Abstract
Objectives: To critically review evidence for associations between long-term cortisol levels, mood, and lifestyle factors.Systematic searches of electronic databases (MEDLINE, EMBASE, PsycINFO, WoS, and CINAHL) were conducted up to 21/11/2020 to identify observational and interventional studies (n = 4971) reporting associations between one or more lifestyle or mood factor with cortisol outcomes measured over ≥4 weeks in healthy adults. Quality of included studies was assessed using Downs and Black checklist. The quality of evidence supporting the associations of lifestyle and mood with long-term cortisol levels was assessed as being of moderate-to-poor quality. Observational studies (n = 25) indicated positive associations for BMI/body weight (ESr, pooled effect size correlation = 0.15, p<.001), physical activity (ESr=0.16, p<.001), perceived stress (ESr=0.114, p = .02), and depression (ESr = 0.133, p = .02), but not stressors (ESr = 0.06, p = .29), anxiety (ESr = 0.08, p = .14), or specific features of stress (ESr = 0.25, p = .10). There was insufficient evidence to reliably estimate associations between long-term cortisol levels and sleep, smoking, alcohol consumption, caffeine consumption, and PTSD. Findings from interventional studies (n = 27) were mixed and did not always support the relationships found in observational studies. The findings of this review were limited by the quality of the evidence. Current evidence for associations between mood and lifestyle factors with long-term levels of cortisol is mixed. For many factors, there was considerable uncertainty regarding the size of association with long-term cortisol due to a paucity of evidence. Future research should aim to (1) follow more consistent sampling protocols between studies and (2) clearly describe the hypothesised mechanisms through which interventions would affect cortisol levels.
Public Interest Statement
Cortisol is a hormone secreted by the adrenal glands. It plays a critical role in how we respond to stressful experiences and to behaviours, such as sleep, physical activity, diet, and smoking that can change during stress. In this review, we have brought together evidence from both observational studies and trials that have looked at the relationship between cortisol measured ≥4 weeks and mood and behaviours. Observational studies suggest greater weight, physical activity, perceived stress, and depression are associated with greater cortisol levels. The relationships between smoking and alcohol consumption and cortisol were inclusive because the evidence was insufficient. Findings from trials were mixed and did not always support the associations found in observational studies. Overall, the current evidence is mixed in quality and quantity. We recommend that future studies clearly describe why they measure cortisol, and directions of the associations between cortisol, mood, and behaviour they expect to find.
1. Introduction
The hypothalamic–pituitary–adrenal (HPA) axis is a critical pathway mediating the relationship between stress and health (Tsigos & Chrousos, Citation2002), and is among the most commonly investigated in the context of acute and chronic stress (McEwen & Wingfield, Citation2003). The final product of the HPA axis, cortisol, is a glucocorticoid steroid hormone secreted by the adrenal cortex, and is often characterised as a ’stress hormone’ because of its critical role in the physiological response to psychological stress. However, when stressors occur, both physiological (e.g. hormone secretions) and behavioural (e.g. alcohol consumption and smoking) responses are triggered which (in the case of an adaptive response), contribute to the restoration and maintenance of homeostasis. For example, stress-related food consumption can contribute to changes in cortisol levels, which, in turn, impact glucose homeostasis and energy deposition (McEwen & Wingfield, Citation2003). Thus, cortisol is associated not only with mood, but also with a range of lifestyle factors that respond to and are affected by stress-related changes in homeostasis and circadian rhythms, e.g. sleep, physical activity, diet etc.
Several earlier reviews have examined the relationship between cortisol, mood, and lifestyle factors. However, these have been limited in a variety of ways, e.g. by focusing on one type of cortisol measure (e.g. hair cortisol); a specific cortisol index (e.g. diurnal cortisol slopes); or a specific population. For example, Adam et al. (Citation2017) examined the association of diurnal cortisol slopes only with mental and physical health outcomes. For mental health outcomes, they found that flatter cortisol diurnal slopes were significantly associated with increased risk of depression, externalising disorders, and internalising disorders. In contrast, Lopresti and colleagues conducted a narrative review describing the relationship between cortisol and diet, sleep and exercise (Lopresti et al., Citation2013). For the diet, they found that diets high in fat and saturated fatty acids were associated with disturbed cortisol diurnal curves, while Omega-3 supplements were associated with lower or blunted cortisol activity. For sleep, they found evidence for higher cortisol levels in individuals with insomnia. For exercise, although some evidence suggests that acute exercise elevated acute levels of cortisol, the relationship between exercise in general and the HPA activity is complex and appears to be influenced by the type, duration, intensity, and chronicity of the exercise and the measure of HPA activity (Lopresti et al., Citation2013).
Although both reviews have helped enhance our understanding of the relationship between cortisol and mood and cortisol and lifestyle, their focus on a specific population and a limited number of indices of cortisol, mood and lifestyle necessarily limit conclusions. This is further compounded by the fact that both reviews focused on acute/short-term measures of cortisol. This, in part, reflects the history of research in this field, which has focused on short-term measures of cortisol (such as awakening cortisol, evening cortisol, or immediate cortisol levels after intervention or challenge). However, the pulsatile nature of the hormone (Young et al., Citation2004) and concerns regarding the health relevance of single isolated measures have increased interest in capturing levels over longer periods of time. (Adam et al., Citation2017)
This has been facilitated by innovations in the field and, in particular, the development of methods to assess cortisol in hair, with hair cortisol concentrations (HCC) being able to provide a description of cortisol levels over periods of one to 6 months (Stalder & Kirschbaum, Citation2012). Wosu et al. (Citation2013) and Stalder, Steudte-Schmiedgen et al. (Citation2017) conducted separate systematic reviews to investigate correlates of HCC. Wosu et al. (Citation2013) examined the association between HCC and socioeconomic factors (e.g. income, education, etc.), occupational stressors (e.g. shift work), age, sex, ethnicity, psychiatric symptoms and disorders (e.g. bipolar disorder and PTSD), adrenocorticoidal conditions (e.g. Cushing syndrome), pregnancy, early life adversity (e.g. childhood maltreatment), BMI, waist-to-hip ratio, alcohol use, cigarette smoking, oral contraceptive use, and physical activity (Wosu et al., Citation2013). Focusing on their findings of direct interest in this review (i.e. indices of mood and lifestyle) they found associations between HCC and psychiatric symptoms and disorders, occupational stressors, physical activity, BMI, waist-to-hip ratio, and alcohol intake. They also reported that the available evidence did not support an association between HCC and cigarette smoking (Wosu et al., Citation2013). They also did not find sufficient evidence to examine the association between diet, sleep quality, caffeine consumption, and HCC.
However, while comprehensive, the Wuso et al. review only narratively synthesised evidence on possible correlates of HCC. In contrast, Stalder, Steudte-Schmiedgen et al. (Citation2017) quantitatively investigated the correlation between HCC and age, sex, BMI, waist-to-hip ratio, hair washing frequency, hair treatment, smoking, oral contraceptive use, blood pressure, previous and current chronic stressors, social support, and depression symptoms. Again focusing on mood and lifestyle indices alone, their meta-analysis showed that HCC was significant and positively associated with previous and current chronic stress, BMI, waist-to-hip ratio, blood pressure, and negatively associated with anxiety disorders. However, no significant associations were found between HCC and other mood disorders, social support, or smoking.
In sum, previous reviews have been limited by their focus on a restricted range of indices of cortisol, mood, and/or lifestyle factors and none, to date, have included evidence from intervention studies. Thus, we sought in this review to address these gaps and provide an updated synthesis of the evidence pertaining to the nature and direction of the associations between long-term levels of cortisol, lifestyle, and mood factors.
2. Method
2.1. Eligibility criteria
2.1.1. Population
This review focussed on healthy adults aged 18–65 years with no medical condition or mental disorder and with no medication intake. This was because conditions, such as Cushing’s syndrome major depressive disorder, and use of certain medications (e.g. steroids), are associated with changes in cortisol levels (Casals & Hanzu, Citation2020; Rothe et al., Citation2020), which may confound the relationship between lifestyle/mood and long-term cortisol. Patients undergoing any type of medical treatment were excluded for similar reasons.
2.1.2. Cortisol measures
This review focused on long-term levels of cortisol only. This included hair cortisol and longitudinal changes in cortisol measured in either blood, saliva, or urine. Hair cortisol is usually measured in 1–3 cm hair segments representing cortisol in the past 1–3 months Stalder & Kirschbaum, Citation2012). Although other cortisol indices, such as cortisol reactivity, CAR, AUCg, and diurnal slope are typically considered as acute cortisol indices (Saxbe, Citation2008), studies that investigated longitudinal changes of such cortisol indices were included in this review. The minimum time frame for studies measuring cortisol in hair is 1 month (4 weeks). Therefore, to assess evidence that could be considered to be comparable, repeated measurements of cortisol using any of the aforementioned acute measures (i.e. blood, saliva, urine, and follicular fluid; Stalder & Kirschbaum, Citation2012) had to cover a minimum period of at least 4 weeks to be included in this review. Studies involving the manipulation of cortisol levels through pharmacological agents were excluded.
2.1.3. Intervention
Interventional studies targeting lifestyle factors (physical activity, diet, caffeine consumption, alcohol consumption, smoking, and sleep), surrogates for lifestyle (e.g. BMI), and measures of mood (e.g. stress, anxiety, depression, and PTSD) were included.
