?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Corrosion is known to negatively affect the structural integrity of reinforced concrete structures. In order to manage the effect of corrosion on structures, some preventive measures must be considered. Among such measures is to test the integrity of the reinforcement used. Three high-yield reinforcements mostly used in Lagos State (Pulkit, LCI and Tiger Thermo Mechanically Treated (TMT)) of three different diameters (12, 16 and 20 mm) were assessed for early deterioration in the reinforced concrete structure. The resistive strengths of Pulkit, LCI and Tiger TMT for the diameters considered were also assessed using gravimetric and electrochemical corrosion techniques in four host environments: freshwater, 1.0 M of NaCl solution, 1.0 M of KCl solution and seawater for a period of 35 days of exposure. The experimental results revealed that Tiger TMT for the three diameters displayed the least corrosion rate with the highest resistive properties. This makes it most suitable for concrete reinforcement in all the three selected media, while LCI shows a high corrosion rate with lower resistive behaviour only in the KCl solution. Pulkit results show the highest corrosion rate with the lowest resistive strength in all the environments. For the sustainability of concrete structures in the Lagoon environment that has some level of salt solution, Tiger TMT is mostly recommended for construction.
PUBLIC INTEREST STATEMENT
This paper concerns early deterioration assessment of selected reinforcement in different host environments, case study: Lagos State, Nigeria. The study is motivated by the urgency to address the early deterioration of selected reinforcement in different environments such as freshwater, NaCl solution, KCl solution and seawater with Lagos State as a case study. When choosing reinforcement for saline environments, it is vital to consider the influence of the saline environment on the steel selected. The knowledge of the reinforcement that displayed the least corrosion rate with the highest resistive properties is vital in selecting reinforcement for the saline environment.
1. Introduction
Reinforcement used in concrete is commonly in a non-corroding passive state (Alabi & Onyeji, Citation2010) and is often used in salty environments where seawater or de-icing salts are present (Ejeh & Jibrin, Citation2012). Chloride disrupts the passive condition layer covering the steel when it moves into the concrete and causes it to rust (Cabrera, Citation1996). One of the worst and most common challenges in construction industries is corrosion in steel, and it is as well a socio-economical and technical problem which still signifies an excessive waste of natural resources (Erdogdu, Kondratova, & Bremner, Citation2004; Silva & Liborio, Citation2006). Steel is thermodynamically unstable in the Earth’s atmosphere, and by reaction with water and oxygen, it will continually tend to deteriorate to a reduced energy state such as oxide or hydroxide (Alo, Oluyamo, Faramika, & Daniyan, Citation2017; Ede, Egunjobi, Bamigboye, & Ogundeji, Citation2015). It is certain that this development will occur, but the uncertainty is how often it will occur in practice. Practically, only the surface atoms of the reinforcement are uncovered to the atmosphere and are therefore free to react (Hansson, Poursaee, & Jaffer, Citation2012). The steel supply the tensile strength needed by reinforced concrete (Ede, Olofinnade, & Opeyemi, Citation2014). The steel subjected to stresses such as flexural and tensile stresses as a result of traffic loads, wind load, thermal cycling and live and dead loads avert the failure of concrete structures (Ede, Adebayo, Bamigboye, & Ogundeji, Citation2015; Webster, Citation2000).
