?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The need for safe and economical methods for wastewater purification has necessitated this research. Blends of different peels: Potato-, apple- and pineapples-peels (PAP-peels) were impregnated with aqueous solutions of ZnCl2 following the variant of the incipient wetness method for activation of activated carbon (AC). Scanning Electron Microscope with attached energy dispersive spectrometer, Atomic Adsorption Spectrometry and Fourier Transform Infrared spectrometer equipment were used for the characterization of the AC produced. The result shows that PAP-peels derived ACs had micro porous characteristics. The study revealed that these new combined adsorbents materials are inexpensive, easily available and they have applications for the removal of Cu, Pb and Cr contained in industrial effluents.
Public Interest Statement
Many heavy metals are essential trace elements for humans, animals and plants in small amounts. But, in larger amounts, they cause acute and chronic toxicity. Their effects led to cancer, learning disabilities, even death. These heavy metals occur from industrial sources such as metal finishing and plating, textile dyes, manufacturing processes, etc. and they also occur naturally. Exploited methods for the removal of heavy metals include the activated sludge process, chemical precipitation, ion exchange, membrane filtration, electrochemical methods, flotation and the adsorption process. The latter which uses activated carbon (AC) as adsorbent is a highly effective technique for wastewater treatment but not cost-effective. Therefore, it is required that all probable sources of low-cost bioadsorbents should be explored. Their viability for the removal of heavy metals should also be studied in detail. This study is a contribution towards the search for such low-cost adsorbents.
1. Introduction
Industries have been forced to reduce to acceptable level, contents of heavy metal in water and industrial wastewaters (Acharya, Sahu, Mohanty, & Meikap, Citation2009). Cr, Cd, Cu and Pb are among those hazardous materials that are most commonly found in industrial wastewaters; therefore their removal is of utmost importance (Acharya et al., Citation2009; Olukanni, Citation2013). Different wastewater treatment techniques have been developed to decrease the amount of wastewater produced (Olukanni, Citation2013; Olukanni & Ducoste, Citation2011; Olukanni & Kokumo, Citation2013). Although various treatment techniques can be employed to remove heavy metals from contaminated wastewater, they have their inherent advantages and limitations in application (Acharya et al., Citation2009; Doyurum & Celik, Citation2006; Gunatilake, Citation2015; Hegazi, Citation2013; Hirunpraditkoon, Tunthong, Ruangchai, & Nuithitikul, Citation2011; Olukanni, Citation2013; Olukanni & Ducoste, Citation2011; Olukanni & Kokumo, Citation2013). The techniques include chemical precipitation, ion-exchange, adsorption, membrane processes and electrolytic methods (Acharya et al., Citation2009; Doyurum & Celik, Citation2006; El-Nemr, El-Sikaily, & Abdelwahab, Citation2009; Hirunpraditkoon et al., Citation2011; Li et al., Citation2008; Olukanni, Citation2013; Olukanni & Ducoste, Citation2011; Olukanni & Kokumo, Citation2013; Ricordel, Taha, Cisse, & Dorange, Citation2001). In recent years, researchers have studied the production of activated carbon (ACs) from cheap and renewable raw materials such as coconut shells, egg shell, wood, maize cob, rice husk, fruit stones, coir pith, used tyres, cotton stalks and bamboo as adsorbents for heavy metals in wastewater treatment (Foo & Hameed, Citation2012a, Citation2012b; Hamadi, Xiao, Farid, & Lu, Citation2001).
In this study, a blend of AC prepared from potato-, apple- and pineapple-peels (PAP-peels) induced for removal of heavy metals in wastewater was investigated, using the chemical activation process in which monitoring of the activation time, temperature and impregnation ratio (IR) was put into consideration.
2. Experimental procedure
Process used in this experiment is the chemical reactivation in which a carbon content material is impregnated with a strong base, strong acid or a salt then carbonized at a lower temperature (450–900)°C in the furnace. The advantage of the preparation of AC using chemical activation process is that low activation temperature and shorter time is required for activating the carbon content material.
2.1. Preparation of substrate
Peels obtained from fresh pineapples, apples and potatoes were washed separately with distilled water to remove inorganic impurities, then oven dried for few minutes to remove moisture content. These peels were then weighed and initial mass were recorded, followed by oven drying for 24 h at 119°C. The peels were then crushed and sieved to required sizes of (1–2) mm, before soaking them in phosphoric acid for 24 h. They were then washed with distilled water until residual liquid reached pH of 7. Figure shows the chopped oven dried peels soaked in phosphoric acid.
