?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Linear polarization resistance and open circuit potential methods were used to assess the inhibitive and adsorption behaviour of Potassium chromate (K2CrO4) and Cetylpyridinium chloride (CPC) on mild steel (MS) in HCl/NaCl solution. Results obtained show that K2CrO4 and CPC inhibit MS significantly against massive degradation. However, under open circuit potential method, inhibition efficiency of K2CrO4 decreased at higher concentration. The synergistic effect of both inhibitors is positive on the protection of mild steel against corrosion. An inhibitive efficiency of over 60% was recorded for the combined admixture. Morphological study showed that the exposed steel with the presence of K2CrO4 and CPC possess some corrosion product with lesser pitting effect compare to unprotected steel with severe surface deterioration and uniform corrosion degradation. The corrosion inhibition performance of K2CrO4 and CPC on mild steel surface was found to follow Langmuir adsorption isotherm model.
Public Interest Statement
The detrimental challenge by corrosion is worth studying in sea water applications, since corrosion problem pose a huge cost for new design and maintenance globally. Mild steel is the most available form of steel due to its low and excellent metallurgical properties however it susceptibility to corrosion is alarming. Appropriate efforts have been made to avert this catastrophes ranging from environmental pollution to loss of life. Research has proven that organic or inorganic chemical compounds with active electronegative group are effective in minimizing the corrosion aggressiveness. Meanwhile current trend has affirmed that the easiest way to develop or improve the properties of new inhibitor is via the synergistic application. Hence, this research aimed at investigating the inhibitive synergistic ability of cetylpyridinium and potassium chromate on corrosion behaviour of mild steel in aqueous dominated solution. This work is a contribution towards finding right improves inhibitor to minimize deterioration and extend life span of steel.
1. Introduction
Mild steel are industrially important especially in water transportation conveying tank, marine vessels, petrochemical septic tank, automobile, pickling’s plants, construction and oil well oxidizing system (Popoola, Fayomi, & Adeleke, Citation2013; Solomon, Umoren, Udosoro, & Udoh, Citation2010). However there are impediments resulting from abundance of sea-salt water and sulphide ion during conveyance especially in marine related application (Shanti & Rajendran, Citation2013). Corrosion by sea water, aqueous corrosion, is an electrochemical process, and steel when in contact with sea water have a specific electrical potential at a specific level of sea water acidity or alkalinity which is the pH. The disadvantage of seawater is in the chloride attack to steel structures. Seawater is an aqueous solution of salts with salinity equals 3.5 %. The composition of artificial ocean water according to ASTM D1141–98(2013) standard is described in Table (Zakowski, Narozny, Szocinski, & Darowicki, Citation2014).
Table 1. Artificial ocean water composition according to the ASTM D1141–98(2013) (Zakowski et al., Citation2014)
More so the presence of H+ within sea water salt concentration aids severe degradation and hydrogen embrittlement (Song & Velu, Citation2007; Zakowski et al., Citation2014). Thus, the consequence of chloride-acid attack or failure due to general corrosion had been widely reported (Quraishi & Sharma, Citation2005). Corrosion is a plight that faces everyone who works with metallic materials (Miksic, Boyle, & Wuertz, Citation2004; Quraishi & Sharma, Citation2005). Researchers are taken various steps to combat oceanic menaces of chloride and acid attack on steels which covered Inhibitive surface treatment (Miksic et al., Citation2004), alloy development (Trethewey & Roberget, Citation1995), use of sacrificial or anode system (Fayomi, Gbenebor, Abdulwahab, Bolu, & Popoola, Citation2013), cathodic or impressed current system (Loto & Popoola, Citation2011), organic and inorganic inhibitors (Ping, Cheng, & He, Citation2010). These methods are often used to mitigate against severe degradation. Moreover replacement of carbon steel has not been successfully achieved due to its importance in manufacturing system such as construction and fabrication industries (Fayomi & Popoola, Citation2014; Killeen & Killen, Citation2005).
The replacement of detrimental effect of seawater for general purposes in the industries, has also not be fully minized due to production processes hence, the need for continuous searching for corrosion inhibitor of mild steel in acid/chloride conditions become needful (Dan et al., Citation2010; Fayomi & Popoola, Citation2014). The mechanism of corrosion inhibition may be by film formation (Abdulwahab, Popoola, & Fayomi, Citation2012) on the substrate that could be effective if the film is continuous and tenacious and if otherwise could aggravate localised or pitting corrosion (Zakowski, Citation2011). Inhibitors could also form salt particles of the aggressive ions in the solution thereby reducing the mobility and the rate of attack of the negative ions on metallic substrate (Abdulwahab et al., Citation2012).
