Abstract
The effect of incorporation of titanium oxide (TiO2) and hydroxyapatite (HA) on the mechanical properties of wollastonite (CaSiO3) was investigated in this study. TiO2/CaSiO3/HA composites were prepared and characterized by means of physical and mechanical properties. Morphology analysis revealed that the wollastonite composite possesses a rough surface. Moreover, the addition of TiO2 and HA to wollastonite was evaluated by means of bulk density and Vickers Hardness. The wollastonite composites containing TiO2 (10–30 wt%) and HA (20–40 wt%) were sintered between 1230°C and 1270°C, with a ramp rate of 10°C/min and a holding time of 2 h. The results indicated that the additives played a significant part in enhancing the physical and mechanical properties of the composite wollastonite in comparison to the pure wollastonite, particularly in composites containing higher percentage of TiO2 (25–30 wt%) and lower HA (20–25 wt%). Furthermore, the results obtained from the XRD analysis revealed that the newly formed apatite particles on the surface of the wollastonite composite were comprise of superfine crystalline and/or defective structure grains.
PUBLIC INTEREST STATEMENT
This paper aims at investigating the usage of wollastonite in becoming a potential biomaterial for clinical applications. Wollastonite as a standalone material does not have enough strength and suffers from a high dissolution rate. Thus, by incorporating additives such as titanium oxide (TiO2) and hydroxyapatite (HA) this problem may be overcome as well as the mechanical properties of wollastonite may be improved. The TiO2/CaSiO3/HA composites were prepared and characterized by means of physical, mechanical properties (i.e. density, hardness, strength).
1. Introduction
Bone-related diseases as well as injuries have become quite frequent in recent times. Due to strenuous activities, bones are under a lot of stress, especially load-bearing bones such as the femur and tibia, and as a result the bone fractures and the surrounding tissues are damaged. This has lead to researchers attempting to develop a biomaterial which is capable of imitating a real bone particularly in terms of durability and reliability (Garcia, Miranzo, & Sainz, Citation2018). Biomaterials, however, are unable to partake in the biological processes which are responsible for bone formation and remodelling (Copp & Shim, Citation1963). The selection of a biomaterial is crucial in determining the outcome of the biological events that take place such as signalling molecules, growth factors and cell migration onto the site of injury (Freyman, Yannas, & Gibson, Citation2001). Currently, metals have been largely utilized for bone replacements. Over time, the properties of the metal implant may deteriorate as metals may support too much load and eventually experience failure. Besides that, metals may also degrade after a period of time due to its surroundings, which in turn causes the release of metal ions into the human body which may lead to side effects such as toxicity and allergic reactions. Wollastonite is seen as a promising biomaterial due to its properties such as biocompatibility with human cells, compatibility with hard tissues as well as not being toxic (Lin et al., Citation2005; Magallanes-Perdomo et al., Citation2010; Risbud, Saheb, Jog, & Bhonde, Citation2001; Tamimi et al., Citation2008; Zhang, Molenda, Fournelle, Murphy, & Sahai, Citation2010). Wollastonite exists in two mineral forms; α-wollastonite (high temperature) and β-wollastonite (low temperature) (Shukur, Al-Majeed, & Obied, Citation2014; Teixeira et al., Citation2014). According to researchers, the release of ions from calcium silicates could promote gene expression and aid in the efficiency of insulin-like growth factor (IGF) which is related to cell proliferation. Generally, wollastonite exhibits good mechanical properties such as hardness and compressive strength. However, according to previous studies, it was found that the high dissolution rate of wollastonite proves to be a drawback as it hinders the biomaterial’s use in clinical applications (Aly, Mohammed, Al-Meer, Elsaid, & Barakat, Citation2016; Liu & Ding, Citation2002a). Addition of other biomaterials to wollastonite could hinder this problem. In this study, TiO2/CaSiO3/HA composites were prepared and characterized by means of physical and mechanical properties.
