?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, three different advanced oxidation processes (AOPs), namely UV, ozone, and a combination of UV/ozone, were applied for rubber wastewater treatment. The kinetics of chemical oxygen demand (COD), total nitrogen and total phosphorous removal in the rubber wastewater treated with the AOPs were investigated. Moreover, the optimum exposure time to treat wastewater with the oxidizing agents (UV, ozone, and both) was revealed. Furthermore, the effluent after the treatment was completed was tested as a potential microalgae culture medium. The reaction rate constant was in a range of 3 × 10−4 to 1.2 × 10−3 s−1, which was higher than the results of the second-order kinetics. UV/ozone combination was the best method to treat the rubber wastewater due to its higher reaction rate constant. The results indicated that a decrease in the COD was observed, following a first-order reaction with a high determination coefficient (R2) (0.936–0.984). The COD removal was 81.44% when the UV/ozone method was used, and the removal of total nitrogen and phosphorus was 69.62% and 25.53%, respectively. Next, Spirulina platensis was cultivated in 30% (v/v) of the effluent obtained from the best method. The results showed that the microalgal growth rate and ODmax were 0.67 and 0.3 day−1, respectively. This research showed that the nutrients in wastewater effluent could be used as food supplements for microalgae production, and therefore, reduce the production costs in large scale microalgae cultivation bioprocesses.
PUBLIC INTEREST STATEMENT
Integrating wastewater treatment with biomass production has gained interest due to its effectivities. The main pollutant in waste water must be reduced while the nutrient (N,P) contained in the waste water is maintained high for microalgae cultivation. The use of advanced oxidation process (AOP) is a promising technique in which UV/ozone will oxidize the pollutant without significantly reduce the nutrients. Microalgae will be utilizing the nutrients in treated waste water through photosynthesis reaction and produce biomass which can be extracted for further valuable products.
Competing interests
The authors declared they have no competing interests.
1. Introduction
Indonesia is the second-largest natural rubber producing country after Thailand. It was reported that 100 kg of latex could produce approximately 85% of clean rubber, 10% of wastewater, and 3%–5% of solid waste, which means the process produces colossal amounts of wastewater during intense rubber processing. For instance, it was reported that 322,986 tons of liquid waste were produced in 2017 based on the amount of rubber production. Rubber wastewater typically comes from different process units, including washing, shredding, grinding, drying, and pressing, and usually contains 2765–4721 mg L−1 chemical oxygen demand (COD), 2210–3120 mg L−1 biological oxygen demand (BOD), 5.98–9.76 mg L−1 nitrogenous compounds, and 0.98–1.75 mg L−1 phosphorus in the form of PO4 (Asia & Akporhonor, Citation2007). These compounds are the primary polluting agents in this wastewater, causing eutrophication, and therefore it needs to be treated before discharge into a river body.
For this purpose, several techniques have been used to treat rubber wastewater and reduce the levels of organic compounds. For instance, Watari et al. (Citation2017) used a pilot-scale up-flow anaerobic sludge blanket to reduce the levels of COD and BOD in rubber wastewater up to 55.6% and 77.8%, respectively. The total suspended solids of rubber wastewater decreased when Mokhtar et al. (Citation2015) used membrane distillation technology as a treatment system. Besides, Rosman et al. (Citation2013) treated rubber wastewater using anaerobic granular sludge and removed the COD up to 96.5%.
Among the wastewater treatment processes, the advanced oxidation processes (AOPs) have become a modern solution with high cleaning performances to reduce the concentrations of the pollutants. In the AOPs, an oxidant is typically dosed to the wastewater and activated by UV radiation by forming highly reactive and unselective hydroxyl radicals (•OH−) (Y. A. Bustos et al., Citation2010; Y. Bustos et al., Citation2014; Magbanua et al., Citation2006). Among the AOPs, the combination of UV and ozone has been widely used as a promising technique in various wastewater treatment process, such as paper board wastewater, domestic wastewater, and polluted river water (Amat & Miranda, Citation2005; Bustos-Terrones et al., Citation2016; Oh et al., Citation2006). In this method, hydrogen peroxide (H2O2) is produced as a result of the reaction between water and ozone, and this makes organic pollutants more susceptible to be attacked by hydroxyl radicals (Rosenfeldt et al., Citation2006; Sharrer & Summerfelt, Citation2007). Besides, UV radiation offers energy to break the chemical bonds of organic pollutants, providing better results in the treatment process (Mailhot et al. Citation2002).