Cetylpyridinium chloride and potassium chromate are inorganic substances that are available in the market among other metallic salts (Oloruntoba, Abbas, & Olusegun, Citation2000). The choice of the two substances is on the fact that they are not poisonous in small quantities and when in ionic form can form positive polar region for the attraction of negative ions especially chloride from seawater. Cetylpyridinium and potassium chromate may also serve for neutralisation of other harmful metals in the seawater environment (Fayomi & Popoola, Citation2013; Oloruntoba et al., Citation2000). Chloride ions had been reported as being less hydrated than sulphate ions hence provides stronger tendency to adsorb on the metal by creating excess negative charge towards the solution phase, which favours synergetic adsorption on the metal surface (Noor, Citation2007; Pandiarajan, Rajendran, & Joseph Rathish, Citation2014; Shylesha, Venkatesha, & Praveen, Citation2011). Meanwhile current trend has affirmed that the easiest way to develop or improve the properties of new inhibitor is via the synergistic application (Fayomi et al., Citation2013; Ridhwan, Rahim, & Shah, Citation2012). Hence, this research is aimed at investigating the inhibitive synergistic ability of cetylpyridinium and potassium chromate on the corrosion behaviour of the mild steel in acid/chloride media.
2. Experimental procedures
2.1. Sample preparation
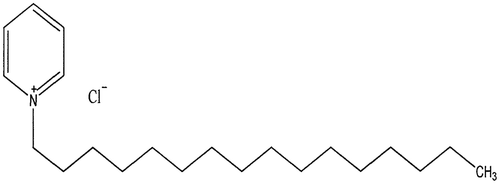
Sectioned mild steel test coupons with spectrometer chemical composition in (wt %) is shown in Table . The dimensions were (30 × 20 × 2) mm before mounting with an epoxy resin. The coupons were polished to a mirror-like nature with emery papers and cleaned in acetone. It was further dried and weighed with an analytical balance. Each sample weight was recorded and labelled correctly. The chemicals used were of analytical reagent grade which were prepared using distilled water. Concentrations of acid were prepared by using double distilled water and the concentration range of inhibitors was 2–10 ml in each of 40 ml 0.5 M HCl mixed with 3.65% NaCl solution. The molecular structures of the prepared inhibitor are shown in Figures and . The electrochemical investigation was performed at 25°C.
Table 2. Chemical composition of mild steel sample immersed in acid/chloride media
Figure 1. Molecular structure of potassium chromate (https://pubchem.ncbi.nlm.nih.gov/compound/potassium_chromate#section=Information-Sources).
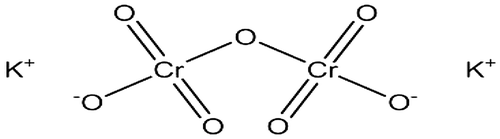
Figure 2. Molecular structure of cetylpyridinium chloride (https://pubchem.ncbi.nlm.nih.gov/compound/cetylpyridinium_chloride#section=IUPAC-Name).
2.2. Linear polarization resistance
Autolab PGSTAT 101 Metrohm potentiostat/galvanostat was used to obtain linear polarization measurements for potential-current trend. The sectioned mild steel coupon was mounted with resin, with a surface area of 1 cm2 and connected with electrode cell containing 40 ml of chloride electrolyte, with and without inhibitor. Graphite rod was used as auxiliary electrode and silver chloride electrode (SCE) function as a reference electrode. Linear polarization resistance potential scan range was from −1.5 V to +1.5 mV at scan rate of 0.0012 V/s. The corrosion potential (Ecorr), and current density (jcorr) data were evaluated from the Tafel plots. The surface coverage (θ) and the percentage inhibition efficiency (% IE) were calculated with Equations (1) and (2).(1)
(1)
The percentage inhibition efficiency (% IE) was calculated from corrosion current density values using the equation.(2)
(2)
where Icorr is inhibited corrosion current densities and Iocorr is Un-inhibited corrosion current density.
2.3. Morphological characterization
The sectioned samples mounted with cold resins sample was prepared mechanically using grade of SiC grit of 80, 150, 400, and 1,200 grits before polishing to 9 μm with Pen Struers diamond paste. Thereafter cleansed with deionized water and placed in a desiccator before used for potentiodynamic polarization according to ASTM standard G1–03(2011). The obtained samples under investigation were then analysed and examined with the help of ME 600T polarising optical microscope with built in camera.