Corrosion will occur when the protective coating around the reinforcement has been removed in the presence of moisture and oxygen (Olawale, Daniel, & Bayode, Citation2016; Tsuru, Yamazaki, & Nishikata, Citation1999; Vedalakshmi, Manoharan, & Song, Citation2009). Alexander, Santhanam and Ballin (Citation2010) defined the corrosion rate as the speed of deterioration of any metal in a specific environment. Corroded products create expansive stress as they occupy more space than the original reinforcement which leads to cracking and spalling of concrete and also reduces the cross-section of the reinforcement (Alexander, Santhanam & Ballin, Citation2010; Andrade, Alonso, Garcia, & Rodriguez, Citation1991; Atanda, Ijitona, & Oluwole, Citation2011; Tsuru et al., Citation1999). Soleymani and Ismail (Citation2004) studied accelerated corrosion rate of concrete specimen containing 16 mm reinforcement and its crack formation with 3% calcium chloride as accelerator and concluded that the cracks produced in concrete as a result of corrosion were not continuous cracks. It first appears at the centre of the specimen and later transmitted to join each other and later spread across the specimen. Alo and Ibitoye (Citation2015) investigated induced stress effect on medium-carbon steel corrosion rate and how the induced stress affects the 0.33% medium-carbon steel in salty environment and concluded that the highest corrosion rate was obtained at uppermost stress level (U-bend shape) while the least corrosion rate manifests in the I-shape samples with the smallest amount of chloride ion in their non-stressed condition. Also, it was noted that as the tensile stress increases, chloride ions of the medium increases with increases in 0.33% carbon steel corrosion rate. Olugbenga, Oluseyi, and Ojo (Citation2011) studied mild steel deterioration subjected to 2 M sulphuric acid and extract from Bambusa glaucescens using the gasometric method. The study revealed that there is a major decrease in the dominant coarsening of the iron oxide phase as the extract concentration increases, while ferrite and pearlite phases dispersed. The current study focuses on the early deterioration of selected reinforcement in different host environments such as lagoon, freshwater, 1.0 M of NaCl and 1.0 M of KCl solutions.
This study further elucidates the resistive strength of the selected reinforcement in different host environments to determine which of the selected reinforcement brand is most suitable to avoid early deterioration of reinforced concrete.
2. Materials and method
2.1. Materials and sample preparation
The materials used for this study are high-yield reinforcement of Pulkit, LCI and Tiger Thermo Mechanically Treated (TMT) of three different diameters of 12, 16 and 20 mm. The samples were machined mechanically and cut into a coupon of the cylindrical dimension of 40 mm length. The coupons were observed wisely to check for rough edges, which could power the corrosion monitoring process.
2.2. Chemical composition of seawater and freshwater
The chemical compositions of both seawater and freshwater used for this study are shown in Tables and
Table 1. Chemical composition of seawater
Table 2. Chemical composition of freshwater
2.3. Weight loss measurement
Thirty-six well-labeled beakers of 250 ml capacity were used with appropriate media solutions. The first 9 beakers were labeled as the freshwater and tagged with 12, 16 and 20 mm, while another 9 beakers also labeled as seawater tagged with 12, 16 and 20 mm and the last 18 beakers equally labeled as NaCl 1.0 M and KCl 1.0 M also tagged with 12, 16 and 20 mm, respectively. The experiment was carried out at room temperature. The samples' initial weights were recorded accurately using analytical balance of 0.1 mg sensitivity and were fully suspended in 500 ml of the experimental media. This was monitored at 3-day interval for 21 days. After the exposure period, the sample was removed, dried, cleaned and weighed. From the weight loss, the corrosion rate and the resistive properties of the samples were calculated using EquationEquation (1)(1)
(1) .
where:
D = density in g/cm3
A = area of sample (17.34) cm2.