2.2. Impregnation of the substrate
The IR of these peels was based on mass ratio of zinc chloride to peels in ratio of 2:1 and 1:2. 32 g of peels was impregnated with the solution of zinc chloride and was kept agitated for 1 h at 80°C the slurry was then filtered and then oven dried for 24 h at 100°C and labelled 1AC500 and 2AC500. According to the IR and amount of time to be carbonised, IR was calculated using Equation (1).
(1)
(1)
where w (ZnCl) is the mass of ZnCl and w (Char) is the mass of Char.
The photochemical and synergetic blend for wastewater purification was investigated. Blends of different peels were used for activation of AC. PAP-peels were impregnated with aqueous solutions of ZnCl2 following a variant of the incipient wetness method. The IR had a strong influence on the pore structure of these ACs, which was easily controlled by simply varying the proportion of ZnCl2 used in the activation. High IRs yielded essentially mesoporous carbons with high surface area and pore volume. Thus, low IR led to essentially micro porous ACs.
2.3. Carbonization of the substrate
The carbonization temperature was carried out in two different temperatures that is 500 and 700°C in a tube furnace. Each sample was heated in a different temperature for 1 h and then allowed to cool for 30 min in the tube furnace after heating. 1AC500 and 2AC500 labeled AC samples were heated separately with a temperature 500°C for 1 h. The final products of AC were then washed with distilled water and dried for a few minutes in an oven to remove moisture then stored in labeled desiccators. Table shows the experimental preparation conditions of the ACs.
Table 1. Experimental preparation conditions of AC
2.3.1. Characteristics of potatoes-, apple-, and pineapple- peels (PAP-Peels)
Potato peels contain an array of nutritionally and pharmacologically active components such as phenolic compounds, glycol alkaloids, and cell wall polysaccharides which may be used as antioxidants, precursors of steroids hormones and dietary fiber (Norlia, Roshazita, Nuraiti, Salwa, & Fatimah, Citation2011). Apple peels contain greater amount of crude fiber, ash and caloric value with great amount of moisture found in the peel. Mineral elements composition revealed that apple peels contains greater amount of potassium and zinc. On the other side, pineapple solid waste constitutes about 40–50% of fresh pineapple fruit and core (Choi, Barford, & McKay, Citation2005). Pineapple waste contains valuable components which are mainly sucrose, glucose, fructose and other nutrients (Bagreev & Bandosz, Citation2001; Bahadir, Bakan, Altas, & Buyukgungor, Citation2007).
2.3.2. Adsorption in wastewater treatment
Adsorption is a natural process by which molecules of a dissolved compound collect on and adhere to the surface of an adsorbent solid. Carbon has been used as an adsorbent for centuries; powder AC is a particularly good adsorbent medium due to its high surface area to volume ratio and it has been also found to be superior compared to other chemical and physical methods for wastewater treatment in terms of its capability for efficiently adsorbing a broad range of pollutants, fast adsorption kinetics and its simplicity of design (Bedmohata, Chaudhari, Singh, & Choudhary, Citation2015). The properties of different carbons can have profound effects on both rate and capacity for adsorption (Chen et al., Citation2011). The percentage adsorption can be calculated by the expression in Equation (2).
(2)
(2)
Therefore, the sorption capacity q (mg/g) is obtained from
(3)
(3)
where v is the volume of the solution (l), m is the amount of sorbent (g), Ci and Ce are the initial and equilibrium concentration in the solution (e.g. Cu, mg L−1).
2.4. Scanning electron microscope (SEM)
SEM was used to study the external morphology of the adsorbent (Fayomi & Popoola, Citation2012). The electron microscope produces images of samples by scanning it with a focused beam of electron. This electron beam was actually scanned in a raster scan pattern, and the beam’s position was combined with the detected signal to produce an image.