3. Results and discussion
3.1. Potentiodynamic polarization study
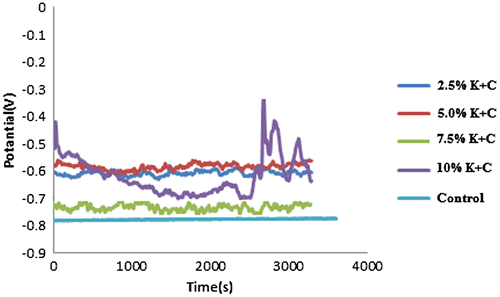
Presented in Figure is the synergistic effect of K2CrO4 and CPC inhibitive activity on mild steel in 0.5 M HCl + 3.65% NaCl contaminated medium under linear polarization system. The open circuit potential plots of the electrochemical influence of K2CrO4/CPC inhibitor on the corrosion of mild steel in its contaminated medium are shown in Figure . Table shows linear polarization Tafel data of admix Potassium chromate and Cetylpyridinium chloride in varying concentration. From the observed Tafel plots the corrosion rate values for K2CrO4/CPC inhibited steel shows less dissolutions. At 0% K2CrO4/CPC spontaneous anodic degradation of the steel sample were seen with obvious possibility of formation of pits within the interfacial surfaces. Although this is expected since metallic corrosion is heterogeneous in nature especially in the presence of aqueous medium (Zakowski et al., Citation2014). However, in other case of inhibited steel, with 2.5–10% inhibitor admix in 0.5 M HCl + 3.65% NaCl solution a remarkable decrease in corrosion rate occurred from 3.120 mm/years of control sample to 1.020 mm/years for 2.5% inhibited steel. Interestingly Cl- ions within the acid and salt solution are liable for the corrosive activity and deterioration that occurred on the steel interface. In practise corrosion inhibiting complexes intermingle with the cells diffusion or redox system hence, the anode–cathode polarization plots in Figure show passive characteristics as results of the inhibiting activity of the K2CrO4 and CPC compound. Thus, the corrosion potential “Ecorr” tend toward more positive region signifying that the contraption of inhibition by K2CrO4 and CPC is by surface active coverage principle as against the control sample. This surface active compound exhibited by the synergistic admix inhibitor act to prevent infringement of hydrogen and chloride ion thereby limiting the precipitation of H and Cl fruition within the surfaces. The corrosion potential, corrosion rate moves with change in corrosion current values as indicated in Figure .
Figure 3. Anodic and cathodic polarization curve for MS in 2.5–10% at 0.5 M HCl + 3.65% NaCl solution.
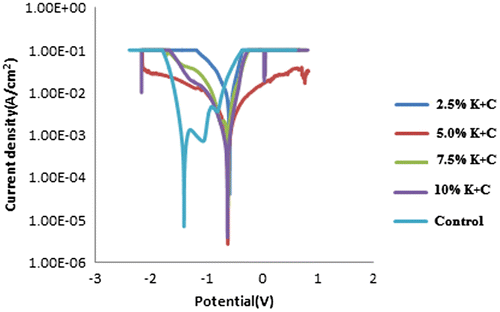
Table 3. Linear polarization data for MS in 2.5–10% at 0.5 M HCl + 3.65% NaCl solution
One notable observation is less change in performance with K2CrO4/CPC as concentration of the inhibitor increases. In general 2.5% K2CrO4/CPC concentration has inhibition efficiency of 99.95%, while at 5.0% K2CrO4/CPC, the inhibition efficiency is 99.94% at 7.5% K2CrO4/CPC, the inhibition efficiency is 99.95%, and at 10.0% K2CrO4/CPC, the inhibition efficiency is 99.94%. In other word, changes in potentials indicate that K2CrO4/CPC has substantially impacted on the mechanism of the corrosion progression as a result of the film laid on the metal surface.
In Figure , the change in corrosion potential against time was observed from the open circuit potential curve. The electrochemical performance of K2CrO4/CPC in 0.5 M HCl + 3.65% NaCl indicated that there were similar corrosion progressions of inhibitive performance of OCP with linear polarization behaviour of steel. This implies that K2CrO4/CPC instigate passivation through adsorption by reducing the active surface area prone to corrosive medium environment.
