Abstract
Healing clay is a rich source of diverse minerals. The relevance of these indigenous minerals in the improvement of antibiotic chemotherapy against prevailing bacterial pathogens is yet to be thoroughly explored. In the present study, healing clay from archaeological context was characterized and used in combination with 19 different antibacterial drugs to test their combined in vitro activity against Mycobacterium smegmatis mc2 155 and a multidrug-resistant (MDR) Mycobacterium smegmatis strain. Among the antibiotics tested, the anti-tuberculosis drug, pyrazinamide (Pzd), showed a drastic antimycobacterial activity against Mycobacterium smegmatis mc2 155 in the presence of 5 µg/µL of the healing clay, whereas ribosome targeted inhibitors such as gentamicin showed significant reduction in activity in the presence of the healing clay. The resistance phenotype of the MDR Mycobacterium smegmatis strain to ampicillin and isoniazid was reversed in the presence of the healing clay. The activity of the other antibiotics was either unaffected, enhanced or reduced in the presence of the healing clay. The activity of ampicillin and isoniazid against the MDR strain in the presence of the healing clay suggest that healing clay might be a useful synergy for these antibiotics against MDR Mycobacterium tuberculosis.
PUBLIC INTEREST STATEMENT
Indigenous clay is a rich source of minerals which have been used for treatment of diseases caused by bacteria. The treatment of infections caused by bacteria is gradually becoming complicated due to the ability of these bacteria to withstand the deadly effect of the drugs. Hence, there is the need to develop new effective drugs or explore alternatives for enhancing the efficiency of the current drugs against prevailing bacterial pathogens. We investigated whether the combination of clay with a selection of drugs is capable of reviving the effectiveness of the drugs against a bacterial strain that belongs to the family responsible for tuberculosis. Our observation indicates that clay is capable of boosting the efficacy of pyrazinamide, which is a drug used for treating tuberculosis patients. The study highlights the potential of exploring clay as a useful synergy for current drugs that are valuable for treating bacterial infections.
1. Introduction
Tuberculosis (TB) is caused by Mycobacterium tuberculosis and it continues to be a global burden (Gupta et al., Citation2018; Sisay et al., Citation2018). Approximately, one-third of the world population are infected with TB and most of these cases are found in Sub-Sahara Africa (Borkowska et al., Citation2017). Although progress has been made based on the significant available data on TB transmission, diagnosis, and treatment, much still remains in terms of improving the treatment regime to effectively decrease the prevalence of the disease. Treatment of TB usually involves multiple drug combination with treatment regimens carefully designed in phases over the period of several months (Sotgiu et al., Citation2015). While this strategy has improved treatment of the disease globally, there are still challenges as approximately 3.6% of newly diagnosed patients show levels of resistance to anti-TB drugs while 20% of previous patients that were treated had multidrug-resistant (MDR) TB (WHO, Citation2014). To overcome these challenges, there is the need to develop experimental models for evaluating mycobacterial cell phenotypes that may impact the efficacy of antibiotics in vivo but remain out of reach. In vitro culture systems arrayed in a great diversity provide a powerful means of simulating some aspects of gene expression profile of mycobacteria during infection. These culture systems may also be instrumental in evaluating the broader efficacy of antibiotics and bioactive compounds.
Clay minerals have been used since pre-historic times for medical intervention including treatments of skin infections, diarrhoea and wound healing (Moosavi, Citation2017; Otto et al., Citation2016; Pavlinakova et al., Citation2018; Shi et al., Citation2018). There are different types of clay spread across the continents and they are inexpensive and environmentally friendly. The surface chemistry of clay minerals as well as their layered architecture can influence cell adhesion and growth phenotypes, and as such, there has been attempts to study the mechanism underlying the antibacterial activity of clay-based materials (Haydel et al., Citation2008; Morrison et al., Citation2016; Williams et al., Citation2011; Zarate-Reyes et al., Citation2018). The increased interest in the antibacterial activity of clay materials or its composite with other biopolymers might eventually lead to development of novel drug delivery biomaterials. It is therefore useful to understand the effect of these biomaterials on cellular growth, differentiation, virulence expression and antibiotic susceptibility in microbial pathogens.