2.1.4. Outcomes
Reported associations between long-term levels of cortisol and lifestyle and/or mood factors served as outcomes. In interventional studies where acute levels of cortisol were measured repeatedly, reported changes in cortisol levels at different time points within and between groups were also regarded as outcome variables. Comparisons of cortisol indices between patients and healthy participants were excluded, where relationships between lifestyle factors and cortisol indices were not measured and reported. Intervention effects on both lifestyle/mood and cortisol were also reported as outcomes for interventional studies. For studies reporting associations between cortisol and multiple lifestyle or mood factors, each association was included based on the lifestyle or mood factors reported. However, if a study reported an association between cortisol and combined lifestyle or mood factors as the outcome, these outcomes were removed due to difficulties inherent in separating the influence of different parameters.
2.1.5. Study design
Both observational and interventional studies are included in this systematic review. To meet the inclusion criteria, interventional studies had to show evidence of changes in at least one lifestyle factor, including BMI, physical activity, diet, caffeine consumption, alcohol consumption, smoking, and sleep or at least one mood factor, including stress, anxiety, depression, PTSD. Non-interventional studies had to investigate the relationship between any of the aforementioned lifestyle or mood factors and cortisol.
2.2. Exclusion criteria
Reviews and conference abstracts were excluded from the review. Editorials, newspaper articles, and other forms of popular media were excluded. Case studies and qualitative studies were excluded as well. Non-English papers were excluded. Failure to meet any one of the above eligibility criteria resulted in exclusion from the review. A number of excluded studies and reasons for exclusion for those excluded following a review of the full text were recorded at each stage.
2.3. Information search
The systematic review was registered with PROSPERO (No. CRD42019112339) and the protocol can be accessed here. Searches were conducted in the following electronic databases to identify relevant articles: MEDLINE, EMBASE, PsycINFO, Web of Science, and CINAHL. The initial search was conducted on 23 January 2018 and and updated on 22 October 2018 which to identify one recently published study. Another update was conducted on 21 November 2020 which did not identify any eligible studies. Reference lists of eligible papers were also searched to locate additional studies that were not identified by the database search but may be eligible for the review.
Medical Subject Heading (MeSH) terms combined with free-text searches were used and adjusted for each database as necessary. The search was limited to full text and English articles. The title and abstract of each article were checked manually against the inclusion/exclusion criteria to exclude irrelevant articles or studies that did not fit the objectives of this review. Given the large number of results returned from the initial search, title, and abstract screening was conducted by one reviewer (RJ) only. Full-text screening and assessment were conducted by two reviewers (RJ and SC) with strong inter-rater reliability (Cohen’s kappa = 0.83) on inclusion/exclusion. Disagreement on inclusion or exclusion for 6 out of 157 studies were resolved via discussion.
2.4. Assessment of risk of bias
Risk of bias was assessed using the 27-item Downs and Black checklist (Downs & Black, Citation1998) which was identified as being suitable for use in systematic reviews of both trials and non-trials (Babu et al., Citation2016; Deeks et al., Citation2003; Donnelly et al., Citation2016). The checklist assesses five domains of the study reporting and design: reporting, external validity, internal validity (bias), internal validity (confounding), and power. Item 27 on the checklist was modified to suit the studies assessed (Huffer et al., Citation2017). For each item, a score of 1 was given if it answers yes and 0 if it answered no or was unable to determine an item. Not applicable (N/A) was recorded on the checklist where the item did not correspond well with the study design. The assessment of the risk of bias was conducted by two reviewers (RJ and SC) independently with 93% agreement on the items. Discrepancies were recorded and discussed between reviewers (RJ and SC) until agreements were achieved. Categorisation of the total risk of bias scores was adapted from Huffer et al. (Citation2017) and is presented in Supplementary Appendix Table 3.
2.5. Data extraction and meta-analysis
Data extraction was conducted independently by two reviewers (RJ and SC). Data extraction for each study included information on publication year, country or region where the study was conducted, sample size, age of study sample, population description, design of the study (observational or interventional), protocol for measuring cortisol (type of cortisol, cortisol indices calculated, time period measured, times of samples, number of samples per day).
Due to the high heterogeneity in the designs of intervention studies (e.g. type of lifestyle, length of follow-up, and type of cortisol measure) meta-analyses were conducted only for observational studies. Meta-analyses using random-effects models were conducted to determine the correlation between long-term levels of cortisol and BMI, physical activity, perceived stress, anxiety, and depression, where data were sufficient and available. Effect sizes were expressed as Pearson’s r for all studies. When r values were available from the study they were entered into the meta-analysis. Otherwise, the r values were calculated from other statistics available (e.g. p-values and sample sizes). When long-term cortisol levels were measured at multiple points in a study (e.g. different trimesters of pregnancy), reported correlations at each time point were treated as independent. When the correlation of cortisol with lifestyle or mood factors was reported separately for subgroups (e.g. men and women), results for each group were treated as independent. Comprehensive Meta-analysis Software (Version 13.0) was used to calculate the pooled correlations (ESrs).
3. Results
3.1. Study characteristics
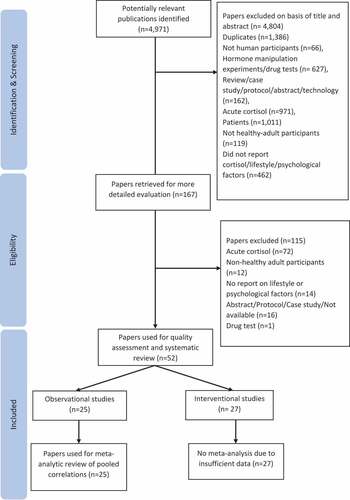
The database search resulted in 4,971 full titles. By 21 November 2020 a total of 52 publications including 25 observational and 27 interventional studies were included in the review and results reported after title and abstract screening and full-text assessment. The flow diagram of the screening and assessment process is presented in . Studies included in the review were conducted between 1999 and 2018, across 16 countries including the US, Brazil, China, and several European countries. Sample sizes ranged from n = 23 to n = 768 (median = 164) for observational studies and from n = 10 to n = 135 (median = 30) for interventions. and present summaries of the studies.
Table 1. Summary of Studies Investigating Long-term Cortisol and Lifestyle Factors
Table 2. Summary of Studies Investigating Long-term Cortisol and Mood
3.1.1. BMI and body weight
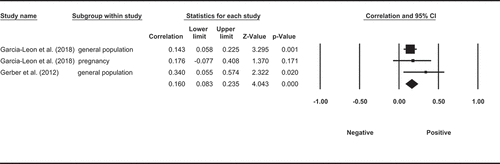
Eight observational studies examined the association between BMI or body weight and long-term cortisol levels in hair. Six studies did not find a significant association between BMI and HCC whilst two studies found that HCC was positively associated with higher BMI. A meta-analysis (), which included available data from six of these eight studies showed a positive and significant pooled correlation between BMI and HCC (ESr, pooled effect size correlation = 0.148, p< 0.001, CI = [0.213, 4.301]). However, the two studies that were not included in the meta-analysis for insufficient data both reported null association of HCC and BMI (n = 85, X. Chen et al. (Citation2015), n = 58, Heinze et al. (Citation2016)).
Figure 2. Random effect meta-analysis for the correlation between long-term levels of cortisol and BMI.
One intervention examined whether a 24-week moderate caloric restriction by diet alone or with an aerobic exercise intervention could result in decreased salivary cortisol levels (Tam et al., Citation2014). They found significant weight loss in the diet groups (both with and without exercise) and significantly higher morning and diurnal cortisol levels at week 24 compared to baseline in all groups. However, group, time, and sex effects on cortisol levels were not found.
In summary, although only two out of the eight observational studies reported a significant positive association between cortisol measured in hair and BMI, this observation was supported by the results of the meta-analysis. However, the intervention showed that although a 25% calorie restriction diet along or with exercise contributed to significant weight loss, it did not induce changes in cortisol levels.
3.1.2. Diets
No observational studies examined the relationship between diet and cortisol levels. Three studies examined the effects of dietary interventions on long-term levels of cortisol, among which two measured cortisol in the blood and one in saliva. The two studies did not specify the hypothesised intervention effects on cortisol. B. D. Brown et al. (Citation2002) compared the effects of cortisol measured in the blood of a high saturated fat Western control diet with two other diets: the control diet plus soy protein (soy diet) and the control diet with polyunsaturated fat (PUFA diet) in women. They found that compared with the control diet, PUFA diet induced a decrease in cortisol, while no significant changes in cortisol measured in blood were found for the soy diet. Torres et al. (Citation2008) compared the effects of salivary cortisol on a low-sodium, high-potassium diet and a high-calcium diet with a moderate-sodium, high-potassium, high-calcium diet. The study reported that higher cortisol levels were positively associated with greater vigour, lower fatigue, and higher levels of urinary potassium and magnesium. Diment et al. (Citation2012) hypothesised that participants who underwent a daily nutritional supplement intervention would have decreased plasma cortisol levels at post-exercise compared to baseline. However, this hypothesised intervention effect was not found. Taken together, the effects of these dietary interventions on long-term levels of cortisol seem to be null. However, due to the high heterogeneity of these studies, definitive conclusions cannot be drawn.
3.1.3. Physical activity
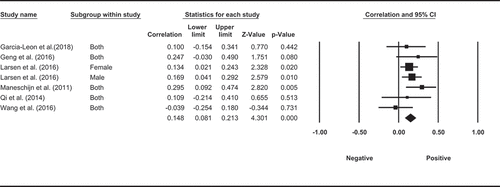
Three observational studies examined the association between physical activity and HCC, with HCC measured over 3 months in all three studies. However, none of the studies measured physical activity for 3 months (i.e. the same period as cortisol). Two studies measured physical activity in participants for a typical week, among which one measured physical activity levels using accelerometers, and found a correlation between greater physical activity and greater HCC, and the other assessed self-reported frequency of exercise per week and did not find significant association between physical activity and HCC. Another study found that participants who were self-reported regular exercisers in general had higher HCC, although the same result was found in a subgroup of pregnant women. Meta-analysis of the three studies (Garcia-Leon et al., Citation2018; Gerber et al., Citation2013; Sumra & Schillaci, Citation2015) showed a significant positive pooled correlation between physical activity and HCC (r = 0.160, p < 0.001, CI = [0.235, 4.043], ).