2. Materials and methods
The TiO2/TCP doped wollastonite (CaSiO3), with different TiO2/TCP concentrations, were synthesized through co-precipitation method. Various weight percentages of TiO2 and TCP were mixed with CaSiO3 by wet milling in ethanol in an ultrasonic machine. Subsequently, the mixture was milled for 1 h, after which the slurry was dried at 60°C in an oven for 12 h. The dried mixture was then sieved through a 212 μm stainless steel sieve to obtain a ready-to-press TiO2/TCP powder. The mixed powder was pressed in circular-hardened steel (20 mm in diameter) and rectangular (80 × 50 × 8 mm) mold and die set under a hydraulic pressure of 500 MPa. The samples were subsequently subjected to cold isostatic pressing (CIP) at a pressure of 200 MPa with a holding time of 5 min.
Pressing was followed by the consolidation of the samples by ambient pressure, while sintering was performed in air using a heating furnace (ModuTemp) at various temperatures ranging from 1230ºC to 1270ºC at ramp-rate of 10ºC/min for both heating and cooling and holding time of 2 h prior to cooling to room temperature. All samples were polished using SiC papers (120, 240, 600, 800) from coarse to rough, followed by polishing with a diamond paste to 6 µm in order to obtain an optically reflective surface.
Phase analysis by X-ray diffraction (Geiger-Flex, Rigaku Japan) of the powders and sintered samples were carried out under ambient conditions using Cu-Kα as the radiation source. The fraction of the monoclinic (m) phase present in the ceramic matrix was determined using the method of Toraya et al. (Toraya, Yoshimura, & Somiya, Citation1984). The morphology of the powders and microstructural evolution of the sintered samples were studied using a Scanning Electron Microscope (Philips XL30 ESEM).
3. Results and discussion
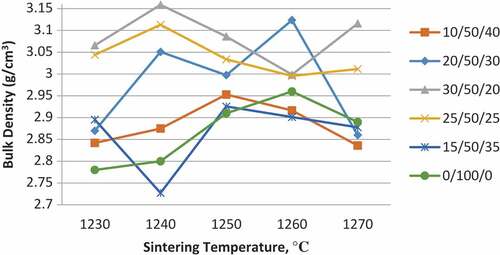
3.1. Bulk density
The densification behavior of the wollastonite composites attribute to the incorporation of TiO2 and HA to the wollastonite, in terms of bulk density, was studied and observed for various sintering temperatures as depicted in Figure . At a low sintering temperature, it was observed that all doped compositions exhibited an increase in density from 1230°C to 1240°C, with results ranging between 2.7 and 3.2 g/cm3. Between this temperature range, the 30 wt% TiO2/50 wt% CaSiO3/20 wt% HA samples achieved the highest bulk density, approximately 3.15 g/cm3. The value obtained was proved to be a significant increase from the theoretical value of density for wollastonite (2.90 g/cm3) (Aly et al., Citation2016), approximately 8.6% higher. Sintering at 1240°C with holding time of 2 h showed enhancement in density all compositions except for the 15 wt% TiO2/50 wt% CaSiO3/35 wt% HA sample. Hence, it could be concluded that the optimum sintering temperature was at 1240°C. The sintering temperature may have played a pivotal part in increasing the bulk density. Moreover, during the sintering process, the porosity of the sample may have been reduced, resulting in higher bulk density.