We have previously characterized healing clay from archaeological context using biophysical techniques and demonstrated that the clay exhibited inhibitory activity against human fetal osteoblast cells (Tiburu et al., Citation2017). In the present study, we further explored the biological significance of clay by testing its effect on the activity of 19 selected antibiotics against Mycobacterium smegmatis mc2 155 and a corresponding MDR strain of the mycobacterium. It is anticipated that insights from interaction of the healing clay with a wide array of antibiotics targeting different cellular pathways in mycobacteria would contribute to our understanding of the mechanism of antibacterial activity of the clay. The differential interactions with the mycobacterial cells using a mixture of the clay and antibiotics would also avail opportunities for developing culture systems to analyze the expression and mechanisms of drug resistance.
2. Materials and methods
2.1. Biophysical Characterization of the healing clay
The healing clay was obtained from the Northern Region of Ghana in West Africa. The clay sample was prepared and characterized using Energy Dispersive X-ray (EDX), Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR), as previously described (Tiburu et al., Citation2017). The EDX spectra and elemental composition of each sample were obtained at accelerating voltages of 5, 10 and 15 kV using a JEOL JSM-7100 F Field Emission Scanning Electron Microscope equipped with a Thermo Scientific Ultra Dry EDX detector. SEM micrographs were obtained at an accelerating voltage of 10 kV using the JEOL JSM-7100 F Field Emission Scanning Electron Microscope. The XRD experiments were conducted on Pan Analytical diffractometer using CuKα radiation at 2θ, scanning from 5 to 80 degrees in steps of 0.05 degrees, with a tube voltage of 45 kV and a current of 40 mA. The FTIR spectrum was recorded with a Nicolet Instrument Co. MAGNA-IR 750 Fourier transform infrared spectrometer.
2.2. Drug susceptibility assay
Mycobacterium smegmatis mc2 155 was used as a drug-susceptible strain. A MDR derivative of Mycobacterium smegmatis mc2 155 (erythromycin-resistant Mycobacterium smegmatis A; Arthur et al., Citation2019) was also used in this study. M7H9 liquid broth and M7H10 agar plates were prepared as previously reported (Arthur et al., Citation2019). The detailed procedure that was used for conducting the drug susceptibility assay has also been reported (Arthur et al., Citation2019). The modification that was applied to the drug susceptibility assay was the uniform spreading of the indicated concentrations of healing clay (1 or 5 µg/µL) onto the agar plates. After spreading with the healing clay suspension, the agar plates were air-dried under asceptic conditions prior to inoculation with the mycobacterial cells.
2.3. Assessment of proliferation and metabolic activity of mycobacterial cells using the Alamar Blue assay
A single colony of mycobacterial cells grown on M7H10 agar plate was inoculated into 50 mL of M7H9 broth. The inoculated broth was incubated at 30°C for 24 h with shaking. The overnight culture was diluted (in triplicate) to an optical density at 600 nm (OD600) of 0.7 in 100 μL of M7H9 broth base in 96-well plates. A suspension of healing clay was added to the diluted cells to a final concentration of either 1 µg/µL, 2 µg/µL, 5 µg/µL or 10 µg/µL, with the exception of the untreated (control) sample. The untreated and treated mycobacterial cultures were incubated at 30°C, with shaking for 20 min, 40 min, 60 min and 120 min after which the OD600 of the cells in the 96-well plate was measured using a Varioskan LUXTM Multimode microplate reader (Thermo Scientific). The Alamar Blue reagent was added to these cells at a final concentration of 10% and fluorescence was measured at excitation and emission wavelengths of 545 nm and 590 nm, respectively.
