?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the present approach, natural limonite iron oxide samples from Baharyia Oasis areas have been collected and ground to the micro-sized scale to be used as pigment for upholding the corrosion resistance behavior and mechanical performance properties of protective paints for steel petroleum structures. The assessment procedure includes measuring of specific gravity, moisture content, oil absorption, particle size distribution, particle shape, pH value, soluble in water, hardness, powder color and chemical composition by XRF analysis of limonite pigment particles. X-ray diffraction (XRD) analysis was implemented to assure the phase identification of the crystalline limonite pigment particles. Dynamic light scattering (DLS) measurements were accomplished to measure the particle size distribution and Zeta-potential of these particles in suspension. Amorphous and lamellar morphology of limonite particles was manifested by scanning electron microscopy (SEM) micrographs. EDX investigation was employed to give a semi-quantitative analysis and the chemical composition of limonite pigment. Different alkyd-based coating formulations were modified using various uniformly dispersing amounts of the processed limonite pigment particles as bi-functional modifier. The anti-corrosion performance properties of the considered limonite modified coating formulations against unmodified blank coating were studied by accelerated corrosion experiment (salt spray chamber) after 500 h exposure in 5% NaCl solution. The obtained results revealed a significant improvement in the rust grade, blistering size, color tolerance and adhesion performance of limonite modified coated steel films compared to unmodified conventional coating. To confirm the previous concept, weight loss method was performed. The mechanical performance properties for these films were investigated by adhesion cross-cut, bend and impact coating tests to confirm their application efficiency. From the recorded data it was found that, the adhesion properties, elongation character and resistance to rapid deformation of these coatings increased significantly to the steel surface with increasing the limonite pigment (%) by weight in the coating formulation. The results obtained compared with the ASTM and paint handbooks and found satisfactory for producing color paint pigment.
Public Interest Statement
Upgrading the added value of natural Iron oxide ores is considered a vital paradigm for the field of coating engineering. Natural iron oxide ore deposits are the main source of limonites, which vary from yellow to brown such as ochre, sienna and umber. Iron oxide pigments (IOPs) are non-toxic, relatively inert, weather-resistant and mainly opaque. They are used in paints, inks, plastics and cosmetics. Characteristics such as bulk density, oil absorption, opacity, and tinting strength determine the suitability of material for a given application. In the present work we have attempted to collect representative samples from Baharyia Oasis areas in Egypt and grind these to super fined micro-sized scale particles. Micro-sized limonite particles as pigment were incorporated in different concentrations by weight in alkyd-based protective paints and evaluated to achieve the protective strategy and mechanical performance properties of these pigmented coatings to be used for steel petroleum structures coating.
1. Introduction
Earthy pigments varying from dull yellow to red and brown are commonly called ochre’s in the economic geology, mining industry, and painting. Ochre’s are defined as clays used to make the earth colors; in pigment terminology, the word ochre is predominantly used as a synonym for yellow ochre (Hradil, Grygar, Hradilová, & Bezdička, Citation2003). Limonite is the significant colored mineral found in a natural state suitable for use as a pigment after being pulverized to the desired size. The name “limonite” was given to many of the yellowish to yellowish brown iron oxides produced during the weathering of iron-bearing rocks or deposited as bog, lake, and shallow marine sediments. The global production of iron oxide pigments in 2000 was estimated to be 1,500,000 Mt., and 87% of the total market is attributed to industrial applications (Will, Citation2004). Limonite is an iron ore consisting of a mixture of hydrated iron (III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO (OH)·nH2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Iron oxides pigments have the advantage of low cost, permanency, and non-toxicity. In the last century, the chemical industry improved on nature by developing a complete range of synthetic iron oxide pigments (IOPs) that surpass the pigments produced from natural iron ores in uniformity, color quality, and chemical purity. An important characteristic of a pigment is its color.
Most of the pigment iron ores characterization is nonreactive and contain only traces of heavy metals, toxic elements and have high degree of light fastness. This term refers to the ability of a pigmented film to resist color changes effects and other physicochemical degradation caused by exposure to weather conditions (Podolsky & Reid, Citation2010). Iron oxides such as magnetite, hematite and goethite are commonly used as pigments for black, red, brown and yellow colors respectively. These pigment types are strong absorbers of ultraviolet radiation and mostly used in automotive paints, wood finishes, construction paints, anticorrosive coatings, plastic industry, nylon, rubber and print ink. The excellent weather fastness, UV absorption properties, high transparency and increase color shades when combined with organic pigments and dyes (Mohapatra & Anand, Citation2010).
Although many techniques have been developed to reduce or minimize the action of corrosion, the use of paints or anti-corrosion coatings is the most efficient, economical and most widely used system at present for this purpose. Iron oxides occupy a special place as pigments or agents “barrier” in the formulation of anti-corrossive paints (Al-Sabagh, Abdou, Migahed, Abd-Elwanees et al., Citation2017a; Abdou, Ayad, Diab, Hassan, & Fadl, Citation2017). The widespread use of iron oxides in the field of anticorrosion paints is because they have a number of attributes that make them ideal candidates for use as protective pigments (CitationAl-Sabagh, Abdou, Migahed, Fadl et al., 2017). Alkyd resins are the most common resins to be used in solvent-based paints. They are basically polyesters and are used for both air-drying and heat-cured paints. Alkyd coating offers benefits of good adhesion, rapid drying, good flexibility, strength and durability at nominal cost; however alkyds cannot endure alkaline conditions (Forsgren, Citation2006; Fabregat et al., Citation2012; Soucek & Johansson, Citation2012). In literature, various researchers have investigated the corrosion resistance of alkyd based coating systems (Ahmed, Abdel-Fatah, & Youssef, Citation2012; Araujo, Margarit, Mattos, Fragata, & de Lima-Neto, Citation2010; Bhanvase & Sonawane, Citation2010; Gonçalves, Baldissera, Rodrigues, Martini, & Ferreira, Citation2011; Yeganeh & Saremi, Citation2015). The protective action in alkyd-based coatings was generally achieved with the use of inhibitors such as metallic pigments, metal oxides and salts at different concentrations. Attempts have been carried out to improve the anticorrosion behavior of the organic paints containing some inorganic pigments such as lamellar micaceous iron oxide (MIO) which is essentially a type of hematite, lamellar aluminum and Zn-dust (Arman, Ramezanzadeh, Farghadani, Mehdipour, & Rajabi, Citation2013; Kakaei et al., Citation2013a, Citation2013b; Nikravesh, Ramezanzadeh, Sarabi, & Kasiriha, Citation2011; Sørensen, Kiil, Dam-Johansen, & Weinell, Citation2009). The incorporation of nanoferrite (Fe2O3) particles in soya alkyd coating enhanced corrosion resistance and physico-mechanical properties (Rahman & Ahmad, Citation2014). Modification of alkyd coatings with aluminum oxide (Al2O3) nano-sized pigment improved anticorrosion behavior, UV resistance and mechanical properties (Dhoke & Khanna, Citation2012; Dhoke, Mangal Sinha, & Khanna, Citation2009). It has been illustrated that application of ZnO nanoparticles improves the anticorrosion behavior of alkyd based coatings (Dhoke & Khanna, Citation2009). Egyptian ilmenite ore was utilized after grinding to micro-sized scale as anticorrosive pigment and mechanical property promoter for internal epoxy coating of gas transmission pipelines (Fadl, Al-Sabagh, Abdou, & Migahed, Citation2016).