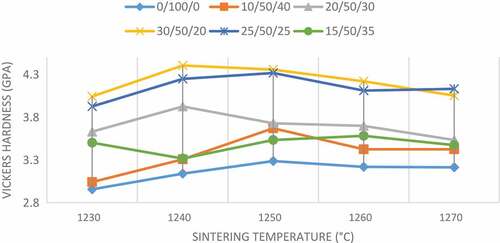
3.2. Vickers hardness
The mechanical properties of the sintered composites were investigated based on Vickers hardness and Compressive strength, relative to the sintering parameters such as the amount of additives and sintering temperatures. The effect of TiO2 and HA additions on the Vickers hardness of wollastonite sintered from 1230ºC to 1270ºC is illustrated in Figure . The Vickers hardness values of the composites varied in a more even manner as compared to that of bulk density, while all samples displayed an increasing trend at low sintering temperatures (1230–1250ºC), followed by a slight decline, as the temperature increased from 1250 to 1270ºC. Pure wollastonite showed the lowest value of hardness (around 3.00 GPa) at the initial sintering temperature (1230ºC), followed by a very minor increase with the increase of temperature, while the highest hardness achieved for this sample was 3.3 GPa. However, the highest hardness achieved among the tested samples was around 4.4 GPa at 1240ºC for the sample composition of 30 wt% TiO2/50 wt% CaSiO3/20 wt% HA. The obtained results indicated that the additives were effective in enhancing the hardness of wollastonite sintered at lower temperatures (between 1240°C and 1250°C). Theoretically, the density and hardness of the wollastonite composites are directly proportional to each other. It can be seen that the sintering temperature range of 1230–1240°C. This was confirmed as the results in Figure , while Figure presented a similar increasing trend, especially for sample compositions 30 wt% TiO2/50 wt% CaSiO3/20 wt% HA and 25 wt% TiO2/50 wt% CaSiO3/25 wt% HA. The increase in density of the composites may verify the existence of strong bonding between the grains in the sintered composites, which suggests that they can achieve greater hardness and material strength. Liu et al. (Liu & Miao, Citation2004) made an observation stating that an increase of hardness with sintering temperature due to the high degree of crystallization resulted in the existence of higher content of wollastonite within the matrix of the composite material.
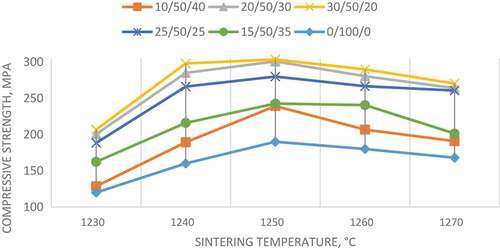
3.3. Compressive strength
Figure indicates the effect of incorporation of TiO2 and HA, as well as sintering temperature, on the compressive strength of wollastonite. The influence of sintering can be clearly observed in the figure, regardless of the percentage of additives. An increasing trend between 1230°C and 1250°C is evident, which is similar to the trends observed in Figures and . The lowest compressive strength observed was seen for the sample containing 10 wt% TiO2/50 wt% CaSiO3/40 wt% HA (around 120 MPa) at the lowest sintering temperature (1230°C), whereas the highest value of strength (around 300 MPa) was observed in the sample containing 30 wt% TiO2/50 wt% CaSiO3/20 wt% HA, rising sharply from 200 to 300 MPa between 1230°C and 1240°C. In reference to Figure , it can be stated that the addition of TiO2 contributed to the improvement in strength, similar to the hardness. This was confirmed since all samples containing TiO2 yielded higher strength values than pure wollastonite sample. In general, the strength of the ceramic is majorly dependant on the porosity and microstructural defects (i.e. grain size) that lie within the sample. Samples with higher porosity rate tend to have lower mechanical properties as well as density, as there are gaps between particles of the samples. This may cause a sample to easily crack since there are no particles to hinder the crack propagation. Through subjecting the samples to optimum sintering parameters (i.e. sintering temperature, holding time), the mechanical properties may increase significantly due to the decrease in porosity. In reference to Figures –, it can be assumed that the optimum temperature to produce the highest mechanical results was 1250°C.