On the other hand, the integration of the treated nutrient-rich wastewater for microalgae cultivation has become a unique opportunity, providing the ability to produce algal biomass and removing nitrogen, phosphorus as well as organic carbon (Nur et al. Citation2015). In another study, Hadiyanto et al. (Citation2012) cultivated Spirulina platensis using the effluent of palm oil mill wastewater (POME) and removed the nutrients up to 45% successfully; however, the study used phytoremediation as the treatment of the wastewater, which requires more time to remediate. However, shorter treatment periods are of interest to the microalgal cultivation processes.
In our study, the aim was to treat rubber wastewater using promising AOPs, namely UV, ozone, and the combination of both, at several flowrates and recycles, and try the effluent as an input of a microalgae production. As the removal of COD, total nitrogen, and total phosphorus has been utilized as performance indicators of such a wastewater treatment system, their kinetics were studied. Finally, the effluent of the best treatment method observed in this study was used as the microalgae cultivation medium.
2. Materials and methods
2.1. The characterization of wastewater
The source of wastewater was a rubber processing plant in PT. Perkebunan Nusantara IX (PTPN IX), which is located in Semarang, Indonesia. All the wastewater samplings were filtered using a thin filter to remove the solid particles before each experiment. The average pH was 5.3 in the rubber wastewater. In the wastewater samples, the average values of COD, BOD, total suspended solids, total nitrogen (Total-N), and total phosphorous (Total-P) were determined as 648.8 mg L−1, 215.8 mg L−1, 630.2 mg L−1, 11.16 mg L−1, and 1.61 mg L−1, respectively.
2.2. Experimental set-up
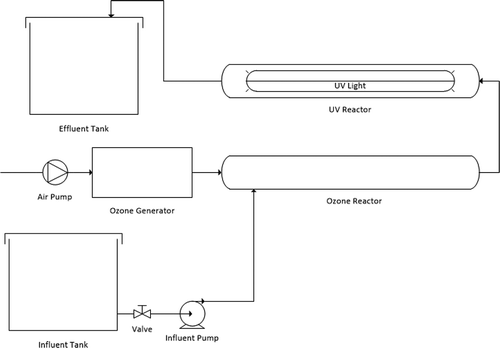
The laboratory-scale batch AOP equipment consisting of a UV annular reactor and an ozone contactor arranged in series was used in this study (Figure ). The UV annular reactor was a glass tube with a UV lamp, which was enclosed by a plexiglass arm, providing a working volume of 1 L. The average intensity of the UV lamp inside was 700 W/cm2. The ozone generator compressed the atmospheric air to the system, and then ozone was bubbled on the ozone contactor at a flow rate of 0.29 L min−1 using a fine-bubble diffuser, resulting in a 0.25 mg min−1 ozone production rate. In our study, when ozone treatment was only used, the UV light was turned off. Conversely, when UV treatment was considered only, the ozone generator was turned off. Hence, no interference was allowed in each ongoing process.
2.3. Experimental design
The experiment was carried out in a continuous process mode, where 2 L of wastewater was pumped into a series of devices passing the ozone contactor and then the UV reactor at room temperature. Rubber wastewater was recycled 5, 10, 15, and 20 times through the reactor. The three treatments examined here were the ozone, the UV, and the combined method (UV/ozone). In the experiments, the flowrates were set to 2.5, 3, and 4 L min−1 for further examination.
2.4. The determination of COD, total-N, and total-P
The COD was measured before and after treatment under specified conditions using HI-83099, HI-839800, and HI-93754B-25 in the Hanna Instrument (Rhode Island, USA) using the dichromate method. Total-N was measured by the Kjeldahl method, while total-P was analyzed using a spectrophotometer (Clesceri et al., Citation1998; Lang, Citation1958).
2.5. The kinetics analysis of COD removal
For the COD removal, two kinetics models were investigated to verify whether the data was fit the first-order or the second-order kinetics, which shown in EquationEquation (1)(1)
(1) and (Equation2
(2)
(2) ), respectively:
where COD was the COD value at time t, COD0 was the initial value, t was the operation time, and k was the kinetic rate constant. All the data were fitted to the models using linear regression, while the coefficient of determination (R2) was used to confirm the adequacy of fit.