2.4. Statistical analysis
The one-way ANOVA test was used to determine whether addition of either 1 µg/µl or 5 µg/µl of clay suspension to the agar plate caused a significant difference in activity of the collection of the 19 antibiotics against the Mycobacterium smegmatis mc2 155 strain (Table ) and the MDR strain (Table ). During the statistical analysis, the mean antimycobacterial activity of each of the collection of 19 antibiotics from the unmodified agar plate was compared to the corresponding mean activities from the agar plates modified with 1 µg/µl and 5 µg/µl of clay suspension to estimate the p-value. The unpaired t-test was also used to determine whether addition of 1 µg/µl or 5 µg/µl of the clay suspension to the agar plate caused a significant difference in the antimycobacterial activity for each antibiotic. For statistical analysis using the unpaired t-test, the p-value was estimated using the mean, standard deviation and sample size of activities from the unmodified and modified agar plates for each indicated antibiotic. A p-value < 0.05 was considered to be statistically significant for both the one-way ANOVA test and the unpaired t-test analysis.
Table 1. Antimicrobial activity of selected antibiotics against M. smegmatis mc2 155 in the absence and presence of archaeological clay nanoparticles
Table 2. Antimicrobial activity of selected antibiotics against Ery M. smegmatis A in the absence and presence of archaeological clay nanoparticles
3. Results
3.1. Analysis of the healing clay using EDX, SEM, XRD and FTIR
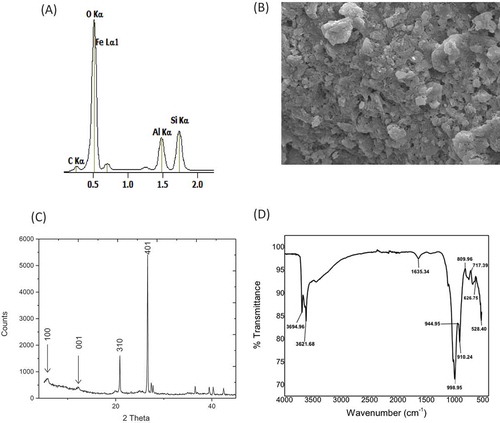
The elements in the clay materials included silicon (Si), aluminum (Al), iron (Fe), oxygen (O) and carbon (C), as investigated using Energy Dispersive X-ray (EDX) and Atomic Absorption Spectroscopy (AAS) (Figure ). The based weight percent ratio of Si to Al was found to be 1.5. The Scanning Electron Microscope (SEM) image of the clay revealed an irregular distribution of particles (Figure ). The X-ray diffraction (XRD) pattern indicates characteristic peaks of d001 and d100 at 7.1 Å and 12.6 Å, respectively, with additional peaks at positions 21 Å and 27 Å at <2θ> angles (Figure ). The XRD pattern and EDX analysis of the clay material showed characteristic peaks revealing a chamosite clay ore with elevated amounts of iron. Finally, the signature peaks of the clay were confirmed using Fourier Transform Infrared Spectroscopy (FTIR) showing unique peaks at 3695 cm−1 and 3622 cm−1 representing NH2 and O-H stretching vibrations, respectively, whereas the peak at 1635 cm−1 revealed Si-O stretching mode (Figure ). The bands located within the range of 650–745 cm−1 were assigned to symmetric T–O–T vibrations within the clay. These results confirm a chamosite clay ore with varying amounts of mineral concentrations (Racha et al., Citation2010). Henceforth, the chamosite clay would be referred to as healing clay in the subsequent sections of the paper.