The aim of this study includes two objects. The first object is to collect a representative sample of Egyptian limonite ore from Baharyia Oasis areas to upgrade its added value by grinding it to the micro-sized scale to be used as a pigment for alkyd-based protective paints. The second object is to investigate the anticorrosion and mechanical performance properties of limonite modified coating formulations on the carbon steel surface to confirm its field application efficiency according to international standards.
2. Materials and methods
2.1. Materials
2.1.1. Chemicals
The employed soya bean short alkyd resin 60% concentration, Appearance: Clear yellow to amber liquid, Odor: Characteristic, bland, fatty oil, color: Yellow to Amber, Vegetable Oil Triglycerides (%): 100, Specific gravity / Relative density at 25°C (g/ml): 0.970–0.975, Flash point (°C): 321, Solubility: Soluble in xylene, was conducted from Chemical Partners Company. Xylene, Color: Colorless, Physical state and appearance: Liquid, Odor: Sweetish, Molecular Formula: C8H10, Specific gravity (g/l): 0.864, Flash point (°C): 26, was obtained from SIGMA ALDRISH Company. Ethylene glycol and acetone were gained from El-Mohandes Company for Chemicals and Trading and used in technical grades.
2.1.2. Bahariya Oasis geological setting
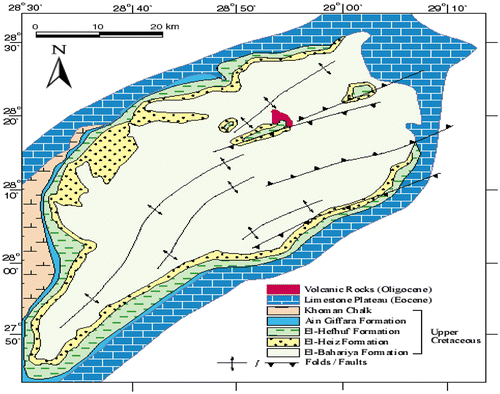
Bahariya Oasis is situated in the middle of the Western Desert between latitudes 27° 48′ and 28° 30′ N and longitudes 28° 32′ and 29° 10′ E, about 370 km southwest of Cairo (Baioumy, Khedr, & Ahmed, Citation2013) as shown in Figures and . The Bahariya is one of the most geologically important areas in the Western Desert, and attracted considerable attention especially in the last four decades, since the discovery of iron ore deposits in the El Gedida, Ghorabi and El Harra localities (Catuneanu, Khalifa, & Wanas, Citation2006). The iron is represented minerlogically by a range of minerals. In ferruginous late rites formed during deep tropical weathering it is commonly present as red-brown hydroxides and oxides: limonite (FeO (OH).nH20), goethite (FeO (OH), hematite (Fe2O3). These are generated under oxidizing conditions from many kinds of country rock. The Bahariya area is a large depression in the Western Desert of Egypt with hot, dry climatic conditions and presence of huge reserves (280 million metric tons) of iron ore deposit .The sedimentary iron ores are found in four areas in the Bahariya Oasis (El-Gedida, Naser, Gebel Ghorabi and El-Harra), Bahariya iron ores have 52% iron percent that is suitable for the iron high ovens in Helwan City factories, now. Iron ores excavated from El-Gedida mine with an annual rate 3.3 Million Tons then, carried about 300 km away to Helwan City factories for steel manufacturing. The El-Gideda mine area is an oval shaped depression up to 15 km2, located within the degraded karst cone hills of the Naqb Formation of Middle Eocene age. The central part of the depression is characterized by a high relief (up to 254 m above sea level). The low Wadi area up to 198 m above sea level surrounds the high central area, which is built up by the Cenomanian clastics at the base, overstepped by the main Lutetian iron ore succession. El-Gedida mine has a structural setting of two normal faults dividing it into three parts, a central plateau and two valleys on its two sides, and has 140 Million Tons of the all 280 Million Tons spare iron ores in Bahariya Oasis, and has about 6 square km surface area with a face wall reaches 45 meters tall (Abouzeid & Khalid, Citation2011; Bahariya Oasis Geological Field Trip, Citation2014) .There is a wide distribution of non-marine limonite ore with an average thickness ranging from 0.5–8 m of Quaternary age covered the Dakhla–Kharga depression. These deposits are recorded as remnants overlying Sabaya and Quseir formations. These indicate that sediments exposed to the deep weathering process, the limonite deposited formed originally from rapid hydrothermal solutions by the discharge of iron-rich Nubian Aquifer water (El-Desoky, El-Rahmany, Farouk, Khalil, & Fahmy, Citation2015).
2.1.3. Egyptian limonite ore sampling and its physic-chemical properties (FeO (OH)·nH20)
Fifty samples were collected from Baharyia areas represent to iron oxide “limonite” ore. The representative sample was used for measuring the ore physical properties, chemical analysis and X-ray diffraction studies. The representative samples were subjected to required tests and the results obtained were compared with the paint pigment manufacturing standards to confirm the limonite ore suitability for producing a color pigments for decorative paints. The pigment tests procedure are explained in a relative text books and standards and summarized as follow: Specific gravity (ASTM-D153, 1989) and (ASTM-C-128, 2001), Moisture content (ASTM-D280, 1987), (ASTM-C-566,1997) and (ISO 11,127–5, 1993), Oil absorption (ASTM D281, 1989), Particle size, Particle shape, Hydrogen ion concentration (pH value) (IS, 2002) and (ASTM E70, 1990), Matter solubility in water, Hardness, and Color of powder. Limonite was analyzed by Thermo ARL ADVANT XP-385 XRF model and its physicochemical properties are shown in Tables and . The viscosity test of the powder samples were measured by mixing the specific proportions of pigment powder as 20, 40 and 60% with linseed oil (ASTM –D1200, 1990), (ASTM-D2196, 1991) and (ASTM-D234, 1991), the viscosity measured with Rotational viscometer and the results indicated in Tables .