2.6. Microalgae cultivation
The cultivation was conducted in a batch mode. Initially, water and optimized wastewater (according to the variables in Table ) were fed into 1 L flasks, namely batch bioreactor. Then, 100 ml of Spirulina platensis culture was fed into the reactor, followed by the addition of urea, Triple Superphosphate (TSP), and sodium bicarbonate (NaHCO3) as nutrients (Table ). All chemicals obtained commercially from a local chemical market in Semarang, Indonesia. Microalgae cultivation was carried out for 14 days. During the cultivation, 18 watts tube lamps were used to provide artificial sunlight by following 12 h light/12 h dark cycle. Aeration system was also used during the cultivation to supply CO2 and mix the culture well. The specific growth rate of S. platensis was calculated using the EquationEquation (3)(3)
(3) (Christwardana & Hadiyanto, Citation2017; Hadiyanto et al., Citation2014) after measuring the cell density at the wavelength of 650–680 nm in a spectrophotometer (Optima SP-300, Tokyo, Japan). All measurements were done in triplicate.
Table 1. The composition of wastewater and nutrients
Here, µ was the specific growth rate. OD2 and OD1 were the optical densities at the end and beginning of the exponential phase, respectively. t1 and t2 were the initial time and the time at the end of the exponential phase, respectively.
3. Results and discussion
3.1. The kinetics results of COD removal
In this study, the COD showed a decrease as a sign of reduced organic matter in wastewater by utilizing different treatments (UV, ozone, UV/ozone) with a specific cycle at various periods. The two crucial outputs of this study were (i) the determination of the most effective treatment to decrease the COD in wastewater and (ii) the determination of the kinetics profile of the decreased COD to reveal whether it followed the first- or second-order kinetics. To do that, 2 L of rubber wastewater was pumped into the reactor at a flow rate of 2.5 L min−1, and then the process was repeated 20 recycles or 960 s. In Figure ), it can be seen that the decrease in COD was faster in the combined method, UV/ozone, than that of in UV or ozone methods only. In detail, when using only ozone, the COD decreased up to 27.73%, and while UV was only used, the decrease ratio was around 70.63%. However, the highest COD removal performance, 81.44%, was seen in the combined UV/ozone, which was higher than that was reported previously by Oh et al. (Citation2007). In their study, 40% COD removal could be achieved with a continuous combined process. Better COD removal efficiency of the UV/ozone combined process was also reported by Chin and Bérubé (Citation2005). As UV/ozone method typically forms radicals during the process, and therefore, leads the highest oxidation potential on wastewater treatment (Amat & Miranda, Citation2005). It was also revealed that the simultaneous usage of UV and ozone was not only useful in the oxidation of organic compounds but also in the inorganic compounds due to the formation and action of hydroxyl radicals (Oh et al., Citation2006, Citation2003). UV light catalyzes the production of hydroxyl radicals from molecular ozone, and therefore, the combined method accelerates the oxidation process. Jung et al. (Citation2008) inferred that ozone has a vital role in the combined UV/ozone process in reducing the COD in wastewater. However, there were pieces of evidence that UV/ozone was not very useful when highly turbid wastewater was treated (Amat & Miranda, Citation2005)
Figure 2. The results of the three AOPs. a) COD reduction of rubber wastewater following b) the first-order and c) the second-order kinetics.
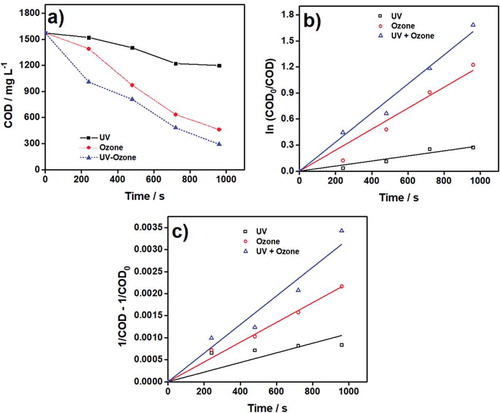
It can be seen that wastewater treated with UV/ozone method had higher k values compared to that of the single methods (Table ). The k values of COD removal when UV, ozone, and UV/ozone techniques were employed were 3 × 10−4, 1.2 × 10−3, and 1.7 × 10–3 s−1, respectively, when first-order kinetics were used. Moreover, when the second-order kinetics were utilized, they were 2 × 10−7, 2 × 10−6, and 7.4 × 10−3 L g−1 s−1, respectively. Since higher k values mean that the COD removal occurs rapidly (Kang & Lee Citation1997), and hence, UV/ozone could remove the COD better than other treatments in our study.