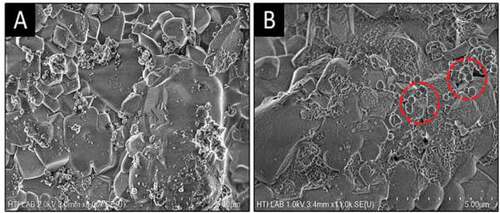
3.4. Morphological analysis
The SEM micrographs of wollastonite composite before and after immersion in SBF are illustrated in Figure . It can be observed that wollastonite composite possess a rough surface, while clusters of TiO2 were evident on the surface in Figure ). This could be due to the fact that TiO2 has higher melting point than the wollastonite (Liu & Ding, Citation2002b). Furthermore, ball-like particles were apparent on the surface of the wollastonite composite after immersion in SBF (Figure )), while these were not observed on the surface of the sample before immersion in SBF (Figure )). These particles are known to be apatite and were formed due to the interaction of calcium ions on the surface of the composite and the SBF solution (Liu & Ding, Citation2002a). The mechanism of apatite formation on the surface of the wollastonite coating in the SBF can be interpreted as follows. When the wollastonite composite is immersed in the SBF, calcium ions in the composite are exchanged with H+ in the solution, leading to formation of silanol (≡Si–OH) on the surface layer, increase in pH, and finally the production of a negatively charged surface with the functional group (≡Si–O¯) (Liu, Ding, & Chu, Citation2004). The ionic activity product of the apatite in the SBF solution is considered to be the key to the formation of apatite on the surface of the substrate (Liu & Ding, Citation2002b). Formation of apatite on the surface of wollastonite composite after immersion in SBF has been reported by Liu et al. (Liu & Ding, Citation2002a), where an increase in the immersion time increased the precipitation of apatite on the surface of the wollastonite composite.
3.5. X-ray diffraction (XRD) analysis
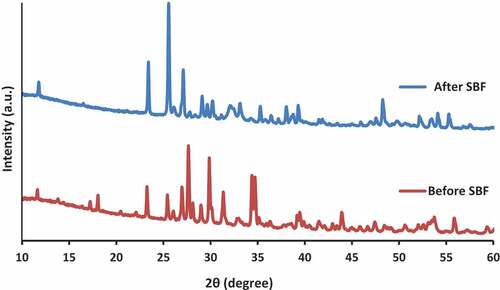
The X-ray diffractograms of wollastonite composite before and after immersion in SBF are presented in Figure . Major crystalline peaks attributed to the structure of wollastonite could be observed at 2θ = 23.2, 27.6, 29.8, and 34.4. However, these peaks were replaced, shifted, or their intensity was reduced after the immersion of sample in SBF solution due to the formation of apatite on the surface of the wollastonite composite. The broadening peaks of apatite crystallite were evident at 2θ = 23.4, 25.5, and 27.1, indicating that the apatite particles were comprise of superfine crystalline and/or defective structure grains. At high supersaturations, where homogeneous nucleation prevails, the critical radius of the crystal may be rather small, and for crystals with large unit cells (such as hydroxyapatite), it may become smaller than one unit cell (Liu & Ding, Citation2002b). On the other hand, the rate of nucleation increases exponentially with the supersaturation. Under such conditions, poorly crystalline or amorphous particles are formed. Amorphous precipitates tend to be metastable and in contact with the mother liquid into a more stable structure. The SBF solution is highly supersaturated with respect to apatite under normal condition. After the immersion of composite in SBF solution, the calcium phosphate phase that accumulates in the surface of the coating, which was initially amorphous (a-CaP), will be crystallized to a hydroxyapatite structure (Liu & Ding, Citation2002b).
4. Conclusion
The present study investigated the effect of TiO2 and HA incorporations on the enhancement of the physical and mechanical properties of wollastonite. The obtained results indicated that properties were significantly enhanced by the addition of 25–30 wt% TiO2 and 20–25 wt% HA. The highest value of density obtained was about 3.15 g/cm3, approximately 8.6% higher than the theoretical density value (2.90 g/cm3). In addition, improvements in the mechanical properties of wollastonite were observed, while sintering at low temperatures (1230–1240ºC) was found to be the optimum temperature to obtain the highest achievable properties. However, sintering above 1250ºC was observed to cause detrimental changes to both physical and mechanical properties. Morphology analysis revealed that the wollastonite composite possesses a rough surface, while apatite was formed on samples which were immersed in SBF. Moreover, the results obtained from the XRD analysis revealed that the newly formed apatite particles on the surface of the wollastonite composite were consist of superfine crystalline and/or defective structure grains.