Table 1. Physical characterization of limonite pigment
Table 2. XRF chemical analysis of the used limonite particles
Table 3. Viscosity of limonite ores forming pigments
2.1.4. Preparation of the carbon steel samples and its composition
The carbon steel samples used in the present work were divided into working specimens with dimensions 5 × 10 cm × 2 mm for salt spray test and weight loss method. The specimens used for cross-cut adhesion, impact and bend coating tests were with dimensions 15 × 10 cm × 0.8 mm. The specimens were mechanically polished with emery paper, washed in acetone and distilled water before coating application and its chemical composition is presented in Table .
Table 4. Chemical composition of testing carbon steel
2.2. Methods and techniques
2.2.1. Preparation of micro-sized limonite pigment particles
Micro-sized limonite particles were prepared by solid-phase milling method. The Lab Testing 911 Metallurgist ball mill was used to grind limonite aggregates obtained from Bahariya Oasis limonite quarry for 3 h in a grinding chamber to obtain the highest degree of ilmenite fineness range at micro-sized scales.
2.2.2. X-ray diffraction (XRD) analysis
X-ray diffraction (XRD) patterns of limonite ore particles were measured by using a Panlytical X’pent PRO (Netherlands) with monochromated Cukα radiation with scattering reflections recorded for 2θ angle between 4 and 79 corresponding to d-spacing between 1.31 and 7.52 Ǻ. To confirm the resolution of the diffraction peaks with standard reproducibility in 2-theta (±0.005), the sample measurements was recorded by using monochromator and detector which were used to generate focusing beam geometry and parallel primary beam. The standard diffraction data were identified according to the International Center for Diffraction Data (ICDD) software with PDF-4 release 2011 database.
2.2.3. Dynamic light scattering (DLS) for particle size distribution measurements
Zeta potential and dynamic light scattering (DLS) measurements were performed on a Zetasizer Nano ZS (Malvern, UK) with a He–Ne laser (633 nm) using a non-invasive backscatter method (detection at 173° scattering angle). Correlation data were fitted, using the method of cumulants, to the logarithm of the correlation function, yielding the diffusion coefficient, D. The hydrodynamic diameters (DH) of the PCVs were calculated using D and the Stokes–Einstein equation (DH = kBT/3π ηD, where kB is the Boltzmann constant, T is the absolute temperature, and η is the solvent viscosity (η = 0.8872 cP for water). The polydispersity index (PDI) of vesicles (represented as 2c/b2, where b and c are first- and second-order coefficients, respectively, in a polynomial of a semi-log correlation function) was calculated by the cumulative analysis. Size distribution of the vesicles was obtained by the non-negative least-squares (NNLS) analysis. The particle size distribution is also important because a tighter size distribution gives greater color purity and brilliance.
2.3. Morphology of limonite pigment particles by scanning electron microscopy (SEM) energy dispersive analysis of X-rays (EDX) characterizations
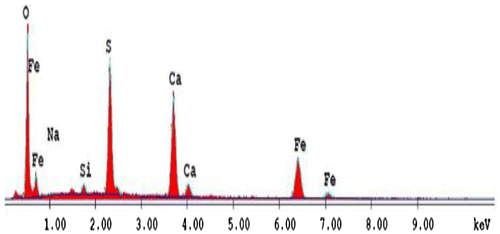
The surface morphology of limonite pigment particles were clarified by scanning electron microscopic (SEM) and energy dispersive analysis of X-rays (EDX) micrographs by JOEL-5410 scanning electron microscope (Japan) utilized for this investigation. SEM micrograph of the investigated particles were carried out at magnifications of 6,000 X. EDX system attached with scanning electron microscope was used for elemental analysis and chemical characterization of material particles. As a type of spectroscopy, it relies on the investigation of sample through interaction between electromagnetic radiation and the matter. So that, a detector was used to convert X-ray energy into voltage signals.
2.4. Modification of the coating
The prepared micro-sized limonite particles were added with different amounts by weight as (5, 10, 15, 20 and 25%) of the total formulations content to the alkyd resin with adding ethylene glycol and xylene using an economic mixer with ultrasonic devices for complete dispersion. Therefore, acetone was added with a contentious stirring for 20 min to form five limonite modified coating formulations (LC-5, LC-10, LC-15, LC-20 and LC-25) against another unmodified coating (blank alkyd coating as varnish). The investigated coating formulations in absence and presence of limonite pigment as a modifier were given in Table .
Table 5. Various limonite coating formulations
2.5. Coating thickness
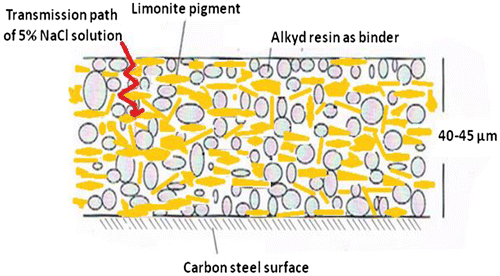
The dry film coating thickness (DFT) was measured on steel panels by Positector 6,000 (USA) in the range from 40–45 μm.
2.6. Anticorrosive properties of unmodified and limonite modified cured coating films
2.6.1. Salt spray test (accelerated corrosion cabinet)
Measurements were performed using salt spray chamber manufactured by Sheen Instruments Ltd. England at 35°C. Coated panels were being placed in the salt spray chamber with relative humidity 95% and test solution 5% NaCl according to ASTM B117-03 and ISO 9227:2012. Cross line in the middle of coated steel panel was made using a cutter to allow the salt solution to transfuse the panel to examine the adherence of paint over the coated area. The corrosion resistance was estimated in terms of blistering degree (ASTM D 714-02), a degree of rusting (ASTM D 610-01), softening (API 5L2 4th edition, July 2002), color tolerances and differences (ASTM D 2244-02), and adhesion degree (ASTM D 3359-97).