Moreover, in this study, the COD removal was fit to the first- and second-order kinetics and the determination coefficients (R2) of all samples were calculated using Excel software. In Figure ), the determination coefficients could be seen for the first-order kinetics when R2 was ranging between 0.936–0.984. While the second-order kinetics were applied (Figure )), R2 values were in the range of 0.865–0.927. Since the R2 of the first-order kinetics was higher than the latter, the COD removal of rubber wastewater followed the first-order kinetic. Table listed all the calculated R2 and k values of our experiments.
Table 2. Coefficient determination and kinetic rate constants of the COD reduction following the first- and the second-order kinetics
3.2. The effect of flow rate on the oxidation process and the performance of the wastewater treatment process
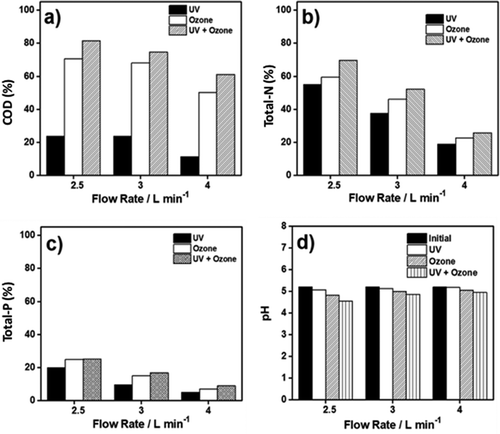
In Figure ), the results of the oxidation process using UV, ozone, and UV/ozone at various flow rates (2.5 to 4 L min−1) done in 20 cycles were presented. When the flow rate was increased, the removal of COD decreased due to the shorter contact time. The highest COD removal (81.44%) was observed when treatment was the UV/ozone at a flow rate of 2.5 L min−1 with a contact time of 960 s. This result was superior compared to the previous studies (Gong et al., Citation2008; Oh et al., Citation2007). They reported that the organic matter removal was up to 80% with longer residence time (150 min).
Figure 3. (a) COD, (b) Total-N, (c) Total-P, and (d) pH reduction of rubber wastewater, which was treated with different methods using various flow rates.
Bustos-Terrones et al. (Citation2016) explained that the ozone treatment alone, without the addition of UV, could not oxidize the COD completely. However, the addition of UV helped ozone to react with water to produce H2O2 and O2. Then, H2O2 continues to react with ozone or UV to produce •OH- (or OH*) (B. S. Oh et al., Citation2006) as discussed in the previous section, following the EquationEquations (4(4)
(4) )–(Equation6
(6)
(6) ):
The presence of hydroxyl radicals enhances the degradation of less reactive materials; consequently, the UV/ozone treatment reduced the COD levels rapidly compared to other methods. Surprisingly, several researchers informed that OH* could contribute to the inactivation of microorganisms (Cho et al., Citation2004; Sjogren & Sierka, Citation1994).
Figure ) showed the reduction of total-N in wastewater after it was treated using the three different methods. Compared to UV or ozone only, the UV/ozone method performed the highest total-N removal (69.62%). Similar to the COD data, it was found to be related to the flow rate. The total-N removal increased when the flow rate of wastewater feed was decreased. Longer contact times between wastewater and UV/ozone led to decreases in the total-N concentration in the wastewater (Peyton & Glaze Citation1988; Mark et al. Citation1996). Furthermore, previous literature revealed that the addition of ozone generated by UV radiation could decrease nitrite concentration up to below 0.1 mg L−1 (Zoschke et al., Citation2014, Citation2012). According to the literature, the reaction between nitrogen (in nitrate form) and UV radiation resulted in nitrate with oxygen (Mack & Bolton, Citation1999; Thomson et al., Citation2004), following the EquationEquation (7)(7)
(7) :
After the reaction between nitrite and ozone, the result was nitrate and oxygen [32], following the EquationEquation (8)(8)
(8) :
In Figure ), the total-P removal results were presented when the three different treatment methods were employed. For the total-P removal process, there were three main findings. First, total-P in wastewater was reduced more when the UV/ozone method was used. Second, total-P removal was increased when the flow rate was decreased. Since the flow rate was low, the contact time was longer. Hence, 25.53% of removal was observed in our study as the highest total-P removal when using the UV/ozone method. Finally, the UV/ozone treatment insignificantly reduced the total-P of wastewater in our study.