3. Results and discussion
3.1. SEM micrographs analysis
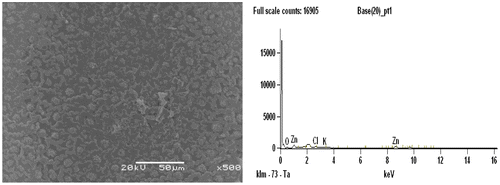
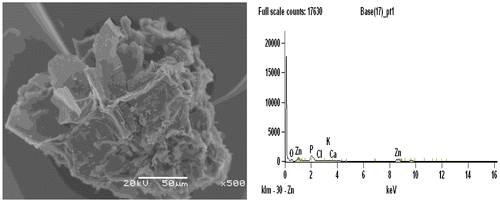
Figures and shows the morphology study by SEM micrographs of ACs, in which cavities, pores and rough surfaces were observed on the surface of the prepared AC. It again shows the effect of the activation temperature and the activating agent influencing the topographical characteristics of the carbon surface. Figure shows the SEM image of AC prepared under optimum conditions (500°C activation temperature, 1hr activation time and 78.22 wt% ZnCl). Small developed pores were found on the surface of the AC which may be due to the lower activation temperature used for activation which significantly resulted to less formation of micro pores. Comparatively, Figure also showed very small pores on the surface of the AC, thus low activation temperature was used for activation. Well-developed micro pores structures which may be favorable to adsorb larger molecules in comparison to other carbons prepared were seen in AC.
3.2. Adsorption capacity of heavy metals in industrial wastewater
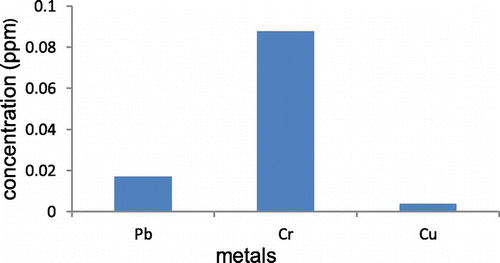
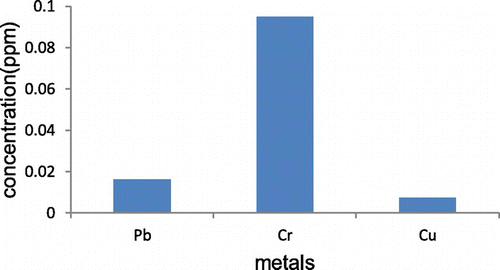
Figure shows the graphs of adsorption capacity of the heavy metals in industrial wastewater. From these graphs, it was observed that the highest metal uptake was copper in which low concentration of this metal is depicted by the results in Figure . Lead is the metal that also had considerable low concentration after adsorption, followed by chromium which experienced lower adsorption capacities (Figure ).
Table shows the adsorption percentage and the adsorption capacity of Cr(III), Cu (II) and Pb(II) from wastewater adsorbed by AC at different activation ratio and carbonisation temperature 1:2 at 500°C. Based on batch equilibrium studies, the uptake capacity of the three heavy metals appears to be greatest for Cu, followed by Lb and then Cr.
Table 2. Adsorption capacities for heavy metals in industrial wastewater
4. Conclusions
From the result obtained, the following conclusions can be drawn:
| (a) | The pyrolysis of PAP-peels impregnated with ZnCl produces materials with a developed structure and adsorption capacities able to adsorb; | ||||
| (b) | The IR has a strong influence on the pore structure of this AC, which are easily controlled by simply varying the proportion of zinc chloride used in the activation; | ||||
| (c) | The experiment revealed that this new combined adsorbents are inexpensive, easily available materials and they have applications for the removal of Cu, Pb and Cr contained in industrial effluents. | ||||
Funding
Covenant University is acknowledged for providing the funds for open access publication.
Additional information
Notes on contributors
O.S.I. Fayomi
O.S.I. Fayomi is a Researcher in the Department of Mechanical Engineering at Covenant University. His research interests are centered on mechanical metallurgy, environmental science and engineering, materials development and processing, mechanical and materials performance, structural integrity, thermomechanical and triboxidation processing.
D.O. Olukanni
D.O. Olukanni is an Associate Professor in the Department of Civil Engineering at Covenant University. His research focus is in water resource and environmental engineering. His current area of research includes: hydraulics and hydrological studies, water supply in rural and urban communities, simulation and optimization of waste water treatment system.
G.U. Fayomi
G.U. Fayomi holds a Master of Science degree in Environmental Management from Lagos State University. Her research interests include water management, urban renewal development and environmental science.
O.O. Joseph
O.O. Joseph is working as a researcher in the Department of Mechanical Engineering at Covenant University. She has a PhD degree in Mechanical Engineering (Materials Engineering Bias). Her research interests include: corrosion and environmental engineering, fracture mechanics and structural integrity, failure analysis of materials, materials performance and characterization.