Figure 3. Random effect meta-analysis for the correlation between long-term levels of cortisol and physical activity.
Six interventions examined the effects of physical activity or exercise on long-term levels of cortisol measured in blood, saliva, and urine, including two yoga interventions and four exercise/training programmes. Only one of these studies hypothesised the direction of intervention effects on cortisol (P. J. Chen et al., Citation2017). Two studies examined the effect of yoga on salivary cortisol through reducing stress, among which one found reduced perceived stress post-intervention and reported that cortisol levels in saliva were higher in the morning and decreased during the day, reaching lower values before sleep, compared to baseline where participants had an increase in cortisol before sleep (Batista et al., Citation2015). Another study did not find a significant effect of yoga on long-term salivary cortisol levels but reported higher levels of long-term cortisol in the intervention group (P. J. Chen et al., Citation2017). Four studies examined the effects of different training programs on cortisol. Mangine et al. (Citation2017) found that greater cortisol levels measured in blood in response to exercise in the high-volume moderate-intensity training group were observed at week 8 after training, compared to the high-intensity low-volume group. Papacosta et al. (Citation2013) observed that evening salivary cortisol fell below baseline at the beginning of the tapering (i.e. gradual reduction in the training load) week and then returned to baseline by the end of the taper training. Tanskanen et al. (Citation2011) reported decreased basal serum cortisol levels from week 4 to week 7 of the training and significantly higher basal serum cortisol levels in participants who were overreaching after 8 weeks of training. Timon et al. (Citation2013) did not observe any significant changes in cortisol in urine following a strength training intervention across the menstrual cycle in women.
The observational studies suggested that physical activity is likely to be positively associated with cortisol measured in hair, which was somewhat supported by results of interventions, with four out of six trials reporting significant changes in cortisol levels after physical activity interventions. However, the types of physical activity and intervention effects on cortisol were all different among these studies. Therefore, we conclude that although physical activity is likely to be associated with cortisol levels, the direction of the relationship remains unclear.
3.1.4. Sleep
There is currently insufficient evidence to conclude the association between sleep and long-term levels of cortisol from both observational and interventional studies. Only one observational study investigated the relationship between HCC over 3 months and self-reported sleep over an unclear time frame and no significant relationship between self-reported sleep quality and HCC was found (Wang et al., Citation2016). Only one intervention examined whether a sleep restriction intervention would reduce early night plasma cortisol (Miller et al., Citation2015). They found that eight out of eleven insomniacs who completed the intervention showed decreases in insomnia severity after the treatment. Contrary to their hypothesis, however, results from six patients showed that cortisol levels in the early morning were significantly higher post-intervention compared to baseline.
3.1.5. Smoking
Three observational studies examined the association between smoking and HCC and none of them found a significant association. Of the three studies, two measured HCC over 3 months and asked participants whether they were smokers. One other study measured HCC over 3–6 months and measured smoking and smoking-related problems in the past 3 months using the Alcohol, Smoking and Substance Involvement Screening Test. Meta-analysis of the association between smoking and HCC was not conducted due to insufficient data.
Only one trial assessed the effect of smoking cessation intervention on cortisol levels measured in hair. Goldberg et al. (Citation2014) found that participants’ HCC was lower at 1-month post-quit compared to the month before the mindfulness-based intervention and that those who stayed abstinent at the 1-month post-quit had significantly lower levels of HCC compared with those in participants who had relapsed. Differences in HCC and smoking cessation between the two intervention groups were not found. Participation in mindfulness training and smoking cessation itself was found to be independently associated with reductions in hair cortisol.
Evidence from observational and interventional studies seemed contradictory: none of the three observational studies reported a significant association between smoking and HCC, but the intervention found reduced HCC among participants of a smoking cessation intervention.
3.1.6. Alcohol consumption
Only one observational study investigated the association between alcohol consumption and HCC and reported that there was no significant correlation between self-reported use of alcohol and HCC measured over 6 months. (Heinze et al., Citation2016). With insufficient evidence, the association between alcohol consumption and long-term levels of cortisol is unclear.
3.1.7. Alcohol withdrawal and cortisol responses to tasks
Two trials involving alcohol did not specifically investigate the effects of alcohol drinking or abstinence on long-term cortisol levels; instead, they both examined alterations in the HPA axis in alcoholic patients in experimental conditions, after long-term alcohol withdrawal. Although the study designs were different, both trials found some evidence that serum cortisol responses to tasks were blunted in alcoholic participants compared with non-alcoholic ones (Coiro et al., Citation2007; Sinha et al., Citation2011).
3.1.8. Caffeine
Evidence is insufficient to determine the influence of caffeine intake on long-term levels of cortisol. No observational study has reported an association between caffeine consumption and long-term levels of cortisol. Only one interventional study examined cortisol responses measured in saliva to a caffeine challenge after controlled levels of daily caffeine intake over 4 weeks (Lovallo et al., Citation2005). They observed that taking caffeine capsules on the test day after 5 days of abstinence resulted in a significant elevation of cortisol throughout the entire day. Significant elevations of cortisol for about 6 hours on the test day were seen in the group that had caffeine intake of 300 mg for five days, but not 600 mg. Lovallo et al. (Citation2005) concluded that daily caffeine intake caused a partial but not complete tolerance to caffeine’s effects on cortisol secretion and that elevated cortisol levels may occur in the afternoon hours in those consuming repeated doses throughout the day.
3.2. Mood correlates of cortisol
Twenty-three observational studies reported a relationship between chronic levels of cortisol and mood factors including stress, anxiety, depression, psychological distress, post-traumatic stress disorder (PTSD), coping efficacy, psychological functioning, mindfulness, and fatigue.
3.2.1. Perceived stress
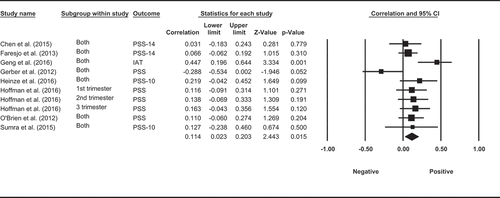
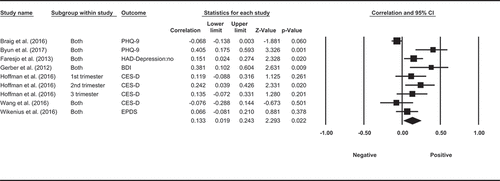
A total of 10 observational studies investigated the association between cortisol and self-reported perceived stress, with 10 using the Perceived Stress Scale (PSS) and one also using a computer task (Implicit Association Test). Four of the ten studies measured HCC over 3 months and self-reported perceived stress over the past month, among which three did not find a significant association, and one reported a weak negative correlation. One study measured HCC over the past 6 months and perceived stress over the past 2 months and did not find significant correlation. One study measured HCC and perceived stress over the same 3 months and did not find significant correlation. One study measured both HCC and perceived stress over the same month where HCC was reported to have no correlation with self-reported stress but was significant positively correlated with implicit stress measured by computer tasks. Two other studies measured HCC for longer periods of time in pregnant women. Hoffman et al. (Citation2016) measured perceived stress with PSS at 16, 22, 28, 34, 40 weeks of gestation and HCC at 16, 28, 40 weeks of gestation. The researchers found that higher levels of first-trimester HCC were prospectively correlated with higher levels of perceived stress at 40 weeks of gestation and that the second-trimester HCC was positively correlated with perceived stress at 16 weeks of gestation. Another study took the maternal hair samples to measure HCC over the past 9 months and asked participants to complete the PSS during a clinical visit at 24–26 weeks of gestation. They did not find any association between HCC and perceived stress (Kramer et al., Citation2009). The final study measured long-term levels of cortisol over 1 month in saliva and self-reported perceived stress over the same month (Byun, Citation2013). They found that salivary cortisol on waking was not correlated with perceived stress, but salivary cortisol in the evening had a positive correlation with higher levels of perceived stress. Despite the mixed observations from these studies, a meta-analysis, which included data from eight studies Faresjö et al. (Citation2013), Gerber et al. (Citation2013), O’Brien et al. (Citation2013), X. Chen et al. (Citation2015), Sumra and Schillaci (Citation2015), Geng et al. (Citation2016), and Heinze et al. (Citation2016), (Hoffman et al., Citation2016) showed a pooled correlation between long-term levels of cortisol and perceived stress (ESr = 0.114, p= 0.0015, CI = [0.023, 0.203], ).
3.2.2. Mood impact of stressors
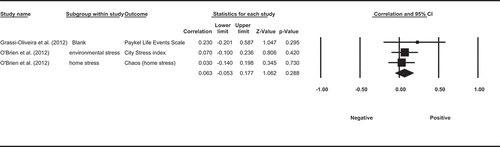
A total of three observational studies measured the association between cortisol and mood impact of stressors. The assessed stressors included negative life events in the previous up to 90 days, chaos in the neighbourhood in the past year, chaos/order of the home environment without a specified time frame, and mental burdening events in the past 3 months. All three studies measured HCC over 3 months. (Grassi-Oliveira et al., Citation2012; O’Brien et al., Citation2013; Ullmann et al., Citation2017) Two of the three studies reported that HCC was positively correlated with self-reported stressful life events or mental burdening events. (Grassi-Oliveira et al., Citation2012; Ullmann et al., Citation2017) One study did not find significant associations between HCC and chaos either in the neighbours or in the home. (O’Brien et al., Citation2013)
A meta-analysis based on available data from two studies (Grassi-Oliveira et al., Citation2012; O’Brien et al., Citation2013) showed that the association between stressors and HCC was not significant (ESr = 0.063, p= 0.288, CI = [−0.053, 0.177], ). To summarise, two out of three studies found a significant positive association between HCC and stressors. However, this observation is not supported by the pooled results from the meta-analysis.