Figure 1. (a) The EDX spectra showing the major peaks of Fe, O, Al, Si and C from the healing clay material. (b) Scanning electron image of the Healing clay material. (c) The XRD spectra of the healing clay showing characteristic crystal lattice peaks at 2-theta angles. (d) FTIR spectra of the clay exhibiting characteristic peaks at 3695 and 3621 1635 and 1000 cm−1
3.2. Healing clay enhances the in vitro antibacterial activity of pyrazinamide
We have previously reported the emergence of two distinct MDR clones of Mycobacterium smegmatis following continuous exposure of the drug-susceptible Mycobacterium smegmatis mc2 155 to increasing concentrations of erythromycin, under in vitro conditions (Arthur et al., Citation2019). The Mycobacterium smegmatis mc2 155 strain is susceptible to 16 out of the 19 commercial antibiotics that were used in this study, at the indicated amount of the antibiotics (Table ). One of the MDR Mycobacterium smegmatis strains was selected and used in this study (erythromycin resistant Mycobacterium smegmatis A); the selected MDR strain is resistant to 10 out of the 19 commercial antibiotics (Table ).
We investigated the effect of the healing clay on the antimycobacterial activity of the selected antibiotics using the disc diffusion assay. A final concentration of either 1 µg/µL or 5 µg/µL of the healing clay was uniformly spread onto each M7H10 agar plate and allowed to dry; the control M7H10 agar plates were not spread with the healing clay. In general, the presence of clay had no significant effect on the collective activity of the selected antibiotics against the Mycobacterium smegmatis mc2 155 strain and the corresponding MDR strain (Tables and ; p-values <0.05 using the one-way ANOVA test). Interestingly, the anti-tuberculosis drug pyrazinamide (Pzd), showed a drastic antimycobacterial activity against the Mycobacterium smegmatis mc2 155 strain in the presence of 5 µg/µL of the healing clay (Table ). This observation was unique to the drug-susceptible strain since pyrazinamide did not exhibit antimycobacterial activity against the MDR strain even in the presence of 5 µg/µL of the healing clay (Table ). It has been reported that the therapeutic effectiveness of pyrazinamide is linked to its ability to eliminate populations of mycobacteria with low metabolic activity (Mitchison, Citation1985). Thus, we postulated that the low metabolic activity of the Mycobacterium smegmatis mc2 155 strain relative to the MDR strain underpin the in vitro antimycobacterial activity of pyrazinamide in the presence of 5 µg/µL of the healing clay.
3.3. The Mycobacterium smegmatis mc2 155 strain has a low metabolic activity relative to the MDR strain
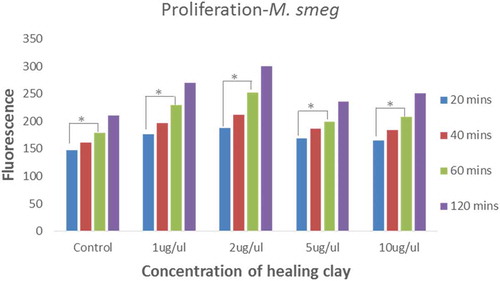
The Alamar Blue assay was used to obtain insights on proliferation and metabolic activity of the drug-susceptible Mycobacterium smegmatis mc2 155 strain and the corresponding MDR strain in the presence and absence of the healing clay (Ahmed et al., Citation1994; O’Brien et al., Citation2000). The fluorescence values obtained for the Mycobacterium smegmatis mc2 155 strain were proportional to the duration of incubation of these mycobacterial cells with the clay suspension (Figure ). Treatment of the Mycobacterium smegmatis mc2 155 strain with the healing clay for at least 60 min resulted in a significant increase in fluorescence relative to the 20-min treatment condition (Figure ).