T = exposure time (h)
W = weight loss (mg)
3. Results and discussion
3.1. The corrosion results for various environments under study
3.1.1. Freshwater
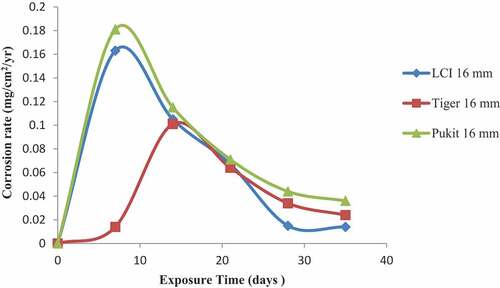
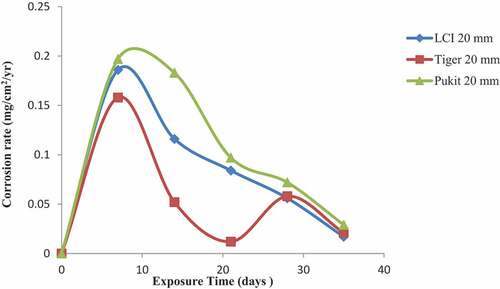
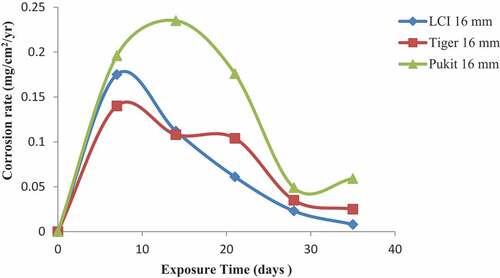
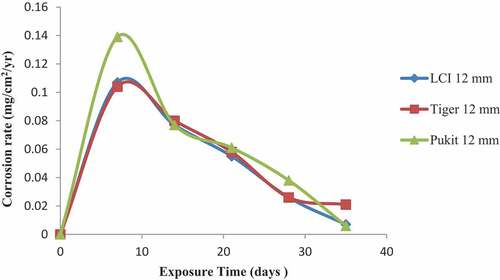
Figures , and show the variation in the corrosion rate of the three brands of reinforcement materials of Tiger TMT, LCI and Pulkit of diameters of 12, 16 and 20 mm immersed in freshwater for a period of 35 days of exposure. It was clearly shown that the corrosion resistance of the Tiger TMT reinforcement materials of 12 mm is drastically high from 7th day with the value of 0.014 mg/cm2/year and 28th day of exposure with 0.026 mg/cm2/year, respectively. It was noted that oxidation at the anode of the sample is relatively high due to the loss of electrons to the environment and then used up at the cathode. Tiger TMT material of 16 mm displayed high corrosion resistance from 7th to 14th days of exposure with the values of 0.014 and 0.012 mg/cm2/year, while the Tiger TMT material of 20 mm equally displayed high corrosion resistance on the 21st day of exposure with the values of 0.012 mg/cm2/year. It is evident that the Tiger TMT reinforcement material has high corrosion resistance in the freshwater because of its high anti-corrosive properties.
Figure 1. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 12-mm-diameter reinforcement in freshwater
3.1.2. Sodium chloride
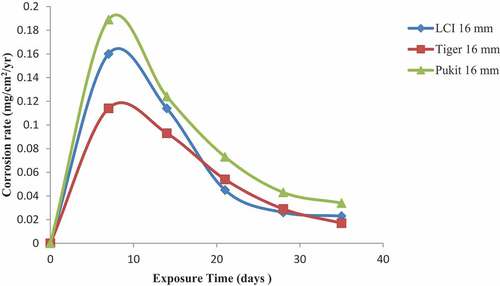
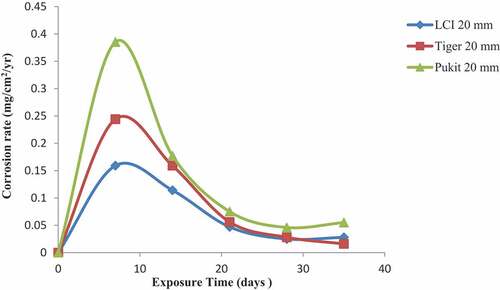
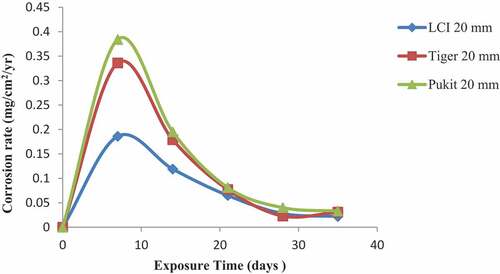
Figures , and show the variation in the corrosion rate of the three brands of reinforcement materials of Tiger TMT, LCI and Pulkit of diameters of 12, 16 and 20 mm, respectively, immersed in NaCl medium for a period of 35 days of exposure. Tiger TMT material of 12 and 16 mm displayed high resistance to corrosion on the 35th day of exposure with a value of 0.09 mg/cm2/year and the latter with the value of 0.017 mg/cm2/year, respectively, while the LCI 20-mm diameter recorded corrosion resistance from 7th to 21st days of exposure with the values of 0.159 and 0.056 mg/cm2/year, respectively. The Pulkit of 16 mm material on the 7th day of exposure recorded 0.189 mg/cm2/year as the high-corroded material compared to others.