3.2. Mechanism of inhibition efficiency and adsorption study
Figure displays the comparative chart of inhibitory efficiency (IE) on mild steel substrate against synergistic inhibitive concentration in 0.5 M HCl + 3.65% NaCl solution. Observed on the chat are the potentiodynamic polarization (PP-Ecorr), Potentiodynamic-corrosion current (PP-Icorr), and polarization-corrosion rates (PP-CR). It was apparent that the inhibitory efficiency from all the processes agreed. More so this confirms the phenomena of adsorption to be linked with the molecular chemical activities of the molecule, ion, and the hydroxyl present.
Figure 5. Synergistic inhibitory efficiency (IE) for MS in 2.5–10% at 0.5 M HCl + 3.65% NaCl solution obtained from potentiodynamic polarization (PP-Ecorr), Potentiodynamic-corrosion current (PP-Icorr), polarization-corrosion rate (PP-CR).
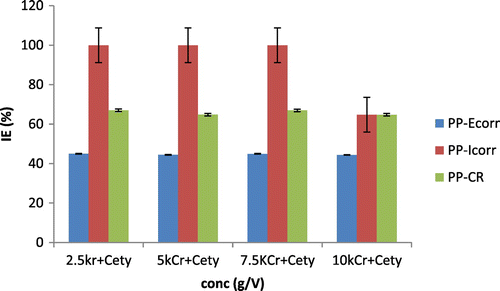
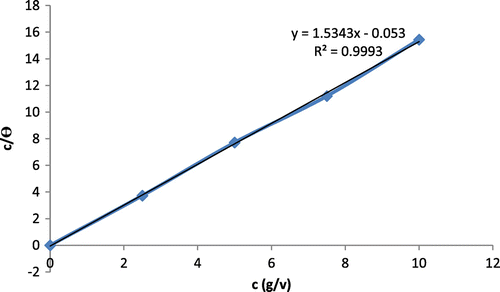
The corrosion rates efficiency increases with increasing concentration of K2CrO4/CPC as inhibitor between 2.5 and 7% but slightly decreased at 5 and 10% concentrations. Icorr rises to about 100 percent while the PP-Ecorr ranges around 40% efficiency with increasing concentration of K2CrO4/CPC. The Langmuir plot (Figure ) on the surface features shows a linear relationship with the increase in the concentration of the inhibitor this confirms a continuous adsorption of the corrosion product on the mild steel substrates and a protection on the mild steel substrate. The linear regression coefficient correction factors (R2) is almost unity – 0.998 which means all the surfaces of the mild steel substrate is covered with adsorbed corrosion products hence, the corrosion protection is achieved.
The adsorption characteristics of this study are centred on the molecular structure and the atom of the adsorbents inhibitor which are traced to physical adsorption between metal surface/charge atoms of the inhibitor (Abdulwahab et al., Citation2012). The change in the current at both anodic and cathodic region acted increasingly with the concentration of the mixed inhibitor at the metal interface reactions. The mechanism of these mixed inhibitory complex compounds is a blend of metal surface blockage, possible salt formation and the electrostatic force repulsion between the main adsorbed species and the medium ion. Hydrogen evolution generation that could have produced a massive impact and deterioration is distorted as a result of inhibitor effort. In an attempt to understand the metal inhibitor interaction and the metallic-complex activities on the coverage site, an adsorption mechanism facilitated according (Fayomi & Popoola, Citation2013) were computed for C/θ and C for potentiodynamic polarization system using Langmuir isotherm and a linear relationship was obtained with correlation regression coefficient of R2 = 0.998, 0.9595 and 0.9992 approximate unity for the addition of potassium chromate, cetylpyridinium chloride and synergistic effect of both inhibitor respectively.