Figure 2. Proliferation of M. smegmatis mc2 155 in the absence and presence of healing clay
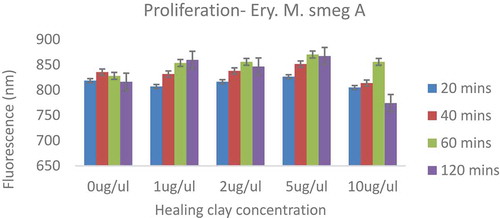
The fluorescence values obtained for the MDR strain were not consistently proportional to the duration of incubation of the cells with the clay suspension (Figure ). Interestingly, the fluorescence values obtained for the MDR strain were approximately four-fold higher than the values obtained for the Mycobacterium smegmatis mc2 155 strain. Since the same optical density (OD600) of mycobacterial cells at exponential phase of growth were used for the Alamar Blue assays, the higher fluorescence values obtained for the MDR strain highlight its high metabolic activity relative to the Mycobacterium smegmatis mc2 155 strain. The data also revealed an inhibitory effect of the healing clay on the MDR strain when incubated with 10 μg/uL of healing clay for 120 min. Collectively, the data illustrate that the concentration of healing clay is relevant for the kind of effect (either inhibitory or stimulatory) it exhibits against Mycobacterium smegmatis. Moreover, these observations corroborate our earlier postulation that the Mycobacterium smegmatis mc2 155 strain has a low metabolic activity relative to the MDR strain.
Figure 3. Proliferation of MDR Mycobacterium smegmatis in the absence or presence of healing clay
3.4. Healing clay renders MDR M. smegmatis susceptible to inhibitors of cell wall synthesis
The drug susceptibility assay revealed that other antibiotics also exhibited notable activity on the mycobacterial strains in the presence of the healing clay. For instance, the resistance phenotype of the MDR M. smegmatis strain to ampicillin and isoniazid was reversed in the presence of the healing clay (Table ). A resistance phenotype to amoxicillin was also detected for the MDR strain in the presence of healing clay (Table ). Since ampicillin, isoniazid and amoxicillin are inhibitors of bacterial cell wall synthesis, these observations suggest that dynamic mechanisms of healing clay render the MDR M. smegmatis to be susceptible or resistant to these antibiotics.
Table 3. Enhancement of antibiotic activity against M. smeg and Ery M. smeg in the presence of healing clay
Table 4. Inhibition of antibiotic activity against M. smeg and Ery M. smeg in the presence of the healing clay
3.5. Ribosome targeted antibiotics are both suppressed and boosted by the healing clay
Gentamicin showed significant reduction in antimycobacterial activity against the Mycobacterium smegmatis mc2 155 strain in the presence of the healing clay (Table ) while paromomycin showed enhanced antimycobacterial activity against the same strain in the presence of the healing clay (Table ). Chloramphenicol also showed significant reduction in activity against the MDR strain in the presence of 5 µg/µL of healing clay (Table ). These observations demonstrate that the antimycobacterial effect exhibited by the ribosome inhibitors is dependent on the specific antibiotic that is selected.
3.6. Modulation of antibiotic effects on bacterial genome stability and transcription by the healing clay
Metronidazole showed enhanced antimycobacterial activity against the Mycobacterium smegmatis mc2 155 strain in the presence of the clay (Table ). Even though 5-fluorouracil and moxifloxacin showed decreased activity against the MDR strain in the presence of clay (Table ), the effect was not statistically significant. We infer that the modulatory effects of healing clay towards this class of antibiotics are more moderate compared to that of pyrazinamide.
4. Discussion
The rapid and spontaneous mutation rate of Mycobacterium tuberculosis, which resulted in emergence of extensively drug resistant strains, provides a platform for exploring alternative chemotherapeutic approaches including the use of minerals to support effective delivery of anti-TB drugs. Minerals from clay have been shown to exhibit antibacterial activity against diverse bacterial strains, including Mycobacterium marinum (Ma’or et al., Citation2006; Somoskovi et al., Citation2004; Williams et al., Citation2008, Citation2004). French green clays have also been used to treat Buruli ulcer, which is a mycobacterium infection (Brunet de Courssou, Citation2002). These studies highlight the potential of using clay for treatment of infections caused by mycobacterial (Londono et al., Citation2017; Schoonen et al., Citation2006). However, studies which explore the synergy of clay and antibiotics against mycobacterial infections are yet to be comprehensively documented.