3.2.3. Other indices of stress
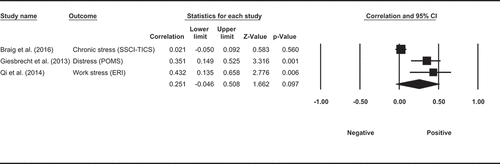
Three observational studies examined the associations between cortisol and other self-reported indices of stress. One study measured HCC over the 3 months before delivery in pregnant women and did not find a significant association between HCC and self-reported chronic stress over the same 3 months (Braig et al., Citation2016). One study measured salivary cortisol in pregnant women at 14, 20, 32 weeks of gestation and measured self-reported distress over the same period. They found that cortisol levels in saliva were positively correlated with distress among pregnant women (Giesbrecht et al., Citation2013). Finally, a study measured HCC and self-reported work stress over the past month and reported that neither the effort nor the reward scores were correlated with HCC (Qi et al., Citation2014). However, they did find that the effort-reward imbalance (1.83*effort-score/reward-score) at work was positively correlated with HCC. To summarise, two of the three studies reported a significant positive association between HCC and specific features of stress, namely distress and effort-reward imbalance at work. This observation was not, however, supported by the meta-analysis of three studies with a non-significantly pooled correlation between specific features of stress and HCC (ESr = 0.251, p= 0.097, CI = [−0.046, 0.508], ).
3.2.4. Anxiety
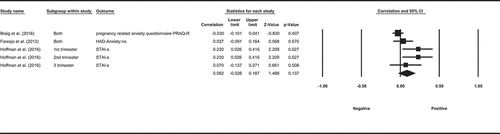
Five observational studies examined the association between cortisol and self-reported anxiety, with mixed findings. All of the studies measured cortisol using hair samples of 3 cm, despite different courses of time overall and different measures of anxiety. Three studies measured HCC over 3 months and none of them found a significant association between HCC either pregnancy-related anxiety over 3 months (Braig et al., Citation2016); not specified time frame (Kramer et al., Citation2009) or anxiety in general (not specified time frame, (Faresjö et al., Citation2013). One other study measured both anxiety and HCC at 16, 22, 28, 34, 40 weeks of gestation and found that self-reported state-trait anxiety measured at 16 and 28 weeks of pregnancy was correlated with HCC in both the second and the third trimester (Hoffman et al., Citation2016). One study measured the HCC over 3–6 months and anxiety, which was measured for approximately 3 months within the aforementioned 3–6 months. This study did not find a significant association between HCC and anxiety (Heinze et al., Citation2016). A meta-analysis based on available data from three studies showed that the pooled correlation between long-term levels of cortisol and anxiety was not significant (ESr = 0.082, p= 0.137, CI = [−0.026, 0.187], ).
3.2.5. Depression
Twelve observational studies examined the association between long-term levels of cortisol and depression measured using seven different scales, half of which found a significant association between depression and cortisol. The ten studies measured HCC, among which three measured HCC using hair samples of 3 cm to 9 cm from pregnant women and did not find a significant association between HCC and depression, and one measured HCC over 6 months using two separate 3 cm samples and depression over the same 6 months, and did not find a significant association between HCC and depression. Two other studies measured HCC from the 3 months prior to conception to the third trimester, with one measuring depression over 1 week at each trimester (Hoffman et al., Citation2016) and one measuring depression shortly after delivery Caparros-Gonzalez et al. (Citation2017) found that HCC during 1st and 3rd trimester was prospectively positively associated with depression. Hoffman et al. (Citation2016) found that HCC during the 1st trimester was prospectively positively associated with depression at 40 weeks of gestation, and both 2nd- and 3rd-trimester HCC were positively associated with depression at 16 and 28 weeks of gestation. Four studies measured HCC over 3 months and depression over an unclear period, among which one reported a significant negative correlation between HCC and depression (Gerber et al., Citation2013), one reported a significant positive correlation between HCC and depression (Faresjö et al., Citation2013), and two reporting no association between HCC and depression (Schalinski et al., Citation2015; Wang et al., Citation2016). Two studies examined the association between cortisol measured in saliva over 1 month and depression, one of which found that cortisol in saliva was higher and the daily cortisol slope steeper in the depressed participants compared to the non-depressed ones (Booij et al., Citation2015). Another study found that although not significantly correlated with depression at the beginning of the study, elevated cortisol levels in saliva in the evening were positively correlated with self-reported depressive symptoms 4 weeks later (Byun, Citation2013).
Meta-analysis was performed with available data from seven studies, with a pooled correlation coefficient of 0.133 (p= 0.022, CI = [0.019, 0.243], ) suggests that long-term levels of cortisol, either measured in hair or saliva, were related to depression.
3.2.6. PTSD
Three observational studies examined the relationship between long-term levels of cortisol and PTSD, where cortisol and self-reported PTSD were measured differently. Cieslak et al. (Citation2011) found that the PTSD at 1-month post-trauma was negatively associated with area under the curve with respect to increase (AUCi) at 3 months and that PTSD at 3 months was positively associated with area under the curve with respect to ground (AUCg) at 1-month post-accident. Stoppelbein and Greening (Citation2015) measured salivary cortisol and self-reported PTSD over the same 12 months. They found a positive correlation between higher levels of cortisol and one of the self-reported PTSD symptoms: numbing symptoms. The third study measured HCC using 3 cm hair samples and clinically assessed the severity of PTSD symptoms over an unclear period and did not find a significant association between the two variables (Schalinski et al., Citation2015). Data were insufficient to conduct a meta-analysis and therefore it was not possible to interrogate the observation further.
3.3. Effects of mood interventions on cortisol
3.3.1. Mindfulness
Six mindfulness interventions were included in this review. All six studies measured long-term cortisol in saliva, and one also measured cortisol in blood. Three studies hypothesised reduced cortisol after intervention. K. W. Brown et al. (Citation2016) reported that the mindfulness intervention significantly improved participants’ perceived stress levels and mood, which did not significantly differ from the social support group during 3-month follow-up. Neither intervention showed changes in the diurnal cortisol response curve. Bergen-Cico et al. (Citation2014) reported that participants who completed all sessions had a significant decrease in the cortisol awakening response (CAR) and AUCi, compared with the control group. They also reported significant associations between changes in cortisol levels and engagement in mindfulness intervention. Changes in mood were not described. Daubenmier et al. (Citation2011) found significant improvement in mindfulness, anxiety, and external-based eating, but not in emotional eating or the average CAR in the mindfulness-based eating intervention group compared to that control participants. Neither group showed significant changes in the cortisol slope or morning serum cortisol concentrations. However, they found that obese participants in the intervention group showed significant reductions in the CAR and maintained body weight, which were not observed for obese participants in the control group. They also reported associations between reduced abdominal fat and improved self-reported mindfulness and chronic stress, and CAR in the intervention group. The three studies did not describe the hypothesised intervention effects on cortisol. Jacobs et al. (Citation2013) found that self-reported mindfulness significantly increased after the intervention, but cortisol levels did not significantly change. However, they found that changes in mindfulness were inversely associated with changes in the average values of the afternoon and bedtime cortisol levels. Lynch et al. (Citation2011) found significantly improved perceived stress, anxiety and depression among participants, along with increased self-reported mindfulness but no significant changes in cortisol levels after the intervention. Marcus et al. (Citation2003) found a non-significant decrease in self-reported perceived stress after intervention among participants. However, the awakening salivary cortisol levels were significantly lower following the intervention. Half of the mindfulness interventions resulted in reductions in cortisol levels following the interventions, but half of the interventions did not find a significant treatment effect on cortisol. Therefore, whether mindfulness-based stress interventions can reduce cortisol levels remains unclear.
3.3.2. Social and emotional support
Holt-Lunstad et al. (Citation2008) examined whether a support enhancement intervention could decrease salivary cortisol as an indicator of improved mood, with a behaviour monitoring control group where participants were instructed to just keep a diary of their physical affection and mood. Stress, mood, or social support were not measured. They did not find intervention effects on salivary cortisol.
3.3.3. Other stress management interventions
Three studies investigated the effects of other stress management methods on long-term levels of cortisol measured in saliva. Two studies did not describe the hypothesised direction of changes in cortisol. They both reported reduced stress (Limm et al., Citation2011; Tsiouli et al., Citation2014) and improved lifestyle (Tsiouli et al., Citation2014) or mood (Limm et al., Citation2011) but neither study observed significant changes in cortisol levels. They also found significant improvement in lifestyle factors, such as sleep, stress-related symptoms, and eating habits in both groups. One study hypothesised that participants in the stress-management intervention group would demonstrate lower intraindividual cortisol variability (i.e. the degree to which an individual’s cortisol output may be erratic on a given day) measured in (Sannes et al., Citation2015). They only measured cortisol as the outcome in this study and reported lower levels of intraindividual cortisol variability among intervention participants compared to the waitlist control group. They also observed improvements towards a steeper cortisol slope and lower AUCg in the intervention group. However, they did not find significant changes in awakening cortisol levels, evening cortisol levels, and CAR following the intervention. At present the evidence for the effects of these stress-management interventions on cortisol is weak.