References
- Acharya, J., Sahu, J. N., Mohanty, C. R., & Meikap, B. C. (2009). Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chemical Engineering Journal, 149, 249–262.10.1016/j.cej.2008.10.029
- Bagreev, F. A., & Bandosz, T. J. (2001). Carbon pH of activated carbon surface as an indication of its suitability for H2S removal from moist air streams. Carbon, 2, 1–39.
- Bahadir, T., Bakan, G., Altas, L., & Buyukgungor, H. (2007). The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme and Microbial Technology, 41, 98–102.10.1016/j.enzmictec.2006.12.007
- Bedmohata, M. A., Chaudhari, A. R., Singh, S. P., & Choudhary, M. D. (2015). Adsorption capacity of activated carbon prepared by chemical activation of lignin for the removal of methylene blue dye. International Journal of Advanced Research in Chemical Science (IJARCS), 2(8), 1–13.
- Chen, Y., Zhu, Y., Wang, Z., Li, Y., Wang, L., Ding, L., Gao, X., Ma, Y., & Guo, Y. (2011). Application studies of activated carbon derived from rice husks produced by chemical-thermal process—A review. Advances in Colloid and Interface Science, 163, 39–52.10.1016/j.cis.2011.01.006
- Choy, K. K. H., Barford, J. P., & McKay, G. (2005). Production of activated carbon from bamboo scaffolding waste-process design, evaluation and sensitivity analysis. Chemical Engineering Journal, 109, 147–165.
- Doyurum, S., & Celik, A. (2006). Pb(II) and Cd(II) removal from aqueous solutions by olive cake. Journal of Hazardous Materials, 138, 22–28.
- El-Nemr, A., El-Sikaily, A., & Abdelwahab, O. (2009). Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. Journal of Hazardous Materials, 161, 100–110.
- Fayomi, O. S., & Popoola, A. P. (2012). An investigation of the properties of Zn coated mild steel. International Journal of Electrochemical Science, 7, 6555–6570.
- Foo, K. Y., & Hameed, B. H. (2012a). Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K 2 CO 3 activation. Bioresource Technology, 104, 679–686.10.1016/j.biortech.2011.10.005
- Foo, K. Y., & Hameed, B. H. (2012b). Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance. Chemical Engineering Journal, 184, 57–65.10.1016/j.cej.2011.12.084
- Gunatilake, S. K. (2015). Methods of removing heavy metals from industrial wastewater. Journal of Multidisciplinary Engineering Science (JMESS), 1, 12–18.
- Hamadi, N. K., Xiao, D. C., Farid, M. M., & Lu, M. G. Q. (2001). Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chemical Engineering Journal, 84, 95–105.10.1016/S1385-8947(01)00194-2
- Hegazi, H. A. (2013). Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC Journal, 9, 276–282.10.1016/j.hbrcj.2013.08.004
- Hirunpraditkoon, S., Tunthong, N., Ruangchai, A., & Nuithitikul, K. (2011). Adsorption capacities of activated carbons prepared from bamboo by KOH activation. International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering, 5, 591–595.
- Li, W., Yang, K., Peng, J., Zhang, L., Guo, S., & Xia, H. (2008). Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Industrial Crops and Products, 28, 190–198.10.1016/j.indcrop.2008.02.012
- Norlia, M. I., Roshazita, C. A., Nuraiti, T. I. T., Salwa, M. Z. M., & Fatimah, M. S. S. (2011). Preparation and characterization of activated carbon. Environment and Chemistry, 184, 3873–4673.
- Olukanni, D. O. (2013). Evaluation of the influence of reactor design on the treatment performance of an optimized pilot-scale waste stabilization pond. International Journal of Engineering & Technology, 3, 189–198.
- Olukanni, D. O., & Ducoste, J. J. (2011). Optimization of waste stabilization pond design for developing nations using computational fluid dynamics. Ecological Engineering, 37, 1878–1888.
- Olukanni, D. O., & Kokumo, K. O. (2013). Efficiency assessment of a constructed wetland using Eichhornia crassipes for wastewater treatment. American Journal of Engineering Research (AJER), 2, 450–454.
- Ricordel, S., Taha, S., Cisse, I., & Dorange, G. (2001). Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling. Separation and Purification Technology, 24, 389–401.10.1016/S1383-5866(01)00139-3