We have previously reported two MDR strains of Mycobacterium smegmatis that could be useful model organisms for screening novel drugs against drug-resistant strains of Mycobacterium tuberculosis (Arthur et al., Citation2019). The drug-susceptible Mycobacterium smegmatis mc2 155 and a corresponding MDR strain were used in this study to obtain insight on the effect of healing clay on the antimycobacterial activity of selected antibiotics. One of the notable observations was the ability of healing clay to resuscitate the in vitro antimycobacterial activity of pyrazinamide against the drug-susceptible strain. Even though pyrazinamide exhibits poor antimycobacterial activity in vitro, it has been shown to exhibit enhanced in vitro activity against Mycobacterium tuberculosis following addition of iron to the growth media (Somoskovi et al., Citation2004). Iron-mediated toxicity in bacterial pathogens is achieved via formation of free radicals that destroy essential cellular components of these pathogens (Schoonen et al., Citation2006). Aluminum in clay materials also exhibits antibacterial activity by compromising the integrity of bacterial membranes (Londono et al., 2017). Thus, the toxic effects of iron and aluminum in the healing clay might have synergized with pyrazinamide to cause the detectable antimicrobial activity against the Mycobacterium smegmatis mc2 155 strain. Despite the increased in vitro activity of pyrazinamide against Mycobacterium smegmatis mc2 155 in the presence of healing clay, the MDR strain was unaffected by the combination of pyrazinamide and healing clay. We demonstrated that this phenomenon might be attributed to the high metabolic activity of the MDR strain relative to the drug-susceptible Mycobacterium smegmatis mc2 155 strain and is consistent with a study which showed that pyrazinamide effectively eliminates tubercular bacilli with low metabolic activity (Mitchison, Citation1985). Subsequent studies would ascertain whether the healing clay synergizes the in vivo activity of pyrazinamide against Mycobacterium tuberculosis.
The 19 antibiotics used in this study include ribosome inhibitors, inhibitors of cell wall synthesis, and compounds that target transcription and bacterial genome stability. This collection of antibiotics affects a majority of the cellular processes targeted by the currently available commercial antibiotics. Besides pyrazinamide, the activity of antibiotics which inhibits bacterial cell wall synthesis was also substantially affected by the healing clay, especially against the MDR strain; the presence of the healing clay revived the antimycobacterial activity of ampicillin and isoniazid while nullifying the activity of amoxicillin. The activity of the other antibiotics was either unaffected, enhanced or reduced in the presence of the healing clay. Importantly, resuscitation of the activity of ampicillin and isoniazid against the MDR strain implies that healing clay might be a useful synergy for these antibiotics against MDR Mycobacterium tuberculosis. Further studies are required to ascertain whether iron, aluminum or other minerals in the healing are the active components underlying the synergy with ampicillin and isoniazid. Moreover, the annulment of the activity of amoxicillin against the MDR strain can provide insight into novel mechanisms of mycobacterial drug resistance.
Thus, the affected antibiotics including ampicillin, isoniazid, pyrazinamide, metronidazole and amoxicillin will be carefully investigated in future studies using clays of different chemical composition to correlate the antibiotics activity to chemical composition of the clay materials. It is hopeful that combining geophargy practices (eating clay) as practiced in most countries while taken TB medications may enhance the efficacy of certain drugs against the MDR strain by reviving their antimycobacterial activity.
5. Conclusions
Healing clay was shown to comprise several minerals. The antimycobacterial activity of pyrazinamide, ampicillin and isoniazid, detected only in the presence of the clay, highlights the potential of the clay components in the development of novel antibacterial chemotherapy. The combination of components of the clay with the antibiotics can also be useful for exploring molecular mechanisms that enhance the susceptibility of mycobacterial to facilitate the development of robust antibiotic chemotherapy.
Additional information
Funding
Notes on contributors
Elvis K Tiburu
The laboratory Elvis K Tiburu is dedicated to the study of nanoparticles prepared from indigenous clay deposits in Ghana which have been associated with healing practices in the past. The nanoparticles synthesized from these mineral deposits are studied for their drug uptake and release properties, as well as direct interactions with cells. The laboratory of Patrick K. Arthur and Vincent Armah (and PKA and VA) is dedicated to drug discovery from fungal sources against microbial pathogens and cancer cells.