3.3.4. Alternative therapies
The two studies examined the effects of 5-week alternative therapies including acupuncture (Huang et al., Citation2012) and massage (Leivadi et al., Citation1999) on reducing stress and changing cortisol levels measured in saliva. Neither study showed significant intervention effects on long-term cortisol levels. The acupuncture intervention did not hypothesise the direction of changes in cortisol levels. Neither the decrease in perceived stress nor the increased CAR found in both the intervention and wait-list control groups were significant. No significant changes in the decline in cortisol or the daily average cortisol concentrations were found between groups following the intervention. The massage intervention hypothesised reduce cortisol levels and other potential stressors, such as anxiety and neck, shoulder, and back pain following the intervention. Participants reported reduced neck, shoulder, and back pain after the treatment sessions but changes in mood, anxiety, or cortisol levels were not observed over time although they found decreased cortisol levels immediately after the intervention sessions in both groups.
3.4. Risk of bias
For the current review, the maximum score of the checklist was 20 for observational studies and 27 for interventional studies, with four sub-sections: quality of reporting (maximum = 8 for observational studies, maximum = 10 for interventions), external validity (maximum = 3 for all of the studies), internal validity bias (maximum = 4 for observational studies, maximum = 7 for interventions), and confounding and selection bias (maximum = 4 for observational studies, maximum = 6 for interventions). The average total score was 9 out of 20 (range: 5–13) for observational studies and 11 out of 27 (range: 7–17) for interventions. Observational studies scoring 10 (n = 11) or above and interventions scoring 14 or above (n = 7) were considered to have moderate quality. The overall quality was considered moderate for observational studies and poor for interventional studies. A summary of the results for risk of bias assessment is presented in Supplementary Appendix Table 3.
4. Discussion
The main aim of this review was to systematically identify and review the available literature on the associations between lifestyle and mood factors and long-term levels of cortisol in healthy adults. The quality and scientific rigour of research conducted over the last two decades in this area were assessed. Overall, the evidence for associations between lifestyle and long-term levels of cortisol was mixed, with considerable heterogeneity between studies on approaches to assessing cortisol, lifestyle and mood factors, and with observational studies meeting criteria for moderate quality and intervention studies meeting criteria for poor quality.
Nonetheless, we can, in this review, glean some evidence in support of a positive association between physical activity and long-term levels of cortisol, with the meta-analysis of observational studies showing a significant pooled correlation. Although the evidence from trials also seemed to suggest that physical activity is likely to induce changes in cortisol levels over time, the direction of the changes remained unclear due to mixed findings. The meta-analysis for observational studies also showed a significant pooled positive correlation between BMI/body weight and long-term levels of cortisol. However, the only trial, which aimed to reduce weight through diet with and without exercise, did not find changes in cortisol levels. Other dietary interventions, regardless of whether they induced changes in body weight or body composition or not, were unlikely to induce changes in long-term cortisol levels. Observational studies suggested that perceived stress and depression were likely to be positively associated with long-term levels of cortisol. However, the observational evidence does not support a significant association between stressors, specific features of stress and anxiety, and cortisol. PTSD is likely to be positively associated with cortisol, although this conclusion is tentative due to insufficient data. Overall, trial evidence examining the effects of psychological interventions on long-term levels of cortisol was insufficient and mixed. Finally, evidence from both observational and interventional studies was insufficient to draw a clear conclusion for the associations between long-term levels of cortisol and smoking, sleep, alcohol consumption, and caffeine consumption.
4.1. Heterogeneity in how lifestyle and mood were measured
One observation from this review was the variability in how lifestyle and mood were measured across studies, which may have contributed to the mixed findings. For example, all three of the observational studies examining the association between physical activity and HCC measured physical activity differently. Due to different types of lifestyle measures being used in these studies, it is difficult to draw a definitive conclusion whether these lifestyles are correlated with cortisol, regardless of findings reported by the studies. Similar to the lifestyle measures, specific mood measures assess different aspects of mood. For example, the PSS measures the general distress and inability to cope (Cohen et al., Citation1983) whereas the Effort-Reward Imbalance Scale measures work stress with a focus on the efforts invested into job performance and rewards received in turn (Siegrist, Citation1996). The associations between cortisol and various mood outcomes might not be consistent (e.g. the pooled correlation is significant for cortisol and perceived stress and depression but not for cortisol and anxiety), indicating that the nature of the mood under investigation, and the source of the negative mood experienced, may be important.
4.2. Heterogeneity in approaches to measure cortisol
Another reason for the mixed findings might be the considerable variety of how long-term levels of cortisol were measured across studies: the type of cortisol measured, the indices examined, and the sampling protocol adopted.
First, different types of cortisol were measured both overall and in studies examining the same lifestyle or mood factor. For example, 80% of the observational studies measured cortisol in hair, other 20% measured cortisol in saliva whilst out of the 27 trials, only 1 measured cortisol in hair, 1 in urine, 6 in blood, and 19 in saliva. Cortisol measured in different samples reveals different information. Although salivary cortisol is often used as an alternative to free cortisol measured in blood, evidence suggested that the correlation between salivary and free serum cortisol varies between different individuals for daily-paired samples (Levine et al., Citation2007). Salivary and urinary cortisol measured over the same 24 h window were likely to provide different information about cortisol secretion (Yehuda et al., Citation2003),and how comparable is hair cortisol to saliva/urine/blood cortisol is still under debate (Kalliokoski et al., Citation2019). Results from this review echoes evidence that cortisol measured in different samples is hardly comparable and should not be used interchangeably (Hellhammer et al., Citation2009; Van Holland et al., Citation2012; Yehuda et al., Citation2003). Secondly, a variety of the cortisol indices were measured, even when studies did measure the same type of cortisol. For example, although all of the six mindfulness interventions measured cortisol in saliva, two measured awakening salivary cortisol and both reported significant intervention effects on cortisol (Bergen-Cico et al., Citation2014; Marcus et al., Citation2003), whereas others where significant intervention effects on cortisol were not found measured diurnal cortisol (K. W. Brown et al., Citation2016), resting p.m. cortisol values (Jacobs et al., Citation2013), or did not specify the measured cortisol indices (Daubenmier et al., Citation2011; Lynch et al., Citation2011). Different cortisol indices capture different characteristics of cortisol activity and should not be used interchangeably (Vedhara et al., Citation2006). With a limited number of studies measuring the effect of the same lifestyle/mood intervention on the same index of cortisol, it is therefore difficult to conclude whether a certain type of intervention affects cortisol or whether the effect, if any, will only be revealed when measured by certain indices of cortisol. This issue has pointed out an important field to work on urgently: to identify which cortisol indices should be focused on while examining the influence of lifestyle and mood. Thirdly, studies adopted a variety of sampling procedures. For example, although four 8-week mindfulness interventions all measured cortisol in saliva, the time samples were collected, number of samples collected per day, and for how many consecutive days, differed. The accuracy and stability of cortisol outcomes depend highly on the sampling protocol when sampled in acute measures such as blood and saliva, which includes the exact timepoint of sampling, the frequency, the length of the sample collection period, and the adherence (Daubenmier et al., Citation2011; Saxbe, Citation2008; Turpeinen & Hämäläinen, Citation2013). Different sampling protocols could bring significant variations of the cortisol results to different studies (Clow et al., Citation2004). Furthermore, lack of clarity about the rigour in timing and sufficiency of sampling in studies failed to report such details could both contribute to the inconsistency in the evidence. These observations further indicated that the ability of these acute measures to reflect the chronic relationship between lifestyles/mood and cortisol or the enduring treatment effects on cortisol is limited. The sampling procedure for hair cortisol is relatively straightforward and consistent across studies: hair samples of a certain length were taken close to the scalp from the posterior vertex area of the head. In this review, we did find significant pooled correlations between HCC and some of the lifestyle and mood factors such as BMI and perceived stress. This suggests that HCC might be a promising method to examine the long-term relationship between cortisol and lifestyle and mood.
4.3. The temporal association between cortisol and lifestyle or mood
It was observed in this review that although several studies measured cortisol and lifestyle/mood concurrently, the time frames captured by the measures did not necessarily correspond. For example, three observational studies examined physical activity and HCC all measured HCC over the previous 3 months, but two of the studies measured physical activity over the past week (Gerber et al., Citation2013; Sumra & Schillaci, Citation2015) and one asked participants whether or not they had engaged in physical activity regularly without specifying a time-frame (Garcia-Leon et al., Citation2018). This brought the issue that, in these studies, whether the examined associations of cortisol and lifestyle/mood reflected the actual association over the time frame when cortisol was captured, or when lifestyle/mood was measured was unclear. Therefore, future studies should clearly report the time frames these variables were measured and cautiously interpret the results. Two studies included in this review found an association between cortisol and subsequent mood, both reporting higher HCC prediction of later-measured depression (Caparros-Gonzalez et al., Citation2017; Hoffman et al., Citation2016). Similar associations between cortisol and major depressive disorder have been reported previously. For example, higher baseline CAR was identified as a significant risk factor for major depressive disorder in adolescents (Adam et al., Citation2010; Ellenbogen et al., Citation2011; Vrshek-Schallhorn et al., Citation2013). There is also evidence suggesting that individual differences during the morning salivary cortisol levels may be a risk factor for subsequent major depressive disorder among women (Harris et al., Citation2000). One study in this review reported an association between mood and subsequent cortisol: higher levels of perceived stress, anxiety, and depression at 16 weeks of gestation were significantly correlated with higher levels of HCC over the second trimester (Hoffman et al., Citation2016). These findings suggested that our understanding of the relationship between cortisol and lifestyle and mood would benefit from prospective studies conducted over long time frames in which it is possible to explore not only the concurrent relationship between these factors, but also conduct time-series analyses to help to disentangle cause and effect, or more likely the circular relationship between cortisol, lifestyle, and mood.