Figure 4. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 12-mm-diameter reinforcement in 1.0 M NaCl
3.1.3. Potassium chloride
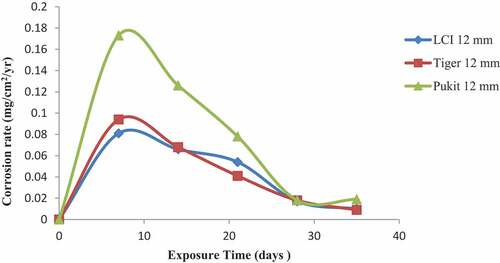
Figures , and show the variation in the corrosion rate of the three brands of reinforcement materials of Tiger TMT, LCI and Pulkit of diameters of 12, 16 and 20 mm, respectively, immersed in 1.0 M of KCl medium for a period of 35 days of exposure. Contrary to the results shown in Figures –, LCI materials displayed high resistance to corrosion in 1.0 M of KCl medium from 14th to 35th days of exposure with the values of 0.0112 and 0.008 mg/cm2/year, and LCI 20 mm also displayed low corrosion rate from 7th to 35th days with the values of 0.186 and 0.033 mg/cm2/year, while Tiger TMT material of 12 mm proved to be more resistant to corrosion from 21st to 28th days of exposure with the rates of 0.046 and 0.021 mg/cm2/year, respectively.
Figure 7. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 12-mm-diameter reinforcement in 1.0 M KCl
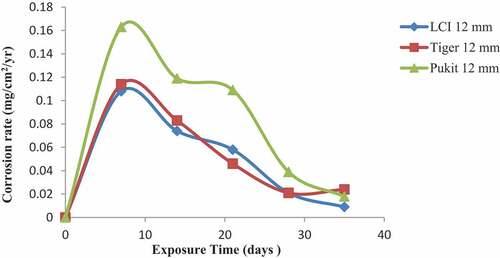
3.1.4. Lagoon
Figures , 1 and 1 show the variation in the corrosion rates of the three brands of reinforcement materials of Tiger TMT, LCI and Pulkit of diameters of 12, 16 and 20 mm, respectively, immersed in the Lagoon medium for a period of 35 days of exposure. Tiger TMT 16 and 20 mm showed high corrosion resistance on the 14th day with the value of 0.014 mg/cm2/year while later on the 28th day recorded 0.008 mg/cm2/year in the Lagoon environment, and Pulkit material displayed the least with more corrosion than others.