2.6.2. Weight loss test
The weight loss method has also been performed for corrosion study. The measurements were made using the salt spray cabinet by the direct exposure of untreated and limonite treated cured coated films for a period of 500 h to the fog of corrosive medium of 5% NaCl solution. The coated mild steel specimens were weighted in an electronic balance with an accuracy of 0.1 mg. After the direct exposure of coated films to the salt spray fog, they were immersed thoroughly in 1 M HCl to remove the rust products and washed with distilled water followed by acetone and dried with air, then weighted again. The performance of the alkyd coating was examined visually and through calculation of the weight loss. Weight loss (∆W) is expressed as the loss in weight per unit area per unit time (g cm−2 h−1). The corrosion rate (CR, mm/y) and protection efficiency (PE%) were determined using Equations (1) and (2) (Hassan, Citation2005; Hsu & Mansfeld, Citation2001; Yu, Gan, & Jiang, Citation2008):(1)
(1)
(2)
(2)
where, w 0 and w 1 are the weight loss values (mg) of unmodified and limonite modified coated samples, respectively, a is the total surface area in cm2, d is the density of the mild steel = 7.85 g/cm3 and t is the exposure time in hours.
2.7. Mechanical performance properties of unmodified and limonite modified cured coating films
The prepared steel specimens were used for measuring (a) the film coating thickness (ASTM D 1005-07); (b) the adhesion cross-cut test (ASTM D 3359-97) by sheen multi-blade cutting tool with six cutting edges spaced 1 mm apart; (c) the resistance to rapid deformation “impact” (ASTM D 2794-93, Reapproved 1999) was measured using a tubular impact tester; d) mandrel flexibility bend test (ASTM D 522-93a, Reapproved 2001) was determined using conical mandrel tester.
3. Results and discussion
3.1. Coating modification by limonite pigment
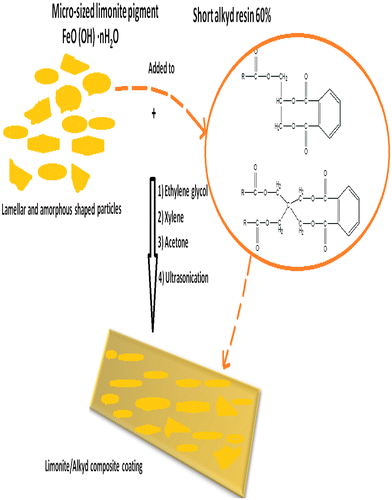
The investigated limonite coating was based on soya bean oil (short oil) alkyd resin 60% diluted in xylene as a binder or vehicle to hold all constituents of coating. Limonite/alkyd composite coating was formulated as shown in Figure . The paint was formulated by adding the micro-sized limonite particles as bi-functional modifier for modifying the anti-corrosion and mechanical properties of alkyd coating. Ethylene glycol was incorporated as leveling agent and to increase the polarity of the coating emulsion to increase the interface surface interaction between the pigment particles and the resin matrix. Xylene solvent was added as a thinner to reach the best dispersion of limonite particles in alkyd resin matrix and to control the paint viscosity. Therefore, Acetone was added to enhance the solubility power of coating constituent. Ultrasonic cavitation produces high shear forces that fracture particle agglomerates into single dispersed particles. The individual micro-sized limonite particles are held together by attraction forces including Vander Waals forces and liquid surface tension. So, the purpose of utilization the ultrasonic devices is to produce de-agglomerated limonite particles which well-dispersed through the alkyd vehicle.
3.2. X-ray diffraction (XRD) analysis of limonite pigment particles
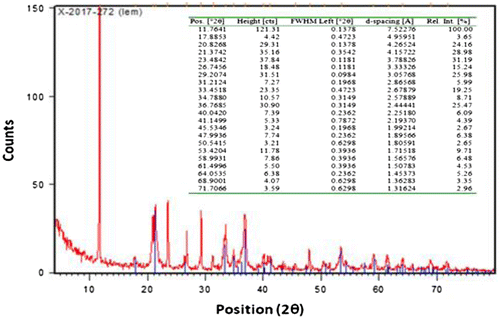
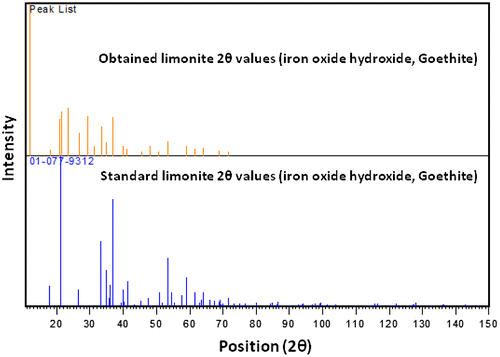
XRD peak patterns of super-fined limonite particles examine the physicochemical make-up of these particles as illustrated in Figures and . The good harmonization between standard diffraction peaks and the obtained peaks ensures the ore phase identification of limonite particles with a chemical composition of FeO (OH)·nH2O. Diffraction peaks as shown in Figure were more intensive and narrower, implying a real crystalline nature of the micro-sized limonite particles. In addition, the broadening at the bottom of diffraction peaks also, denotes that the crystalline sizes were small and in good agreement with the characteristics of submicro-sized particles.
3.3. Dynamic light scattering (DLS) measurements
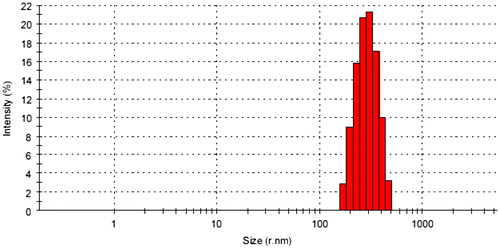
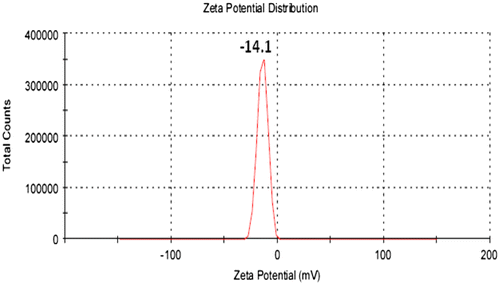
DLS analysis provides the ability to measure the particle size distribution in suspension. Figure shows the relation between the particle size and the distribution intensity of the limonite particles. According to Figure it can be noted that the fined limonite particles with a random movement in suspension locate in the micro-sized scale (submicro-sized scale) with particle intensity around 100%. The dispersion in suspension gives an idea about the distribution ability of these particles through any coating binder and the interface-interaction property that will be established. Figure shows the Zeta-potential distribution values for the micro-sized limonite particles. From the obtained results, the mean Zeta-potential peak value was obtained at −14.1 mV and this value express about the steric stabilization of dispersed limonite particles in the suspension. Then, the aggregation of limonite particles will be prevented in suspension.