4.4. Other issues from the interventions
The overall picture of the effects of lifestyle or psychological interventions on long-term levels of cortisol was mixed, with only 18 out of 27 trials showing significant effects on long-term levels of cortisol. The insufficient or mixed evidence and methodological limitations. First, most of the trials described cortisol as a biomarker of mood hypothessing that the interventions would influence mood, which might be captured by cortisol. However, this hypothesised mechanism was not always affected in these studies, for instance, one stress-reduction intervention where lower awakening salivary cortisol levels were found did not result in significant improvement in self-reported stress (Marcus et al., Citation2003). A few studies did not measure or fully report changes in the targeted mechanisms. For example, one of the two yoga studies only measured stress using cortisol but not self-reported stress (P. J. Chen et al., Citation2017). Similarly, one intervention targeted at stress among veterans with PTSD did not report changes in stress or PTSD (Bergen-Cico et al., Citation2014). Cortisol levels, captured either short term or long term, may not correlate well with self-reported mood measures, hence these different measures should not be treated interchangeably (Van Holland et al., Citation2012). If an intervention did not measure, report or result in any changes in the hypothesised mechanism, regardless of whether the effect on cortisol was significant, it is impossible to distinguish whether it was indeed due to the intervention being effective/ineffective or other reasons. Second, many trials failed to hypothesise the direction of intervention effects on cortisol. Out of the 27 trials, less than half (n = 12) hypothesised the direction of the effect on cortisol, only half of these (n = 6) reported findings that were in line with their hypotheses (Bergen-Cico et al., Citation2014; Daubenmier et al., Citation2011; Goldberg et al., Citation2014; Miller et al., Citation2015); reductions during the evening cortisol (Papacosta et al., Citation2013; Sannes et al., Citation2015). The other six studies, which did hypothesise a direction either did not find a significant intervention effect on cortisol (Diment et al., Citation2012; Holt-Lunstad et al., Citation2008; P. J. Chen et al., Citation2017; Leivadi et al., Citation1999; K. W. Brown et al., Citation2016) or found it in the opposite direction (Tam et al., Citation2014). Given that there are a limited number of studies reporting significant effects in the expected direction, it is difficult to conclude whether a specific type of intervention is effective in changing long-term cortisol levels. However, the six interventions that confirmed their hypotheses had clearer descriptions of which cortisol indices they measured. It is likely that a lifestyle or mood intervention that changes cortisol levels may benefit from a more specific hypothesis involving the indices of cortisol and the direction of possible effects.
4.5. Strengths and limitations
This is the first systematic review to synthesise the available literature on the associations between lifestyle and mood factors and long-term cortisol levels in healthy adults. This is also the first systematic review to synthesis relevant evidence from both observational and interventional studies. By doing so, it contributes to the understanding of the role of cortisol, when assessed over the long term, in lifestyle and mood. This review also points to the outstanding gaps between and within observational and interventional studies in cortisol research, which have revealed several directions for future research. Another strength of this review is the robust methods that were used throughout the review process, and the quality as well as the scientific rigour of studies were assessed taking into consideration both the study design and reporting. However, the studies included in this review were highly heterogeneous, thus the meta-analytic approach was only adopted for observational studies of BMI, physical activity, perceived stress, stressors, and other indices of stress, anxiety, and depression. Findings from the meta-analyses led to the conclusion that BMI, physical activity, perceived stress, and depression were significant correlates of chronic levels of cortisol. However, due to the mixed findings in relevant trials, this conclusion should be interpreted with caution. Another limitation should be noted that the overall quality of reviewed studies was only moderate for observational studies and poor for interventional studies. The lack of quality was salient in the external validity and bias in both the design and reporting of the studies, which further emphasised the need for robust research in this area. As was pointed out by a previous review, the lack of consensus within the literature regarding what cortisol outcomes are most meaningful to health is still a challenge in cortisol research (Saxbe, Citation2008). An urgent step for future research will be to address the limitations and concerns discussed in this review and to rethink our knowledge of cortisol being a biomarker of stress and behaviour. We were unable to duplicate the title and abstract screening due to the size of the search. However, a second reviewer was involved in full-text screening, which increased the certainty that relevant papers were included.
4.6. Recommendations for future research
Finally, several recommendations for future research are made. First, more research is needed to investigate the relationships between long-term cortisol levels and sleep, smoking, alcohol consumption, and caffeine consumption. Nonetheless, there is emerging evidence for a positive association between BMI/body weight, physical activity, perceived stress and depression and cortisol, suggesting that intervention trials in these areas may be fruitful areas of enquiry. Such trials would benefit from (1) using more consistent protocols to measure cortisol over the longer terms, such as HCC; (2) clearer description of the hypotheses and aims of the study in relation to cortisol, (3) measurement of cortisol levels at baseline or over longer periods pre-intervention as reference. Hypothesised mechanisms (e.g. the mediating mood factors) for the interventions to be effective should also be clearly described, measured, and reported. For the association between cortisol and mood, it is possible that the long-term association between these two factors is prospective. Current evidence to support this observation, however, is insufficient. Future research is encouraged to explore this area with two focuses: (1) the prospective association itself and (2) whether it is mood that predicts cortisol or vice versa.
Author contributions
All authors contributed to the study's design. RJ and SC contributed to the systematic searches and data extraction processes. RJ drafted the first draft of the manuscript. All authors contributed to and approved the final version of the manuscript.
Disclosure statement
KV is a member of the independent scientific advisory board for Cortigenix.
Data Availability Statement
All available data including the data extraction tables for all included studies, and raw data for meta-analysis are available here: https://osf.io/36fnt/
Additional information
Funding
Notes on contributors
Ru Jia
The authors’ research interests include experimental and applied research examining the relationship between psychological factors and health, disease, and treatment outcomes as well as the development of brief and complex interventions to improve these outcomes. The hormone cortisol is one of the key biological pathways by which psychological functioning can influence health and has, therefore, has been the focus of much of the research of the group. This has included examining its role in influencing vaccine effectiveness and in vitro fertilisation outcomes, and most recently examining the role of cortisol in influencing the effects of mental health on physical health during the COVID-19 pandemic. (102 words)
References
- Adam, E. K., Doane, L. D., Zinbarg, R. E., Mineka, S., Craske, M. G., & Griffith, J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35(6), 921–52. https://doi.org/10.1016/j.psyneuen.2009.12.007
- Adam, E. K., Quinn, M. E., Tavernier, R., McQuillan, M. T., Dahlke, K. A., & Gilbert, K. E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. https://doi.org/10.1016/j.psyneuen.2017.05.018
- Babu, A. S., Padmakumar, R., Maiya, A. G., Mohapatra, A. K., & Kamath, R. L. (2016). Effects of exercise training on exercise capacity in pulmonary arterial hypertension: A systematic review of clinical trials. Heart, Lung & Circulation, 25(4), 333–341. https://doi.org/10.1016/j.hlc.2015.10.015
- Batista, J. C., Souza, A. L., Ferreira, H. A., Canova, F., & Grassi-Kassisse, D. M. (2015). Acute and chronic effects of tantric yoga practice on distress index. The Journal of Alternative and Complementary Medicine, 21(11), 681–685. https://doi.org/10.1089/acm.2014.0383
- Bergen-Cico, D., Possemato, K., & Pigeon, W. (2014). Reductions in cortisol associated with primary care brief mindfulness program for veterans with PTSD. Medical Care, 52(12 Suppl 5), S25–31. https://doi.org/10.1097/MLR.0000000000000224
- Booij, S. H., Bos, E. H., Bouwmans, M. E., van Faassen, M., Kema, I. P., Oldehinkel, A. J., & de Jonge, P. (2015). Cortisol and α-amylase secretion patterns between and within depressed and non-depressed individuals. PloS one, 10(7), e0131002.
- Braig, S., Grabher, F., Ntomchukwu, C., Reister, F., Stalder, T., Kirschbaum, C., Rothenbacher, D., & Genuneit, J. (2016). The association of hair cortisol with self-reported chronic psychosocial stress and symptoms of anxiety and depression in women shortly after delivery. Paediatric and Perinatal Epidemiology, 30(2), 97–104. https://doi.org/10.1111/ppe.12255
- Brown, K. W., Coogle, C. L., & Wegelin, J. (2016). A pilot randomized controlled trial of mindfulness-based stress reduction for caregivers of family members with dementia. Aging & Mental Health, 20(11), 1157–1166. https://doi.org/10.1080/13607863.2015.1065790
- Brown, B. D., Thomas, W., Hutchins, A., Martini, M. C., & Slavin, J. L. (2002). Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutrition and Cancer, 43(1), 22–30. https://doi.org/10.1207/S15327914NC431_2
- Byun, E. (2013). Effects of uncertainty on perceived and physiological stress and psychological outcomes in stroke-survivor caregivers. In Publicly accessible Penn dissertations. University of Pennsylvania. 616.