Figure 10. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 12-mm-diameter reinforcement in Lagoon
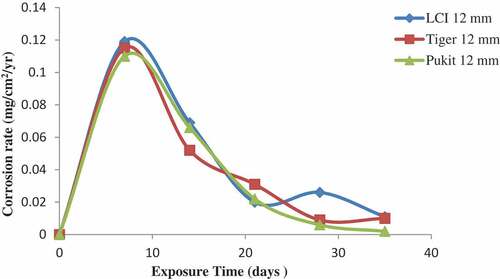
Figure 11. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 16-mm-diameter reinforcement in Lagoon
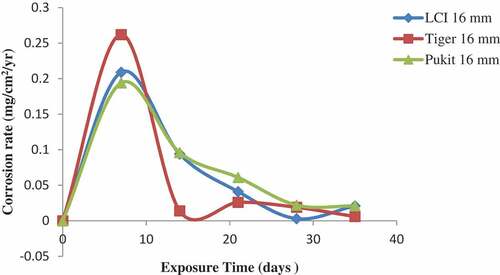
Figure 12. Plot of corrosion rate against exposure time of LCI, Tiger TMT and Pulkit 20-mm-diameter reinforcement in 1.0 M Lagoon
The plots of Figures –1 showed that the Tiger TMT material has a high value of corrosion resistance in freshwater, NaCl (1.0 M) and Lagoon, while LCI showed good chemical and mechanical properties in the 1.0 M of KCl solution. From the experimental analysis, it was concluded that Tiger TMT of 20-mm diameter recorded low corrosion rates of 0.008 mg/cm2/year on the 28th day of exposure in the Lagoon environments. Pulkit-reinforced material of 12, 16 and 20 mm diameters displayed high corrosion rates throughout the media for the period of 35 days of exposure due to the oxidation taken place at the sites and loss of electrons to the environment.
4. Conclusions
Considering the deterioration assessment of selected reinforcement materials of different diameters of 12, 16 and 20 mm in the Lagoon, 1.0 M of NaCl and 1.0 M of KCl media and freshwater:
It is clear that Tiger TMT-reinforced material possesses high resistance to corrosion in freshwater, NaCl medium and Lagoon, followed by LCI. Though LCI corrodes slowly at the initial stage, but as the days of exposure increase, its corrosion rate increases compared to the Tiger TMT brand.
LCI has high resistance to corrosion in 1.0 M of KCl medium compared to Tiger TMT, which displayed little or no retardation in resistance to corrosion in some stages throughout the 35 days of exposure.
Finally, based on the results from this study, the Tiger TMT material is the most suitable and appropriate reinforced material with low corrosion rates for construction in freshwater, NaCl and Lagoon environments.
Acknowledgements
The authors wish to thank the Chancellor and Management of Covenant University for the platform made available for this research.
Additional information
Funding
Notes on contributors

David O. Olukanni
David O. Olukanni is a Professor in the Department of Civil Engineering, Covenant University, Ota, Nigeria. His research interests are in the areas of environmental sustainability, solid waste management, modeling and simulation of wastewater treatment system, water supply in rural and urban communities, waste to wealth (recovery of materials as alternative sources of energy and recycled materials) and water, sanitation and hygiene studies.
Babatunde Ige
Babatunde Ige is a research student in the Department of Civil Engineering, Covenant University, Ota, Ogun State. His research interests are in the areas of concrete infrastructure and environmental sustainability.
Gideon O. Bamigboye
Gideon O. Bamigboye is a Senior Lecturer in the Department of Civil Engineering, Covenant University, Ota, Ogun State, Nigeria. His research focuses on innovative construction materials, environmental sustainability and concrete infrastructure among others.
Bamidele Durodola
Bamidele Durodola is a Senior Technologist in the Chemistry Laboratory, Chemistry Department, Covenant University, Ota, Nigeria. His research interest is in the area of environmental sustainability.
References
- Alabi, A. G. F., & Onyeji, L. I. (2010). Analysis and comparative assessment of locally produced reinforcing steel bars for structural purposes. Journal of Research Information in Civil Engineering, 7, 49–10.
- Alexander, M. G., Santhanam, M., & Ballim, Y. (2010). Durability design and specification for concrete structures—The way forward. International Journal of Advances in Engineering Sciences and Applied Mathematics, 2, 95–105. doi:10.1007/s12572-011-0027-x
- Alo, A., & Ibitoye, S. (2015). Effect of induced stress on the corrosion rate of medium carbon steel in saline environment. Journal of Chemical Engineering and Materials Science, 6, 52–59. doi:10.5897/JCEMS
- Alo, F. L., Oluyamo, S. S., Faramika, O. P., & Daniyan, A. A. (2017). The study of water and corrosion properties of two grades of carbon steel used in construction industries in Nigeria. International Journal of Materials Engineering, 7, 77–82.