It has been known that there are two forms of phosphorus in the wastewater; the dissolved form and the particulate form. Orthophosphate, the primary form of dissolved phosphorus reacts with excess water and forms phosphoric acid. When the pH of the wastewater drops, phosphoric acid starts to exist in the wastewater. In our study, the pH decreased from 5.2 to 4.65, and it was illustrated in Figure ).
3.3. The effect of wastewater concentration on microalgae cultivation
The rubber wastewater, which was treated with UV/ozone at a flow rate of 2.5 L min−1 for 20 cycles, was tested as a potential medium for Spirulina platensis cultivation. For this aim, various concentrations (0, 30, 50, and 80%, v/v) of wastewater were tested. In general, the microalgae concentration decreased when higher concentrations of wastewater were used within the medium (Figure ). The values of ODmax for Spirulina platensis were 0.82, 0.67, 0.45, and 0.28 when the cells were cultivated in 0, 30, 50, and 80% of wastewater, respectively. Moreover, their specific growth rates were 0.32 day−1, 0.30 day−1, 0.29 day−1, and 0.26 day−1, respectively.
Figure 4. The growth of Spirulina platensis for 10 days of cultivation. In this study, 0, 30, 50, and 80% (v/v) of wastewater treated with UV/ozone was used as a growth medium.
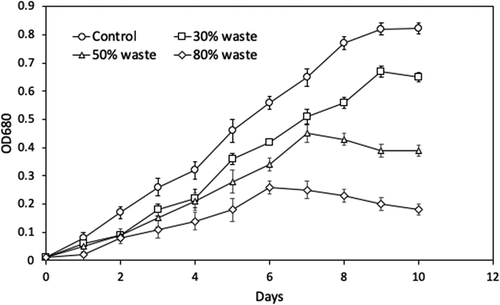
Unpredictably, the ODmax and the growth rate of microalgae, which were cultivated in 30% wastewater was comparable to the microalgae, which were cultivated in freshwater, whereas only half of the nutrient composition was used. This observation proved that the microalgae, which were cultivated with 30% rubber wastewater, could save nutrients up to 50%. The decrease in ODmax and growth rate was probably due to the antimicrobial compounds existed in wastewater. Since the UV/ozone method produces hydroxyl radicals, the microorganisms can be inactivated. Similarly, this might be the case in our experiments. Hence, the microalgae were inactivated or killed when the wastewater concentration was increased. Consequently, using wastewater effluent at high concentrations as a microalgae cultivation medium was not recommended.
4. Conclusion
Three different advanced oxidation processes (UV, ozone, UV/ozone) were evaluated to treat rubber processing wastewater at various flow rates for different cycles (to find out the best contact time), and then the effluent obtained from the treatment having a flow rate of 2.5 L min−1 for 20 cycles was utilized as the microalgae cultivation medium. The COD removal followed the first-order kinetics, and the rate constant (k) values were 3 × 10−4 to 1.2 × 10−3 s−1. The R2 values were 0.936 to 0.984. Best removal performances in COD, total-N, and total-P were calculated as 81.44%, 69.62%, and 25.53%, respectively, with the UV/ozone treatment. A synergistic effect in the combined process (UV/ozone) showed that the removal efficiency was higher than the standalone process (UV or ozone only). The increase in the flow rate of feed into the reactor led to a decrease in organic matter. In the S. platensis cultivation, the values of ODmax and growth rate were 0.67 and 0.3 day−1, respectively, when 30% of wastewater (v/v) was utilized. Similarly, the values were 0.8 and 0.32 day−1, respectively, when microalgae were cultivated in freshwater. It can be concluded that the rubber wastewater treated with the UV/ozone method can be used as a cultivation medium for Spirulina platensis and save the external nutrient requirement for microalgae cultivation. It is worth noting that the initial pH of the rubber wastewater was low, and we did not take this into account in the study. Thus, this will be done in our future work.
Acknowledgements
The authors thank C-BIORE for their facilities and guidance.