- Caparros-Gonzalez, R. A., Romero-Gonzalez, B., Strivens-Vilchez, H., Gonzalez-Perez, R., Martinez-Augustin, O., Peralta-Ramirez, M. I., & Slattery, D. A. (2017). Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PLoS One, 12(8), e0182817. https://doi.org/10.1371/journal.pone.0182817
- Casals, G., & Hanzu, F. A. (2020). Cortisol measurements in cushing’s syndrome: Immunoassay or mass spectrometry? Annals of Laboratory Medicine, 40(4), 285–296. https://doi.org/10.3343/alm.2020.40.4.285
- Chen, X., Gelaye, B., Velez, J. C., Barbosa, C., Pepper, M., Andrade, A., Gao, W., Kirschbaum, C., & Williams, M. A. (2015). Caregivers’ hair cortisol: A possible biomarker of chronic stress is associated with obesity measures among children with disabilities. BMC Pediatrics, 15(1), 9. https://doi.org/10.1186/s12887-015-0322-y
- Chen, P. J., Yang, L., Chou, C. C., Li, C. C., Chang, Y. C., & Liaw, J. J. (2017). Effects of prenatal yoga on women’s stress and immune function across pregnancy: A randomized controlled trial. Complementary Therapies in Medicine, 31, 109–117. https://doi.org/10.1016/j.ctim.2017.03.003
- Cieslak, R., Benight, C. C., Luszczynska, A., & Laudenslager, M. L. (2011). Longitudinal relationships between self-efficacy, post-traumatic distress and salivary cortisol among motor vehicle accident survivors. Stress and Health, 27(3), e261–e268. https://doi.org/10.1002/smi.1379
- Clow, A., Thorn, L., Evans, P., & Hucklebridge, F. (2004). The awakening cortisol response: Methodological issues and significance. Stress, 7(1), 29–37. https://doi.org/10.1080/10253890410001667205
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Coiro, V., Casti, A., Jotti, G. S., Rubino, P., Manfredi, G., Maffei, M. L., Melani, A., Volta, E., & Chiodera, P. (2007). Adrenocorticotropic hormone/cortisol response to physical exercise in abstinent alcoholic patients. Alcoholism: Clinical and Experimental Research, 31(5), 901–906. https://doi.org/10.1111/j.1530-0277.2007.00376.x
- Daubenmier, J., Kristeller, J., Hecht, F. M., Maninger, N., Kuwata, M., Jhaveri, K., Lustig, R. H., Kemeny, M., Karan, L., & Epel, E. (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity, 2011, 651936. https://doi.org/10.1155/2011/651936
- Deeks, J. J., Dinnes, J., D’Amico, R., Sowden, A. J., Sakarovitch, C., Song, F., Petticrew, M., & Altman, D. G. (2003). Evaluating non-randomised intervention studies. Health Technology Assessment, 7(27), 1–173. iii-x. https://doi.org/10.3310/hta7270
- Diment, B. C., Fortes, M. B., Greeves, J. P., Casey, A., Costa, R. J. S., Walters, R., & Walsh, N. P. (2012). Effect of daily mixed nutritional supplementation on immune indices in soldiers undertaking an 8-week arduous training programme. European Journal of Applied Physiology, 112(4), 1411–1418. https://doi.org/10.1007/s00421-011-2096-8
- Donnelly, J. E., Hillman, C. H., Castelli, D., Etnier, J. L., Lee, S., Tomporowski, P., Lambourne, K., & Szabo-Reed, A. N. (2016). Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Medicine & Science in Sports & Exercise, 48(6), 1197–1222. https://doi.org/10.1249/MSS.0000000000000901
- Downs, S. H., & Black, N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health, 52(6), 377–384. https://doi.org/10.1136/jech.52.6.377
- Ellenbogen, M. A., Hodgins, S., Linnen, A. M., & Ostiguy, C. S. (2011). Elevated daytime cortisol levels: A biomarker of subsequent major affective disorder? Journal of Affective Disorders, 132(1–2), 265–269. https://doi.org/10.1016/j.jad.2011.01.007
- Faresjö, Å., Theodorsson, E., Chatziarzenis, M., Sapouna, V., Claesson, H. P., Koppner, J., Faresjö, T., & Dowd, J. B. (2013). Higher perceived stress but lower cortisol levels found among young Greek adults living in a stressful social environment in comparison with Swedish young adults. PLoS One, 8(9), e73828. https://doi.org/10.1371/journal.pone.0073828
- Garcia-Leon, M. A., Peralta-Ramirez, M. I., Arco-Garcia, L., Romero-Gonzalez, B., Caparros-Gonzalez, R. A., Saez-Sanz, N., Santos-Ruiz, A. M., Montero-Lopez, E., Gonzalez, A., Gonzalez-Perez, R., & Nater-Mewes, R. (2018). Hair cortisol concentrations in a Spanish sample of healthy adults. PLOS ONE, 13(9), e0204807. https://doi.org/10.1371/journal.pone.0204807
- Geng, L., Xiang, P., Yang, J., Shen, H., & Sang, Z. (2016). Association between hair cortisol concentration and perceived stress in female methamphetamine addicts. Journal of Psychosomatic Research, 91, 82–86. https://doi.org/10.1016/j.jpsychores.2016.10.011
- Gerber, M., Kalak, N., Elliot, C., Holsboer-Trachsler, E., Pühse, U., & Brand, S. (2013). Both hair cortisol levels and perceived stress predict increased symptoms of depression: An exploratory study in young adults. Neuropsychobiology, 68(2), 100–109. https://doi.org/10.1159/000351735
- Giesbrecht, G. F., Poole, J. C., Letourneau, N., Campbell, T., & Kaplan, B. J. (2013). The buffering effect of social support on hypothalamic-pituitary-adrenal axis function during pregnancy. Psychosomatic Medicine, 75(9), 856–862. https://doi.org/10.1097/PSY.0000000000000004
- Goldberg, S. B., Manley, A. R., Smith, S. S., Greeson, J. M., Russell, E., Van Uum, S., Koren, G., & Davis, J. M. (2014). Hair cortisol as a biomarker of stress in mindfulness training for smokers. The Journal of Alternative and Complementary Medicine, 20(8), 630–634. https://doi.org/10.1089/acm.2014.0080
- Grassi-Oliveira, R., Pezzi, J. C., Daruy-Filho, L., Viola, T. W., Francke, I. D., Leite, C. E., & Brietzke, E. (2012). Hair cortisol and stressful life events retrospective assessment in crack cocaine users. The American Journal of Drug and Alcohol Abuse, 38(6), 535–538. https://doi.org/10.3109/00952990.2012.694538
- Harris, T. O., Borsanyi, S., Messari, S., Stanford, K., Cleary, S. E., Shiers, H. M., Brown, G. W., & Herbert, J. (2000). Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry, 177(6), 505–510. https://doi.org/10.1192/bjp.177.6.505
- Heinze, K., Lin, A., Reniers, R., & Wood, S. J. (2016). Longer-term increased cortisol levels in young people with mental health problems. Psychiatry Research, 236, 98–104. https://doi.org/10.1016/j.psychres.2015.12.025
- Hellhammer, D. H., Wüst, S., & Kudielka, B. M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34(2), 163–171. https://doi.org/10.1016/j.psyneuen.2008.10.026
- Hoffman, M. C., Mazzoni, S. E., Wagner, B. D., Laudenslager, M. L., & Ross, R. G. (2016). Measures of maternal stress and mood in relation to preterm birth. Obstetrics & Gynecology, 127(3), 545–552. https://doi.org/10.1097/AOG.0000000000001287
- Holt-Lunstad, J., Birmingham, W. A., & Light, K. C. (2008). Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine, 70(9), 976–985. https://doi.org/10.1097/PSY.0b013e318187aef7
- Huang, W., Taylor, A., Howie, J., & Robinson, N. (2012). Is the diurnal profile of salivary cortisol concentration a useful marker for measuring reported stress in acupuncture research? A randomized controlled pilot study. The Journal of Alternative and Complementary Medicine, 18(3), 242–250. https://doi.org/10.1089/acm.2010.0325
- Huffer, D., Hing, W., Newton, R., & Clair, M. (2017). Strength training for plantar fasciitis and the intrinsic foot musculature: A systematic review. Physical Therapy in Sport, 24, 44–52. https://doi.org/10.1016/j.ptsp.2016.08.008
- Jacobs, T. L., Shaver, P. R., Epel, E. S., Zanesco, A. P., Aichele, S. R., Bridwell, D. A., Rosenberg, E. L., King, B. G., Maclean, K. A., Sahdra, B. K., Kemeny, M. E., Ferrer, E., Wallace, B. A., & Saron, C. D. (2013). Self-reported mindfulness and cortisol during a Shamatha meditation retreat. Health Psychology, 32(10), 1104–1109. https://doi.org/10.1037/a0031362
- Kalliokoski, O., Jellestad, F. K., & Murison, R. (2019). A systematic review of studies utilizing hair glucocorticoids as a measure of stress suggests the marker is more appropriate for quantifying short-term stressors. Scientific Reports, 9(1), 11997. https://doi.org/10.1038/s41598-019-48517-2
- Kramer, M. S., Lydon, J., Séguin, L., Goulet, L., Kahn, S. R., McNamara, H., Genest, J., Dassa, C., Chen, M. F., Sharma, S., Meaney, M. J., Thomson, S., Van Uum, S., Koren, G., Dahhou, M., Lamoureux, J., & Platt, R. W. (2009). Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. American Journal of Epidemiology, 169(11), 1319–1326. https://doi.org/10.1093/aje/kwp061
- Leivadi, S., Hernandez-Reif, M., Field, T., O’Rourke, M., D’Arienzo, S., Lewis, D., Pino, N. D., Schanberg, S., & Kuhn, C. (1999). Massage therapy and relaxation effects on university dance students. Journal of Dance Medicine & Science, 3(3), 108–112. https://www.ingentaconnect.com/content/jmrp/jdms/1999/00000003/00000003/art00003
- Levine, A., Zagoory-Sharon, O., Feldman, R., Lewis, J. G., & Weller, A. (2007). Measuring cortisol in human psychobiological studies. Physiology & Behavior, 90(1), 43–53. https://doi.org/10.1016/j.physbeh.2006.08.025
