.013
- Cohen, J., & Barch, D. (1999). Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive task. Journal of Abnormal Psychology, 108, 120–133. doi:10.1037/0021-843X.108.1.120
- De Lissnyder, E., Koster, E. H. W., Everaert, J., Schacht, R., Van den Abeele, D., & De Raedt, R. (2012). Internal cognitive control in clinical depression: General but no emotion-specific impairments. Psychiatry Research, 199, 124–130. doi:10.1016/j.psychres.2012.04.019
- Edwards, B. G., Barch, D. M., & Braver, T. S. (2010). Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Frontiers in Human Neuroscience, 4, 32. doi:10.3389/fnhum.2010.00032
- Eugène, F., Joormann, J., Cooney, R. E., Atlas, L. Y., & Gotlib, I. H. (2010). Neural correlates of inhibitory deficits in depression. Psychiatry Research, 181, 30–35. doi:10.1016/j.pscychresns.2009.07.010
- Goeleven, E., De Raedt, R., Baert, S., & Koster, E. H. W. (2006). Deficient inhibition of emotional information in depression. Journal of Affective Disorders, 93, 149–157. doi:10.1016/j.jad.2006.03.007
- Gotlib, I., & Joormann, J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. doi:10.1146/annurev.clinpsy.121208.131305
- Grimm, S., Beck, J., Schuepbach, D., Hell, D., Boesiger, P., Bermpohl, F., … Northoff, G. (2008). Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry, 63, 369–376. doi:10.1016/j.biopsych.2007.05.033
- Gupta, R., & Kar, B. (2012). Attention and memory biases as stable abnormalities among currently depressed and currently remitted individuals with unipolar depression. Frontiers in Psychiatry, 3, 99. doi:10.3389/fpsyt.2012.00099
- Hallion, L. S., & Ruscio, A. M. (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin, 137, 940–958. doi:10.1037/a0024355
- Hammar, A., & Ardal, G. (2009). Cognitive functioning in major depression–a summary. Frontiers in Human Neuroscience, 3, 26. doi:10.3389/neuro.09.026.2009
- Harvey, P.-O., Fossati, P., Pochon, J.-B., Levy, R., Lebastard, G., Lehéricy, S., … Dubois, B. (2005). Cognitive control and brain resources in major depression: An fMRI study using the n-back task. NeuroImage, 26, 860–869. doi:10.1016/j.neuroimage.2005.02.048
- Holmes, E. A., Lang, T. J., & Shah, D. M. (2009). Developing interpretation bias modification as a “cognitive vaccine” for depressed mood: Imagining positive events makes you feel better than thinking about them verbally. Journal of Abnormal Psychology, 118, 76–88. doi:10.1037/a0012590
- Koenigs, M., & Grafman, J. (2009). The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research, 201, 239–243. doi:10.1016/j.bbr.2009.03.004
- Kojima, M., Furukawa, T. A., Takahashi, H., & Kawai, M. (2002). Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Research, 110, 291–299. doi:10.1016/S0165-1781(02)00106-3
- Koster, E. H. W., Leyman, L., De Raedt, R., & Crombez, G. (2006). Cueing of visual attention by emotional facial expressions: The influence of individual differences in anxiety and depression. Personality and Individual Differences, 41, 329–339. doi:10.1016/j.paid.2005.12.022
- Lamm, C., Pine, D. S., & Fox, N. A. (2013). Impact of negative affectively charged stimuli and response style on cognitive-control-related neural activation: An ERP study. Brain and Cognition, 83, 234–243. doi:10.1016/j.bandc.2013.07.012
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Technical Report A-8). Gainesville, FL: University of Florida.
- Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
- Msetfi, R. M., Murphy, R. A., Kornbrot, D. E., & Simpson, J. (2009). Impaired context maintenance in mild to moderately depressed students. Quarterly Journal of Experimental Psychology, 62, 653–662. doi:10.1080/17470210802486092
- Ochsner, K., & Gross, J. (2005). The cognitive control of emotion. Trends in cognitive sciences, 9, 242–249. doi:10.1016/j.tics.2005.03.010
- Peirce, J. W. (2009). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2. doi:10.3389/neuro.11.010.2008
- Rogers, M. A., Kasai, K., Koji, M., Fukuda, R., Iwanami, A., Nakagome, K., & Kato, N. (2004). Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research, 50, 1–11. doi:10.1016/j.neures.2004.05.003
- Schlaghecken, F., & Martini, P. (2012). Context, not conflict, drives cognitive control. Journal of Experimental Psychology: Human Perception and Performance, 38, 272–278. doi:10.1037/a0025791
- Servan-Schreiber, D., Cohen, J. D., & Steingard, S. (1996). Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of General Psychiatry, 53, 1105–1113. doi:10.1001/archpsyc.1996.01830120037008
- Zhong, M., Wang, X., Xiao, J., Yi, J., Zhu, X., Liao, J., … Yao, S. (2011). Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biological Psychology, 88, 233–242. doi:10.1016/j.biopsycho.2011.08.007