3.4. Morphology of limonite pigment particles by SEM and EDX micrographs
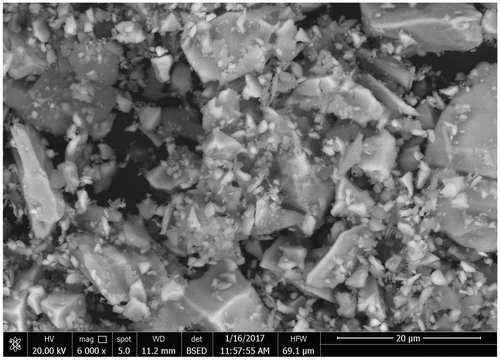
SEM and EDX micrographs of micro-sized limonite particles were illustrated in Figures and . These clearly show the realistic nature of the limonite particles used in this study. These characteristic images revealed that, the limonite particles had amorphous shape and some of them had lamellar nature as shown in Figure with a shield of the overlapping plates during the highly-dispersion of these particles through the coating binder. These SEM images ensure the protective action can be expected after incorporation of the limonite pigment particles through the alkyd resin. EDX micrograph as shown in Figure gave an idea about the composition of the limonite pigment particles and illustrated a semi-quantitative analysis for these particles.
3.5. Anticorrosive properties of unmodified and limonite modified cured coating films
3.5.1. Salt spray test (accelerated corrosion cabinet)
Salt spray accelerated corrosion test is generally suitable as corrosion protection method for rapid analysis for discontinuities, pores and damage in organic and inorganic coatings. Some inhibitive pigments are relatively weak-effective for the anticorrosion purpose in the presence of high concentrations of chlorides such as seawater and there is a decrease in the corrosion protection of organic coatings after short immersion time (Herbert & Uhlig, Citation1985). Due to fragile protective effect of alkyd varnish layer used in formulation process of protective coatings used for steel petroleum structures, a hydrolytic degradation of the coating binder exposed to corrosive electrolyte was made. Coating degradation leads to increase in number of pores and crevices. Therefore, the corrosion resistance was also studied to investigate the effect of the limonite pigment particles on the protective performance of the used alkyd coating. Results of salt spray test after 500 h of exposure by 5% NaCl solution are depicted in Table and shown in Figure . It can be seen that the coating degradation after exposure to salt spray is more distinct in case of blank alkyd coating without limonite particles as anticorrosive pigment than those modified with it. Visual comparison of photographic reference standards used to evaluate and measure the degree of blistering, softening, adhesion grade and the percentage of the rusted area showed that, blank alkyd coating without limonite pigment shows the presence of blisters (#6 size and medium dense frequency), softening, a degree of rust (3), and loss of adhesion (2B). This indicates that, blank alkyd coating had undergone a drastic chemical changes during exposure in which responsible for the failure. With the incorporation of limonite pigment particles in the coating formulation with different loading levels, no any defects were observed such as rust, regular blistering distributed on the surface of the coated film, or loss of adhesion and reached the maximum protective behavior for LC-25 coating. The data depicted in Table indicated that in case of LC-25 coating, the rust grade gave the maximum value at grade (10), the blistering size appeared at #8 with few frequency, 3% color tolerance changes, no softening and the adhesion grade of 5B. The inhibitive performance of the limonite coating (LC) films as a function of the rust grade increased and supported with increasing the loading level (%) by weight of the limonite pigment in the following order: LC-25 > LC-20 > LC-15 > LC-10 > LC-5 > Blank alkyd coating as varnish.
Table 6. Accelerated corrosion experiment of unmodified and limonite modified alkyd coating films after 500 h exposure in salt spray cabinet using 5% NaCl soln
Figure 10. Accelerated corrosion salt spray test for unmodified and limonite modified cured alkyd coating films.

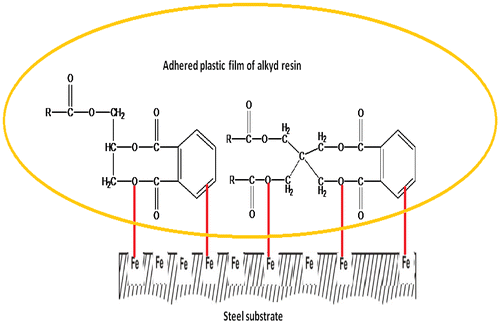
This improvement in the protective action was attributed the well-dispersion of limonite pigment particles with amorphous and lamellar shapes through the alkyd base matrix in which restrict the diffusion of the corrosive electrolyte through the limonite modified cured alkyd coating films acting as an effective barrier. It may be also attributed to, the well-dispersed limonite particles having a small size absorb more resin on their surfaces which promote the density of the coating films, thereby diminishing the transport paths for the corrosive electrolyte to penetrate the coating and consequently reducing the corrosion process (Kalendová, Citation2003; Shi, Liu, Han, & Wei, Citation2007; Yang, Liu, & Han, Citation2005). The synergistic anticorrosion effect of limonite pigment particles and alkyd coating in hindrance the corrosion of carbon steel is illustrated by the protective mechanism as shown in Figure and summarized as follows. First, the superior corrosion resistance offered by the combined effect of adsorption of π- electrons of the aromatic rings and lone pair of electrons of oxygen atoms present in functional groups of alkyd base matrix and form a strong coherent plastic film and increase the adhesion forces between the metal surface and the coating as shown in Figure . This plastic film reduces the transfer of the corrosive species to metal surface by forming a protective layer and imparts excellent chemical stability.
Figure 12. Sketch illustrates the plastic film formed on the carbon steel surface by the alkyd resin.
Second, the barrier effect of the limonite cured coated films could be attributed to the presence of limonite pigment particles with amorphous and lamellar shapes which aligned parallel to the steel surface through the alkyd base matrix producing a shield of the overlapping plates. This alignment enhanced protection by providing a protective barrier, increasing the film reinforcement and enhancing the inter-coat adhesion. Barrier effect observed from coating films as shown in Figure was attributed to the amorphous nature of limonite particles in which a physical barrier is formed that prevents the ingress of water, oxygen and ions to inhibit corrosion of the steel and degradation of the binder (Mathiazhagan & Joseph, Citation2011).