- Limm, H., Gündel, H., Heinmüller, M., Marten-Mittag, B., Nater, U. M., Siegrist, J., & Angerer, P. (2011). Stress management interventions in the workplace improve stress reactivity: A randomised controlled trial. Occupational and Environmental Medicine, 68(2), 126–133. https://doi.org/10.1136/oem.2009.054148
- Lopresti, A. L., Hood, S. D., & Drummond, P. D. (2013). A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. Journal of Affective Disorders, 148(1), 12–27. https://doi.org/10.1016/j.jad.2013.01.014
- Lovallo, W. R., Whitsett, T. L., Al’absi, M., Sung, B. H., Vincent, A. S., & Wilson, M. F. (2005). Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosomatic Medicine, 67(5), 734–739. https://doi.org/10.1097/01.psy.0000181270.20036.06
- Lynch, S., Gander, M.-L., Kohls, N., Kudielka, B., & Walach, H. (2011). Mindfulness-based coping with university life: A non-randomized wait-list-controlled pilot evaluation. Stress and Health, 27(5), 365–375. https://doi.org/10.1002/smi.1382
- Mangine, G. T., Hoffman, J. R., Gonzalez, A. M., Townsend, J. R., Wells, A. J., Jajtner, A. R., Beyer, K. S., Boone, C. H., Wang, R., Miramonti, A. A., LaMonica, M. B., Fukuda, D. H., Witta, E. L., Ratamess, N. A., & Stout, J. R. (2017). Exercise-induced hormone elevations are related to muscle growth. Journal of Strength and Conditioning Research, 31(1), 45–53. https://doi.org/10.1519/JSC.0000000000001491
- Marcus, M. T., Fine, M., Moeller, F. G., Khan, M. M., Pitts, K., Swank, P. R., & Liehr, P. (2003). Change in stress levels following mindfulness-based stress reduction in a therapeutic community. Addictive Disorders & Their Treatment, 2(3), 63–68. https://doi.org/10.1097/00132576-200302030-00001
- McEwen, B. S., & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. https://doi.org/10.1016/S0018-506X(02)00024-7
- Miller, C. B., Kyle, S. D., Gordon, C. J., Espie, C. A., Grunstein, R. R., Mullins, A. E., Postnova, S., Bartlett, D. J., & Langguth, B. (2015). Physiological markers of arousal change with psychological treatment for insomnia: A preliminary investigation. PLoS One, 10(12), e0145317. https://doi.org/10.1371/journal.pone.0145317
- O’Brien, K. M., Tronick, E. Z., & Moore, C. L. (2013). Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and Health, 29(4), 337–344. https://doi.org/10.1002/smi.2475
- Papacosta, E., Gleeson, M., & Nassis, G. P. (2013). Salivary hormones, IgA, and performance during intense training and tapering in judo athletes. Journal of Strength and Conditioning Research, 27(9), 2569–2580. https://doi.org/10.1519/JSC.0b013e31827fd85c
- Qi, X., Zhang, J., Liu, Y., Ji, S., Chen, Z., Sluiter, J. K., & Deng, H. (2014). Relationship between effort-reward imbalance and hair cortisol concentration in female kindergarten teachers. Journal of Psychosomatic Research, 76(4), 329–332. https://doi.org/10.1016/j.jpsychores.2014.01.008
- Rothe, N., Steffen, J., Penz, M., Kirschbaum, C., & Walther, A. (2020). Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: A systematic review. Neuroscience and Biobehavioral Reviews, 114, 232–270. https://doi.org/10.1016/j.neubiorev.2020.02.024
- Sannes, T. S., Dolan, E., Albano, D., Ceballos, R. M., & McGregor, B. A. (2015). Stress management reduces intraindividual cortisol variability, while not impacting other measures of cortisol rhythm, in a group of women at risk for breast cancer. Journal of Psychosomatic Research, 79(5), 412–419. https://doi.org/10.1016/j.jpsychores.2015.09.012
- Saxbe, D. E. (2008). A field (researcher’s) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review, 2(2), 163–190. https://doi.org/10.1080/17437190802530812
- Schalinski, I., Elbert, T., Steudte-Schmiedgen, S., Kirschbaum, C., & Schmahl, C. (2015). The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One, 10(8), e0136921. https://doi.org/10.1371/journal.pone.0136921
- Siegrist, J. (1996). Adverse health effects of high-effort/low-reward conditions. Journal of Occupational Health Psychology, 1(1), 27–41. https://doi.org/10.1037/1076-8998.1.1.27
- Sinha, R., Fox, H. C., Hong, K. I., Hansen, J., Tuit, K., & Kreek, M. J. (2011). Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry, 68(9), 942–952. https://doi.org/10.1001/archgenpsychiatry.2011.49
- Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair–state of the art and future directions. Brain , Behavior, and Immunity, 26(7), 1019–1029. https://doi.org/10.1016/j.bbi.2012.02.002
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., and Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274.
- Stoppelbein, L., & Greening, L. (2015). A longitudinal study of the role of cortisol in posttraumatic stress disorder symptom clusters. Anxiety, Stress, & Coping, 28(1), 17–30. https://doi.org/10.1080/10615806.2014.923844
- Sumra, M. K., & Schillaci, M. A. (2015). Stress and the multiple-role woman: Taking a closer look at the “superwoman”. PLoS One, 10(3), e0120952. https://doi.org/10.1371/journal.pone.0120952
- Tam, C. S., Frost, E. A., Xie, W., Rood, J., Ravussin, E., & Redman, L. M. (2014). No effect of caloric restriction on salivary cortisol levels in overweight men and women. Metabolism, 63(2), 194–198. https://doi.org/10.1016/j.metabol.2013.10.007
- Tanskanen, M. M., Kyröläinen, H., Uusitalo, A. L., Huovinen, J., Nissilä, J., Kinnunen, H., Atalay, M., & Häkkinen, K. (2011). Serum sex hormone-binding globulin and cortisol concentrations are associated with overreaching during strenuous military training. Journal of Strength and Conditioning Research, 25(3), 787–797. https://doi.org/10.1519/JSC.0b013e3181c1fa5d
- Timon, R., Corvillo, M., Brazo, J., Robles, M. C., & Maynar, M. (2013). Strength training effects on urinary steroid profile across the menstrual cycle in healthy women. European Journal of Applied Physiology, 113(6), 1469–1475. https://doi.org/10.1007/s00421-012-2575-6
- Torres, S. J., Nowson, C. A., & Worsley, A. (2008). Dietary electrolytes are related to mood. British Journal of Nutrition, 100(5), 1038–1045. https://doi.org/10.1017/S0007114508959201
- Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. https://doi.org/10.1016/S0022-3999(02)00429-4
- Tsiouli, E., Pavlopoulos, V., Alexopoulos, E. C., Chrousos, G., & Darviri, C. (2014). Short-term impact of a stress management and health promotion program on perceived stress, parental stress, health locus of control, and cortisol levels in parents of children and adolescents with diabetes type 1: A pilot randomized controlled trial. Explore (NY), 10(2), 88–98. https://doi.org/10.1016/j.explore.2013.12.004
- Turpeinen, U., & Hämäläinen, E. (2013). Determination of cortisol in serum, saliva and urine. Best Practice & Research: Clinical Endocrinology & Metabolism, 27(6), 795–801. https://doi.org/10.1016/j.beem.2013.10.008
- Ullmann, E., Licinio, J., Barthel, A., Petrowski, K., Stalder, T., Bornstein, S. R., & Kirschbaum, C. (2017). Persistent LHPA activation in German individuals raised in an overprotective parental behavior. Scientific Reports, 7(1), 2778. https://doi.org/10.1038/s41598-017-01718-z
- van Holland, B. J., Frings-Dresen, M. H., & Sluiter, J. K. (2012). Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. International Archives of Occupational and Environmental Health, 85(8), 849–852. https://doi.org/10.1007/s00420-011-0727-3
- Vedhara, K., Tuinstra, J., Miles, J. N., Sanderman, R., & Ranchor, A. V. (2006). Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology, 31(3), 299–311. https://doi.org/10.1016/j.psyneuen.2005.08.006
- Vrshek-Schallhorn, S., Doane, L. D., Mineka, S., Zinbarg, R. E., Craske, M. G., & Adam, E. K. (2013). The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine, 43(3), 483–493. https://doi.org/10.1017/S0033291712001213
- Wang, H. L., Visovsky, C., Ji, M., & Groer, M. (2016). Stress-related biobehavioral responses, symptoms, and physical activity among female veterans in the community: An exploratory study. Nurse Education Today, 47, 2–9. https://doi.org/10.1016/j.nedt.2016.06.017
- Wosu, A. C., Valdimarsdóttir, U., Shields, A. E., Williams, D. R., & Williams, M. A. (2013). Correlates of cortisol in human hair: Implications for epidemiologic studies on health effects of chronic stress. Annals of Epidemiology, 23(12), 797–811.e792. https://doi.org/10.1016/j.annepidem.2013.09.006
- Yehuda, R., Halligan, S. L., Yang, R. K., Guo, L. S., Makotkine, I., Singh, B., & Pickholtz, D. (2003). Relationship between 24-hour urinary-free cortisol excretion and salivary cortisol levels sampled from awakening to bedtime in healthy subjects. Life Sciences, 73(3), 349–358. https://doi.org/10.1016/S0024-3205(03)00286-8
- Young, E. A., Abelson, J., & Lightman, S. L. (2004). Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology, 25(2), 69–76. https://doi.org/10.1016/j.yfrne.2004.07.001