References
- Ahmed, S. A., Gogal, R. M., Jr., & Walsh, J. E. (1994). A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. Journal of Immunological Methods, 170(2), 211–11. https://doi.org/10.1016/0022-1759(94)90396-4
- Arthur, P. K., Amarh, V., Cramer, P., Arkaifie, G. B., Blessie, E. J. S., Fuseini, M. S., Carilo, I., Yeboah, R., Asare, L., & Robertson, B. D. (2019). Characterization of two new multidrug-resistant strains of Mycobacterium smegmatis: Tools for routine in vitro screening of novel anti-mycobacterial agents. Antibiotics (Basel), 8. doi: 10.3390/antibiotics8010004
- Borkowska, D. I., Napiorkowska, A. M., Brzezinska, S. A., Kozinska, M., Zabost, A. T., & Augustynowicz-Kopec, E. M. (2017). From latent tuberculosis infection to tuberculosis. news in diagnostics (QuantiFERON-Plus). Polish Journal Of Microbiology / Polskie Towarzystwo Mikrobiologow = The Polish Society of Microbiologists, 66(1), 5–8. https://doi.org/10.33073/pjm-
- Brunet de Courssou, L. (2002). 5th WHO advisory group meeting on Buruli ulcer treatment with clay.
- Gupta, N., Garg, S., Vedi, S., Kunimoto, D. Y., Kumar, R., & Agrawal, B. (2018). Future path toward TB vaccine development: Boosting BCG or re-educating by a new subunit vaccine. Frontiers in Immunology, 9(13), 2371. https://doi.org/10.3389/fimmu.2018.02371
- Haydel, S. E., Remenih, C. M., & Williams, L. B. (2008). Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogens. The Journal of Antimicrobial Chemotherapy, 61(2), 353–361. https://doi.org/10.1093/jac/dkm468
- Londono, S. C., Hartnett, H. E., & Williams, L. B. (2017). Antibacterial activity of aluminum in clay from the Colombian Amazon. Environmental Science & Technology, 51(4), 2401–2408. https://doi.org/10.1021/acs.est.6b04670
- Ma’or, Z., Henis, Y. A., Long, Y., Orlov, E., Sorenson, K. B., & Oren, A. (2006). Antimicrobial properties of Dead Sea black mineral mud. International Journal of Dermatology, 45(5), 504–511. https://doi.org/10.1111/ijd.2006.45.issue-5
- Mitchison, D. A. (1985). The action of antituberculosis drugs in short-course chemotherapy. Tubercle, 66(3), 219–225. https://doi.org/10.1016/0041-3879(85)90040-6
- Moosavi, M. (2017). Bentonite clay as a natural remedy: A brief review. Iranian Journal of Public Health, 46, 1176.