3.5.2. Weight loss method
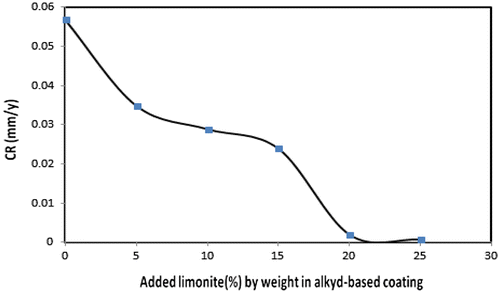
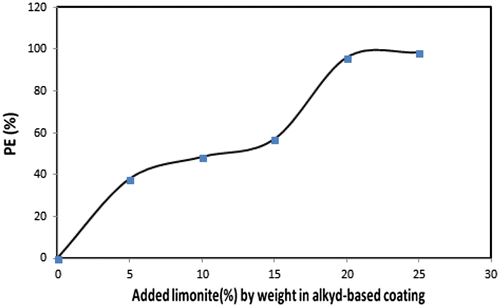
Table shows the weight loss values of unmodified and limonite modified coated steel films during the direct exposure to the salt spray fog for a period of 500 h. The results revealed that, blank alkyd coated film without limonite pigment showed the maximum weight loss value at 127.5 mg with corrosion rate (CR) of 0.057 mm/y. The highest weight loss in case of blank alkyd coated film was attributed to the spreading of pinholes on the coating surface due to the absence of limonite pigment in which leave permeable sites then, corrosion products were formed on these sites and by removing them, made the blank coating film offered the maximum weight loss value. With increasing the limonite pigment loading levels till 25% by weight from the total ratio in the coating formulation (LC-25 coating), the least weight loss value at 2 mg with corrosion rate (CR) of 0.0008 mm/y and protection efficiency (PE) of 98.4% were observed. These results were attributed to the presence of the highest loading level of the amorphous and lamellar-shaped limonite particles which aligned parallel to the steel surface through the alkyd base matrix producing a shield of the overlapping plates in which increase the density of cured film then, enhancing the inter-coat adhesion (Gabilondo & López, Citation2007). So, the chlorides and various aggressive ions could not penetrate the LC-25 coating film and offered the maximum protection efficiency. Figures and showed the decrease in corrosion rate values and the increase in protection efficiency values with increasing the limonite pigment amount by weight with respect to the total coating formulation.
Table 7. Weight loss data of unmodified and limonite modified alkyd coating films before and after 500 h exposure in salt spray cabinet using 5% NaCl soln
3.6. Mechanical performance properties of unmodified and limonite modified cured coating films
3.6.1. Cross-cut adhesion test
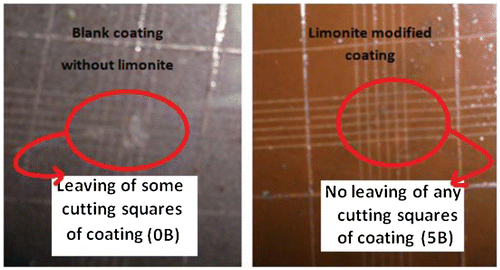
The results of cross-cut test were shown in Table to improve with increasing the concentration (%) by weight of limonite pigment through alkyd coating films. So, LC-25 achieved the maximum adhesion property (5B) against completely deterioration in adhesion character in case of blank conventional alkyd coating. This positive result in the mechanical property as shown in Figure can be attributed to the increasing of the interface surface interaction between the micro-sized limonite particles and alkyd base matrix in which enhancing the inter-coat adhesion. And this could be due to a superb dispersion and high compatibility between limonite and alkyd resin matrix. The limonite pigment particles may act to prohibit alkyd disaggregation during the curing and result in more homogenous coating (Huong, Citation2006; Shi, Nguyen, Suo, Liu, & Avci, Citation2009).
Table 8. Mechanical performance properties of unmodified and limonite modified alkyd coating films
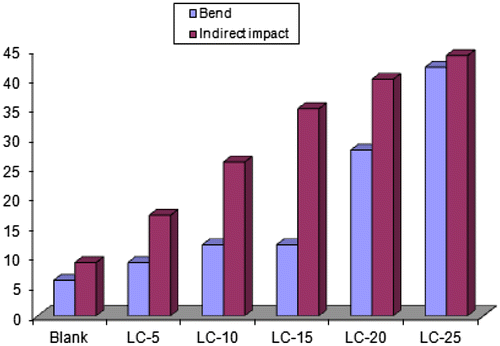
3.6.2. Impact damage test (resistance to rapid deformation)
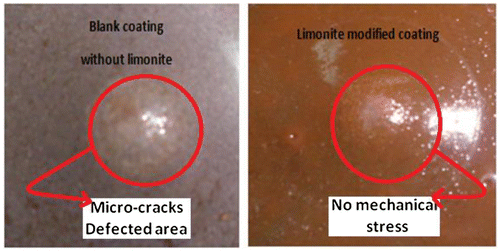
Impact resistance was evaluated to study the load distribution property of coating systems. The results of indirect impact were shown in Figure to improve with increasing the concentration of limonite pigment through alkyd coating films. This positive result in the mechanical property as depicted in Table can be attributed to the increasing in the interface surface interaction between the micro-sized limonite pigment particles and alkyd base matrix then, decreasing the formation of micro-voids between these particles and the coating matrix and decreasing the origin of any cracks and the under mechanical stress.
3.6.3. Bend test (flexibility)
Based on the qualitative measurements, it could be noted that the limonite coating films showed reasonably an increase in flexibility (bend) with increasing the limonite pigment content as illustrated in Table . These results may be ascribed to the following mechanisms. First, the well-dispersion of limonite pigment particles tends to occupy holidays such as pinholes and voids in the thin-film coating and serve as the bridges in the interconnected matrix, causing a reduction in the total free volumeand an enhancement in cross-linking density of the cured film. As such, the cured limonite coating has reduced chain segmental motions and improved stiffness. Second, the limonite pigment particles may act to prevent alkyd disaggregation during the curing process and result in more homogenous coating (Huong, Citation2006; Shi et al., Citation2009). Finally, the limonite pigment particles resulted in a reduced viscosity of alkyd solution in xylene and thus led to a thinner coating layer on the steel surface, which diminished the internal stress of the cured coating. The ingress in the mechanical properties (bend and impact) with increasing the concentration of the limonite pigment particles in the alkyd coating films was shown in Figure .