Correction
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
D. Ragurajan
My team comprises of 4 people. We specialise in ceramic materials for biomedical applications. Over the years, we have experimented on various types of ceramics such as zirconia, titanium oxide, zinc oxide, as well as cerium oxide in order to find which material is more suited for biomedical applications. The materials we research on are normally synthesized in our lab in Universiti Tenaga Nasional. Mechanical and physical tests are done in our lab while biomedical tests are conducted at Hospital Universiti Kebangsaan Malaysia with our collaborator. This paper focuses on the potential of wollastonite being a biomaterial which can hopefully then be used as an implant material in the human body to replace other biomaterials such as metals or polymers.
References
- Aly, I. H. M., Mohammed, L. A. A., Al-Meer, S., Elsaid, K., & Barakat, N. A. M. (2016). Preparation and characterization of wollastonite/titanium oxide nanofiber bioceramic composite as a future implant material. Ceramics International, 42, 11525–7.
- Copp, D. H., & Shim, S. S. (1963). The homeostatic function of bone as a mineral reservoir. Oral Surgery, Oral Medicine, and Oral Pathology, 6, 738–744.
- Freyman, T. M., Yannas, I. V., & Gibson, L. J. (2001). Cellular materials as porous scaffolds for tissue engineering. Progress in Materials Science, 46, 273–282.
- Garcia, E., Miranzo, P., & Sainz, M. A. (2018). Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceramics International, 44, 12896–12904.
- Lin, K., Zhai, W., Ni, S., Chang, J., Zeng, Y., & Qian, W. (2005). Study of the mechanical property and in vitro biocompatibility of CaSiO3 ceramics. Ceramics International, 31, 323–326.
- Liu, J., & Miao, X. (2004). Sol- gel derived bioglass as a coating material for porous alumina scaffolds. Ceramics International, 30, 1781–1785.
- Liu, X., & Ding, C. (2002a). Plasma sprayed wollastonite/TiO2 composites coatings on titanium alloys. Biomaterials, 23, 4065–4077.
- Liu, X., & Ding, C. (2002b). Morphology of apatite formed on surface of wollastonite coating soaked in simulated body fluid. Materials Letters, 57, 652–655.
- Liu, X., Ding, C., & Chu, P. K. (2004). Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials, 25, 1755–1761.
- Magallanes-Perdomo, M., De Aza, A. H., Mateus, A. Y., Teixeira, S., Monteiro, F. J., De Aza, S., & Pena, P. (2010). In vitro study of the proliferation and growth of human bone marrow cells on apatite-wollastonite-2M glass ceramics. Acta Biomaterialia, 6, 2254–2263.
- Risbud, M., Saheb, D. N., Jog, J., & Bhonde, R. (2001). Preparation, characterization and in vitro biocompatibility evaluation of poly(butylene terephthalate)/wollastonite composites. Biomaterials, 22, 1591–1597.
- Shukur, M. M., Al-Majeed, E. A., & Obied, M. M. (2014). Characteristic of wollastonite synthesized from local raw materials. International Journal of Engineering & Technology, 4, 426–429.
- Tamimi, F., Kumarasami, B., Doillon, C., Gbureck, U., Le Nihouannen, D., Cabarcos, E. L., & Barralet, J. E. (2008). Brushite-collagen composites for bone regeneration. Acta Biomaterialia, 4, 1315–1321.
- Teixeira, S. R., Souza, A. E., Carvalho, C. L., Reynoso, V. C. S., Romero, M., & Ma, J. (2014). Characterization of a wollastonite glass-ceramic material prepared using sugar cane bagasse ash (SCBA) as one of the raw materials. Materials Characterization, 98, 209–214.
- Toraya, H., Yoshimura, M., & Somiya, S. (1984). Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction. Journal of the American Ceramic Society. American Ceramic Society, 67, C-119-C–121.
- Zhang, N. L., Molenda, J. A., Fournelle, J. H., Murphy, W. L., & Sahai, N. (2010). Effects of pseudowollastonite (CaSiO3) bioceramic on in vitro activity of human mesenchymal stem cells. Biomaterials, 31, 7653–7665.