- Morrison, K. D., Misra, R., & Williams, L. B. (2016). Unearthing the antibacterial mechanism of medicinal clay: A geochemical approach to combating antibiotic resistance. Scientific Reports, 6(1), 19043. https://doi.org/10.1038/srep19043
- O’Brien, J., Wilson, I., Orton, T., & Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal Of Biochemistry / FEBS, 267(17), 5421–5426. https://doi.org/10.1046/j.1432-1327.2000.01606.x
- Otto, C. C., Kilbourne, J., & Haydel, S. E. (2016). Natural and ion-exchanged illite clays reduce bacterial burden and inflammation in cutaneous meticillin-resistant Staphylococcus aureus infections in mice. Journal of Medical Microbiology, 65(1), 19–27. https://doi.org/10.1099/jmm.0.000195
- Pavlinakova, V., Fohlerova, Z., Pavlinak, D., Khunova, V., & Vojtova, L. (2018). Effect of halloysite nanotube structure on physical, chemical, structural and biological properties of elastic polycaprolactone/gelatin nanofibers for wound healing applications. Materials Science & Engineering. C, Materials for Biological Applications, 91, 94–102. https://doi.org/10.1016/j.msec.2018.05.033
- Racha, A., Bojja, S., & Parthasarathy, G. (2010). Chamosite, a naturally occurring clay as a versatile catalyst for various organic transformations. Clay Minerals, 45(3), 281–299. https://doi.org/10.1180/claymin.2010.045.3.281
- Schoonen, M. A. A., Cohn, C. A., Roemer, E., Laffers, R., Simon, S. R., & O’Riordan, T. (2006). Mineral- induced formation of reactive oxygen species. Reviews in Mineralogy and Geochemistry, 64(1), 179–221. https://doi.org/10.2138/rmg.2006.64.7
- Shi, R., Niu, Y., Gong, M., Ye, J., Tian, W., & Zhang, L. (2018). Antimicrobial gelatin-based elastomer nanocomposite membrane loaded with ciprofloxacin and polymyxin B sulfate in halloysite nanotubes for wound dressing. Materials Science & Engineering. C, Materials for Biological Applications, 87, 128–138. https://doi.org/10.1016/j.msec.2018.02.025
- Sisay, S., Mekonen, A., Abera, A., Berhan, Y., Kebede, T., & Ferede, A. (2018). An evaluation of collaboration in the TB and HIV control programme in Oromia Region, Ethiopia: Seven years of retrospective data. International Journal of Infectious Diseases: IJID : Official Publication of the International Society for Infectious Diseases, 77, 74–81. https://doi.org/10.1016/j.ijid.2018.10.001
- Somoskovi, A., Wade, M. M., Sun, Z., & Zhang, Y. (2004). Iron enhances the antituberculous activity of pyrazinamide. The Journal of Antimicrobial Chemotherapy, 53(2), 192–196. https://doi.org/10.1093/jac/dkh042
- Sotgiu, G., Centis, R., D’Ambrosio, L., & Migliori, G. B. (2015). Tuberculosis treatment and drug regimens. Cold Spring Harbor Perspectives in Medicine, 5(5), a017822. https://doi.org/10.1101/cshperspect.a017822
- Tiburu, E. K., Kankpeyeng, B. W., Nkumbaan, S. N., Salifu, A., & Zhuang, J. (2017). novel nanocrystal clay materials with potential bone cells growth enhancement or inhibition characteristics in vitro. JBBBE, 30, 45–60. https://doi.org/10.4028/www.scientific.net/JBBBE.30.45
- WHO. (2014). Antimicrobial resistance: Global report on surveillance.
- Williams, L. B., Haydel, S. E., Geise, R. F., & Eberl, D. D. (2008). Chemical and mineralogical characteristics of French green clays used for healing. Clays and Clay Minerals, 56(4), 437–452. https://doi.org/10.1346/CCMN.2008.0560405
- Williams, L. B., Holland, M., Eberl, D. D., Brunet, T., & Brunet de Courssou, L. (2004). Natural antibacterial clay minerals. Mineral Society Bulletin. London. 139, 3–8.
- Williams, L. B., Metge, D. W., Eberl, D. D., Harvey, R. W., Turner, A. G., Prapaipong, P., & Poret-Peterson, A. T. (2011). What makes a natural clay antibacterial? Environmental Science & Technology, 45(8), 3768–3773. https://doi.org/10.1021/es1040688
- Zarate-Reyes, L., Lopez-Pacheco, C., Nieto-Camacho, A., Palacios, E., Gomez-Vidales, V., Kaufhold, S., Ufer, K., Garcia Zepeda, E., & Cervini-Silva, J. (2018). Antibacterial clay against gram-negative antibiotic resistant bacteria. Journal of Hazardous Materials, 342, 625–632. https://doi.org/10.1016/j.jhazmat.2017.08.078