4. Conclusions
Natural limonite pigment samples from Baharyia Oasis areas have been collected and ground to the micro-sized scale and were incorporated for upgrading the anticorrosion and mechanical properties of alkyd- based coating for steel petroleum structures. Limonite characterization was performed by different assessment methods. Various limonite/alkyd composite coating were prepared for the present investigation. Visual comparison of photographic reference standards used by salt spray accelerated corrosion test affirmed that blank alkyd coating (without limonite) had undergone a drastic chemical changes during exposure in which responsible for the failure. With the incorporation of limonite pigment particles in the coating formulation with different loading levels, no any defects were observed such as rust, regular blistering distributed on the surface of the coated film, or loss of adhesion. Weight loss test manifested that LC-25 coating gave the least corrosion rate (CR) value at 0.0008 mm/y and protection efficiency (PE) 98.4%. With increasing the amount (%) by weight of limonite pigment, There is a synergistic increase in the adhesion properties, elongation character and resistance to rapid deformation of the modified coatings This positive result in the mechanical property can be attributed to the increasing of the interface surface interaction between the micro-sized limonite particles and alkyd base matrix in which enhancing the inter-coat adhesion.
Funding
This work was supported by the Egyptian Petroleum Research Institute.
| Abbreviations | ||
| XRD | = | X-ray diffraction |
| XRF | = | X-ray fluorescence |
| DLS | = | Dynamic light scattering |
| SEM | = | Scanning electron microscopy |
| EDX | = | Energy dispersive analysis of x-rays |
| IOPs | = | Iron oxide pigments |
| CR | = | Corrosion rate |
| PE | = | Protection efficiency |
| DFT | = | Dry film thickness |
| PDI | = | The polydispersity index |
| NNLS | = | Non-negative least squares |
| LC | = | Limonite coating |
Acknowledgement
The authors wish to express deep thank to Egyptian Petroleum Research Institute (EPRI) for equipment and chemicals support.
Additional information
Notes on contributors
M.I. Abdou
M.I. Abdou is a professor and Head of production department in Egyptian Petroleum Research Institute (EPRI). He is a director of Technical Support Center (Petroleum-industry) and employs a head of coating production, application and development project. He works in the fields upgrading the added value of Egyptian ores, drilling fluids applications, steel petroleum structures inspection, and corrosion monitoring operations
Hany El-Sayed Ahmed
Hany El-Sayed Ahmed is an assistant-professor, production department, EPRI. He works in the fields of drilling mud engineering and coating applications.
M.A. Wahab Gaber
M.A. Gaber is an assistant-professor in exploration department, EPRI. He interests by the natural fillers application in coating industry.
A.M. Fadl
A.M. Fadl is a researcher in production department, EPRI. He is a deputy- director of Technical Support Center (Petroleum-industry) and coating project. He works in the fields of coating engineering and technology, upgrading the added value of Egyptian ores and corrosion monitoring operations.
References
- Abdou, M. I. , Ayad, M. I. , Diab, A. S. M. , Hassan, I. A. , & Fadl, A. M. (2017). Influence of surface modified ilmenite/melamine formaldehyde composite on the anti-corrosion and mechanical properties of conventional polyamine cured epoxy for internal coating of gas and oil transmission pipelines. Progress of Organic Coating , 113 , 1–14.10.1016/j.porgcoat.2017.08.003
- Abouzeid, A.-Z. M. , & Khalid, A.-A. M. (2011). Mineral industry in Egypt-part I: Metallic mineral commodities. Natural Resources , 2 , 35–53.
- Ahmed, N. M. , Abdel-Fatah, H. T. M. , & Youssef, E. A. (2012). Corrosion studies on tailored Zn-Co aluminate/kaolin core–shell pigments in alkyd based paints. Progress of Organic Coating , 73 , 76–87.10.1016/j.porgcoat.2011.09.003
- Al-Sabagh, A. M. , Abdou, M. I. , Migahed, M. A. , Abd-Elwanees, S. , Fadl, A. M. , & Deiabc, A. (2017). Investigations using potentiodynamic polarization measurements, cure durability, ultra violet immovability and abrasion resistance of polyamine cured ilmenite epoxy coating for oil and gas storage steel tanks in petroleum sector. Egyptian Journal of Petroleum . in press. doi:10.1016/j.ejpe.2017.07.006
- Al-Sabagh, A. M. , Abdou, M. I. , Migahed, M. A. , Fadl, A. M. , Farag, Ahmed A. , Mohammedy, M. M. , … Deiabc, A. (2017). Influence of ilmenite ore particles as pigment on the anticorrosion and mechanical performance properties of polyamine cured epoxy for internal coating of gas transmission pipelines. Egyptian Journal of Petroleum . in press. doi:10.1016/j.ejpe.2017.07.005
- Araujo, W. S. , Margarit, I. C. P. , Mattos, O. R. , Fragata, F. L. , & de Lima-Neto, P. (2010). Corrosion aspects of alkyd paints modified with linseed and soy oils. Electrochimica Acta , 55 , 6204–6211.10.1016/j.electacta.2010.03.088
- Arman, S. Y. , Ramezanzadeh, B. , Farghadani, S. , Mehdipour, M. , & Rajabi, A. (2013). Application of the electrochemical noise to investigate the corrosion resistance of an epoxy zinc-rich coating loaded with lamellar aluminum and micaceous iron oxide particles. Corrosion Science , 77 , 118–127.10.1016/j.corsci.2013.07.034
- Bahariya Oasis Geological Field Trip . (2014). Geological Filed trip report. Shebeen El-Kom: El-Monfya University, Faculty of Science.
- Baioumy, H. M. , Khedr, M. Z. , & Ahmed, A. H. (2013). Mineralogy, geochemistry and origin of Mn in the high-Mn iron ores (pp. 63–76). Egypt, Ore Geology Reviews: Bahariya Oasis.
- Bhanvase, B. A. , & Sonawane, S. H. (2010). New approach for simultaneous enhancement of anticorrosive and mechanical properties of coatings: Application of water repellent nano CaCO3-PANI emulsion nanocomposite in alkyd resin. Chemical Engineering Journal , 156 , 177–183.10.1016/j.cej.2009.10.013
- Catuneanu, O. , Khalifa, M. A. , & Wanas, H. A. (2006). Sequence stratigraphy of the lower cenomanian bahariya formation, Bahariya Oasis, Western Desert, Egypt. Sedimentary Geology , 190 , 121–137.