3.3. Microstructural examination
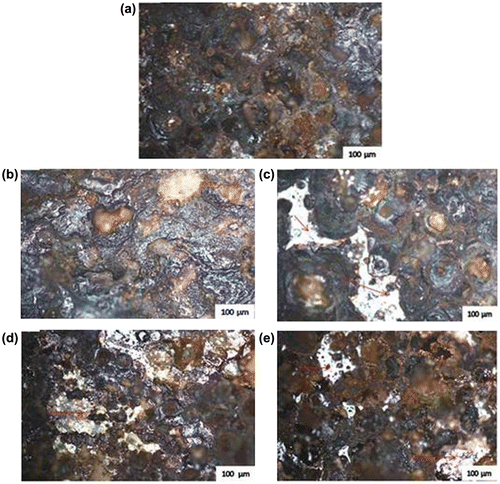
Figure (a)–(e) shows the micrograph of mild steel after exposure to 0.5 M HCl + 3.65% NaCl in the presence of K2CrO4/CPC varying concentration. The images show that there is a film formation on the samples of the inhibited steel (see Figure (b)–(e)) resulting to the protection of the mild steel against severe corrosion attack. Figure (a) shows the micrograph of the uninhibited mild steel after the corrosion test. Surface deterioration was observed due to the redox chemical reactions of corrosive ions emanating from metal solution interaction. It is obvious that the activity of medium spell negatively on the metal ions causing the release of valence electrons of Fe2+ cations into the acid medium thereby weakening the grey interfacial lattices. It is good to mention that a clear illustration of the micrograph especially for the as received sample in Figure (a) shows that pitting effect and intergranular corrosion is invariably accountable for the corrosion of MS. Little micro-pits but scale of film along metal surface were noticed from the inhibited metals in Figure (b)–(e), this thus indicated that synergistic action of K2CrO4/CPC ions and its heteroatoms adsorbs onto the mild steel.
4. Conclusions
| • | K2CrO4/CPC synergistic admix performed effectively well in the contaminated acid-chloride medium thereby inhibiting the mild steel. | ||||
| • | K2CrO4/CPC was found to be mixed type due to functional groups and heteroatoms of the compound. | ||||
| • | Maximum corrosion inhibitor efficiency of 99.95% was obtained for K2CrO4/CPC concentrate. | ||||
| • | Langmuir isotherm of a linear relationship with correlation regression coefficient of R2 = 0.9992 approximately unity was obtained for the addition K2CrO4/CPC on mild steel. | ||||
Funding
This work is based upon the financial support of National Research Foundation and effort by surface engineering research centre (SERC), Department of Chemical Metallurgical and Materials Engineering, the Tshwane University of Technology, Pretoria, South Africa. Covenant University is acknowledged for providing the funds for open access publication.
Additional information
Notes on contributors
O.S.I. Fayomi
O.S.I. Fayomi is a senior researcher in the Department of Mechanical Engineering at Covenant University and Research Fellow with Surface Engineering Research Centre (SERC) Tshwane University of Technology, Pretoria, South Africa. His research interests are centered on surface structural integrity, corrosion engineering, mechanical metallurgy, environmental science and engineering, materials development and processing, nanotechnology and triboxidation processing.
A.P.I. Popoola
A.P.I. Popoola is a professor of Metallurgical Engineering in the Department of Chemical, Metallurgical and Materials Engineering at Tshwane University of Technology, Pretoria, South Africa. She is the leader of Advanced Engineering Materials and Surface Technologies unit and her research includes: Surface science and corrosion engineering among others.
T. Oloruntoba
D.T. Oloruntoba is working as a senior lecturer in the Department of Metallurgical and Materials Engineering, Federal University of Technology Akure. His research interest is corrosion engineering.
A.A. Ayoola
A.A. Ayoola holds a PhD degree in Chemical Engineering. He is a senior lecturer in the Department of Chemical Engineering, Covenant University, Ota, Nigeria. His research interest includes biofuel (Renewable) Energy and Corrosion Science.
References
- Abdulwahab, M., Popoola, A. P. I., & Fayomi, O. S. I. (2012). Inhibitive effect by Ricinu’s communis on the HCl/H3PO4 acid corrosion of aluminium alloy. International Journal of Electrochemical Science, 7, 11706–11717.
- Dan, T., Shoji, T., Lu, Z., Sakaguchi, K., Wang, J., & Han, E. (2010). Effects of hydrogen on the anodic behavior of Alloy 690 at 60°C. Corrosion Science, 52, 1228–1236.10.1016/j.corsci.2009.11.039
- Fayomi, O. S. I., Abdulwahab, M., Durodola, B. M., Popoola, A. P. I., Joshua, T. O., Alao, A. O., … Inegbenebor, O. A. (2013). Study of the electrochemical behavior and surface interaction Of AA6063 Type Al-Mg-Si alloy by sodium molybdate in simulated sea water environment. BEST: International Journal of Management, Information Technology and Engineering, 1, 159–166.
- Fayomi, O. S. I., Gbenebor, O. P., Abdulwahab, M., Bolu, C. A., & Popoola, A. P. I. (2013). Structural modification, strengthening mechanism and electrochemical assessment of the enhanced conditioned AA6063-type Al-Mg-Si alloy. Journal of New Materials for Electrochemical Systems, 16, 59–64.