- Andrade, C., Alonso, C., Garcia, D., & Rodriguez, J. (1991). Remaining lifetime of reinforced concrete structures: Effect of corrosion on the mechanical properties of the steel. Life prediction of corrodible structures, NACE, Cambridge. 23.
- Atanda, P., Ijitona, O., & Oluwole, O. (2011). Corrosive effect of pineapple juice on the fatigue and hardness properties of austempered ductile iron. International Journal of Materials Engineering, 1, 21–25.
- Cabrera, J. G. (1996). Deterioration of concrete due to reinforcement steel corrosion. Cement and Concrete Composites, 18, 47–59. doi:10.1016/0958-9465(95)00043-7
- Ede, A. N., Adebayo, S. O., Bamigboye, G. O., & Ogundeji, J. (2015). Structural, economic and environmental study of concrete and timber as structural members for residential buildings in Nigeria. The International Journal of Engineering and Science (IJES), 4, 76–84.
- Ede, A. N., Egunjobi, E. O., Bamigboye, G. O., & Ogundeji, J. (2015). Assessment of quality of steel reinforcing bars used in Lagos, Nigeria. International Research Journal of Innovative Engineering, 1, 1–8.
- Ede, A. N., Olofinnade, M. O., & Opeyemi, J. (2014). Experimental investigation of yield strength of steel reinforcing bars used in Nigeria concrete structures. International Journal of Scientist and Engineers Research, 5, 76–83.
- Ejeh, S. P., & Jibrin, M. U. (2012). Tensile tests on reinforcing steel bars in the Nigerian construction industry. IOSR Journal of Mechanical and Civil Engineering (IOSR-JMCE), 4, 6–12. doi:10.9790/1684-0420612
- Erdogdu, S., Kondratova, I. L., & Bremner, T. W. (2004). Determination of chloride diffusion coefficient of concrete using open-circuit potential measurements. Cement Concrete Research, 34, 603–609. doi:10.1016/j.cemconres.2003.09.024
- Hansson, C. M., Poursaee, A., & Jaffer, A. S. (2012). Corrosion of reinforcing bar in concrete. In The master builder (pp. 106–118). Retrieved from http//www.masterbuilder.com
- Olawale, A., Daniel, K., & Bayode, O. (2016). Corrosion of NST60Mn and NST50 steels electroplated with copper in selected water environments. FUOYE Journal of Engineering and Technology, 1, 67–74.
- Olugbenga, A., Oluseyi, O., & Ojo, S. (2011). Assessing the deterioration behaviour of mild steel in 2 M sulphuric acid using Bambusa glaucescens. International Journal of Applied Engineering Research, 2, 406–418.
- Silva, F. G., & Liborio, J. B. (2006). A study of steel bar reinforcement corrosion in concrete with SF and SRH using electrochemical impedance spectroscopy. 209–215. doi:10.1002/biot.200500019
- Soleymani, H., & Ismail, M. (2004). Comparing corrosion measurement methods to assess the corrosion activity of laboratory OPC and HPC concrete specimens. Cement and Concrete Research, 34, 2037–2044. doi:10.1016/j.cemconres.2004.03.008
- Tsuru, T., Yamazaki, T., & Nishikata, A. (1999). Organic coating for cathodic protection in atmospheric condition. Journal of Corrosion Science and Engineering, 2, 19–23.
- Vedalakshmi, R., Manoharan, S. P., & Song, H. W. (2009). Application of harmonic analysis in measuring the corrosion rate of rebar in concrete. Corrosion Science, 51, 2777–2789. doi:10.1016/j.corsci.2009.07.014
- Webster, M. P. (2000). The assessment of corrosion-damaged concrete structure. Birmingham: University of Birmingham.