- Dhoke, S. K. , & Khanna, A. S. (2009). Study on electrochemical behavior of nano-ZnO alkyd-based waterborne coatings. Journal of Applied Polymer Science , 113 , 2232–2237.10.1002/app.v113:4
- Dhoke, S. K. , & Khanna, A. S. (2012). Electrochemical impedance spectroscopy (EIS) study of nano-alumina modified alkyd based waterborne coatings. Progress of Organic Coating , 74 , 92–99.10.1016/j.porgcoat.2011.11.020
- Dhoke, S. K. , Mangal Sinha, T. J. , & Khanna, A. S. (2009). Effect of nano-Al2O3 particles on the corrosion behavior of alkyd based waterborne coatings. Journal of Coating Technology and Research , 6 , 353–368.10.1007/s11998-008-9127-3
- El-Desoky, H. , El-Rahmany, M. , Farouk, S. , Khalil, A. , & Fahmy, W. (2015). Geochemical characteristics of goethite bearing deposits in the Dakhla – Kharga oases, Western Desert, Egypt. IJSEAS , 1 (8), 72–85.
- Fabregat, M. G. , Azambuja, D. S. , Aleman, C. , & Armelin, E. (2012). Evaluation of an environmentally friendly anticorrosive pigment for alkyd primer. Progress of Organic Coating , 73 (321), 17–28.
- Fadl, A. M. , Al-Sabagh, A. M. , Abdou, M. I. , & Migahed, M. A. (2016). Method for preparation epoxy lotion from ilmenite ore to protect steel structures from corrosion . ARE Patent No. 27679.
- Forsgren, A. (2006). Corrosion control through organic coatings . Milton Park: Taylor & Francis Group, LLC.10.1201/CRCCORRTECHN
- Gabilondo, N. , & López, M. J. A. (2007). Curing kinetics of amine and sodium hydroxide catalyzed phenol-phenol formaldehyde resins. Journal of Thermal Analysis and Calorimetry , 90 , 229–236.10.1007/s10973-006-7747-3
- Gonçalves, G. S. , Baldissera, A. F. , Rodrigues, L. F. , Martini, E. M. A. , & Ferreira, C. A. (2011). Alkyd coatings containing polyanilines for corrosion protection of mild steel. Synthetic Metals , 161 , 313–323.10.1016/j.synthmet.2010.11.043
- Hassan, H. H. (2005). Effect of chloride ions on the corrosion behavior of steel 0.1 M citrate. Electrochimica Acta , 51 , 526–535.10.1016/j.electacta.2005.05.011
- Herbert, H. , & Uhlig, R. (1985). Corrosion & Corrosion control (3rd ed.). (pp. 151–153). New York, NY : John Wiley and Sons.
- Hradil, D. , Grygar, T. , Hradilová, J. , & Bezdička, P. (2003). Clay and iron oxide pigments in the history of painting. Applied Clay Science , 22 , 223–236.10.1016/S0169-1317(03)00076-0
- Hsu, C. H. , & Mansfeld, F. (2001). Atmospheric corrosion of B6 bronze evaluated by thin layer activation technique. Corrosion , 57 , 747–748.10.5006/1.3280607
- Huong, N. (2006). Improvement of bearing strength of laminated composites by nanoclay and Z-pin reinforcement ( PhD. dissertation). University of New South Wales, Australia.
- Kakaei, M. N. , Danaee, I. , & Zaarei, D. (2013a). Investigation of corrosion protection afforded by inorganic anticorrosive coatings comprising micaceous iron oxide and zinc-dust. Corrosion Engineering, Science and Technology , 48 , 194–198.10.1179/1743278212Y.0000000060
- Kakaei, M. N. , Danaee, I. , & Zaarei, D. (2013b). Evaluation of cathodic protection behavior of waterborne inorganic zinc‐rich silicates containing various contents of MIO pigments. Anti-Corrosion Methods and Materials , 60 , 37–44.10.1108/00035591311287438
- Kalendová, A. (2003). Effects of particle sizes and shapes of zinc metal on the properties of anticorrosive coatings. Progress in Organic Coatings , 46 (4), 324–332.10.1016/S0300-9440(03)00022-5
- Mathiazhagan, A. , & Joseph, R. (2011). Nanotechnology- A new prospective in organic coating review. International Journal of Chemical Engineering and Applications , 2 (4), 226.
- Mohapatra, M. , & Anand, S. (2010). Synthesis and applications of nano-structured iron oxides/hydroxides – a review. International Journal of Engineering Science and Technology , 2 (8), 127–146.
- Nikravesh, B. , Ramezanzadeh, B. , Sarabi, A. A. , & Kasiriha, S. M. (2011). Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corrosion Science , 53 , 1592–1603.10.1016/j.corsci.2011.01.045
- Podolsky, G. , & Reid, Austin H, Jr. (2010). Industrial minerals and rocks (pp. 1453–1469). Amsterdam: Elsevier
- Rahman, O. U. , & Ahmad, S. (2014). Physico-mechanical and electrochemical corrosion behavior of soya alkyd/Fe2O3 nanocomposite coatings. RSC Advances , 4 , 14936–14947.10.1039/C3RA48068B
- Shi, H. , Liu, F. , Han, E. , & Wei, Y. (2007). Effect of nanopigments on the corrosion resistance of alkyd coatings. Journal of Material Science and Technology , 23 (4), 551–558.
- Shi, X. , Nguyen, T. A. , Suo, Z. , Liu, Y. , & Avci, R. (2009). Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surface and Coating Technology , 204 , 237–245.10.1016/j.surfcoat.2009.06.048
- Sørensen, P. A. , Kiil, S. , Dam-Johansen, K. , & Weinell, C. E. (2009). Anticorrosive coatings: A review. Journal of Coating Technology and Research , 6 , 135–176.10.1007/s11998-008-9144-2
- Soucek, M. , & Johansson, M. K. G. (2012). Alkyds for the 21st century. Progress of Organic Coating , 73 , 273–289.10.1016/j.porgcoat.2012.01.004
- Will, R. (2004). Iron oxides for colorants and chemical applications . Portland, ME : Intertech.
- Yang, L. H. , Liu, F. C. , & Han, E. H. (2005). Effects of P/B on the properties of anticorrosive coatings with different particle size. Progress of Organic Coating , 53 (2), 91–98.10.1016/j.porgcoat.2005.01.003
- Yeganeh, M. , & Saremi, M. (2015). Corrosion inhibition of magnesium using biocompatible Alkyd coatings incorporated by mesoporous silica nanocontainers. Progress in Organic Coatings , 79 , 25–30.10.1016/j.porgcoat.2014.10.015
- Yu, J. , Gan, F. , & Jiang, L. (2008). Inhibition effect of 2-amino-5-mercapto-1,2,4-triazol on copper corrosion. Corrosion , 64 , 900–904.10.5006/1.3294405