- Fayomi, O. S. I., & Popoola, A. P. I. (2013). Assessing the inhibitory potential of natural silicon oil on brass degradation in 1 M H2SO4. Research Journal of Chemistry and Environment, 10, 94–100.
- Fayomi, O. S. I., & Popoola, A. P. I. (2014). The inhibitory effect and adsorption mechanism of Roasted Elaeis guineensis.as green inhibitor on the corrosion process of AA6063 Al-Mg-Si alloy in simulated solution. Silicon. doi:10.1007/s12633-014-9177-3.
- Killeen, J., & Killen, J. (2005). Behaviour of high corrosion resistance Zr-based alloys. In Proceedings of the Technical Meeting of International Atomic Energy Agency (pp. 1–200), Buenos Aires.
- Loto, C. A., & Popoola, A. P. I. (2011). Effect of anode and size variations on the cathodic protection of mild steel in sea water and sulphuric acid. International Journal of the Physical Sciences, 6, 2861–2868.
- Miksic, B., Boyle, R., & Wuertz, B. (2004). Efficacy of vapor phase corrosion inhibitor technology in manufacturing. The Journal of science and Engineering Corrosion, 60, 515–522.10.5006/1.3287755
- Noor, E. A. (2007). Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extracts of Fenugreek leaves. International Journal of Electrochemical Science, 2, 996.
- Oloruntoba, D. T., Abbas, J. A., Olusegun, S. J. (2000). Water hyacinth (eichhornia crassipes) leaves extract as corrosion Inhibitor for AISI 1030 steel in sea water. In S. Agyyepong, S. A. R. Leiringer, W. Huhges (Eds.), Proceedings of the 4th West Africa built environment research (WABER) conference (pp. 1129–1138). Abuja: Laryea.
- Pandiarajan, M., Rajendran, S., & Joseph Rathish, R. (2014). Corrosion inhibition by potassium chromate-Zn2+ system for mild steel in simulated concrete pore solution research. Journal of Chemical Sciences, 4, 49–55.
- Ping, Z., Cheng, G., & He, Y. (2010). Ni-P-SiC composite coatings electroplated on carbon steel assisted by mechanical attrition. Acta Metallurtica Sinica (English Letters), 23, 1–10.
- Popoola, A. P. I., Fayomi, O. S. I., & Adeleke, A. A. (2013). Evaluation of Persea Americana green extract inhibitory performance on aluminium alloy in 1 M H2SO4/3.65% NaCl acid-chloride solutions. Research Journal of Chemistry and Environment, 17, 45–51.
- Quraishi, M. A., & Sharma, H. K. (2005). Thiazoles as corrosion inhibitors for mild steel in formic and acetic acid solutions. Journal of Applied Electrochemistry, 35, 33–39.10.1007/s10800-004-2055-8
- Ridhwan, A. M., Rahim, A. A., & Shah, A. M. (2012). Synergistic effect of halide ions on the corrosion inhibition of mild steel in hydrochloric acid using mangrove tannin. International Journal of Electrochemical Science, 7, 8091–8104.
- Shanti, T., & Rajendran, S. (2013). Corrosion resistance of mild steel simulated concrete pore solution in the presence of carboxy methyl cellulose. Journal of Chemical, Biological and Physical Sciences, 3, 2550–2556.
- Shylesha, B. S., Venkatesha, T. V., & Praveen, B. M. (2011). Corrosion inhibition study of mild steel by new inhibitor in different corrosion medium. Research Journal of Chemical Sciences, 1, 46–50.
- Solomon, M. M., Umoren, S. A., Udosoro, I. I., & Udoh, A. P. (2010). Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corrosion Science, 52, 1317–1325.10.1016/j.corsci.2009.11.041
- Song, H.-W., & Velu, S. (2007). Corrosion monitoring of reinforced concrete structures - A review. International Journal of Electrochemical Science, 2, 1–28.
- Trethewey, K. R., & Roberget, P. R. (1995). Expert overview corrosion management in the twenty-first century. British Corrosion Journal, 30, 192–198.10.1179/bcj.1995.30.3.192
- Zakowski, K. (2011). Studying the effectiveness of a modernized cathodic protection system for an offshore platform. Anti-Corrosion Methods and Materials, 58, 167–172.10.1108/00035591111148876
- Zakowski, K., Narozny, M., Szocinski, M., & Darowicki, K. (2014). Influence of water salinity on corrosion risk the case of the southern Baltic Sea coast. Environmental Monitoring and Assessment, 186, 4871–4879.10.1007/s10661-014-3744-3