Additional information
Funding
Notes on contributors

Hadiyanto Hadiyanto
Hadiyanto, is currently full professor at Chemical Engineering Department, Diponegoro University. He is also a head of Center of Biomass and Renewable Energy (CBIORE) where the group itself focus on research of biomass for food, energy and other valuable products.
One main resources used in the group is microalgae where its biomass has been intensively studied for biodiesel, bioactive compounds and food nutrients.
References
- Amat, A., & Miranda, L. (2005). Use of ozone and/or UV in the treatment of effluents from board paper industry. Chemosphere, 60(8), 1111–11. https://doi.org/10.1016/j.chemosphere.2004.12.062
- Asia, I. O., & Akporhonor, E. E. (2007). Characterization and physicochemical treatment of wastewater from rubber processing factory. International Journal of the Physical Sciences, 2(3), 61–67. http://academicjournals.org/journal/IJPS/article-stat/117B41112942
- Bustos, Y., Vaca, M., López, R., Bandala, E., Torres, L., & Rojas-Valencia, N. (2014). Disinfection of primary municipal wastewater effluents using continuous UV and ozone treatment. Journal of Water Resource and Protection, 6(1), 16–21. https://doi.org/10.4236/jwarp.2014.61003
- Bustos, Y. A., Vaca, M., López, R., & Torres, L. G. (2010). Disinfection of a wastewater flow treated by advanced primary treatment using O3, UV and O3/UV combinations. Journal of Environmental Science and Health, Part A, 45(13), 1715–1719. https://doi.org/10.1080/10934529.2010.513241
- Bustos-Terrones, Y., Rangel-Peraza, J. G., Sanhouse, A., Bandala, E. R., & Torres, L. G. (2016). Degradation of organic matter from wastewater using advanced primary treatment by O3 and O3/UV in a pilot plant. Phys Chemistry Earth A/B/C, 91(1), 61–67. https://doi.org/10.1016/j.pce.2015.12.006
- Chin, A., & Bérubé, P. R. (2005). Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Research, 39(10), 2136–2144. https://doi.org/10.1016/j.watres.2005.03.021
- Cho, M., Chung, H., Choi, W., & Yoon, J. (2004). Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Research, 38(4), 1069–1077. https://doi.org/10.1016/j.watres.2003.10.029
- Christwardana, M., & Hadiyanto, H. (2017). The effects of audible sound for enhancing the growth rate of microalgae Haematococcus pluvialis in vegetative stage. HAYATI Journal of Biosciences, 24(3), 149–155. https://doi.org/10.1016/j.hjb.2017.08.009
- Clesceri, L. S., Eaton, A. D., Greenberg, A. E., & Franson, M. A. H., & American Public Health Association. (1998). Water Environment Federation. Standard methods for the examination of water and wastewater, 1220. Washington DC: American Public Health Association.
- Gong, J., Liu, Y., & Sun, X. (2008). O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water Research, 42(4–5), 1238–1244. https://doi.org/10.1016/j.watres.2007.09.020
- Hadiyanto, H., Azimatun Nur, M. M., Hartanto, G. D. (2012) Cultivation of chlorella sp. As biofuel sources in palm oil mill effluent (POME). International Journal of Renewable Energy Development, 1(2),45-49. doi:10.14710/ijred.1.2.45-49
- Hadiyanto, H., Soetrisnanto, D., & Christwardhana, M. (2014). Phytoremediation of palm oil mill effluent using pistia stratiotes plant and algae Spirulina sp for biomass production. IJE TRANSACTIONS C: Aspects, 27(12), 1809-1814. http://old.ije.ir/Vol27/No12/C/abstract-1833.html.
- Jung, Y. J., Oh, B. S., & Kang, J. W. (2008). Synergistic effect of sequential or combined use of ozone and UV radiation for the disinfection of Bacillus subtilis spores. Water Research, 42(6–7), 1613–1621. https://doi.org/10.1016/j.watres.2007.10.008
- Kang, J. W., & Lee, K. H. (1997). A kinetic model of the hydrogen peroxide/UV process for the treatment of hazardous waste chemicals. Environmental Engineering Science, 14(3), 183–192. https://doi.org/10.1089/ees.1997.14.183
- Lang, C. A. (1958). Simple micro determination of Kjeldahl nitrogen in biological materials. Analytical Chemistry, 30(10), 1692–1694. https://doi.org/10.1021/ac60142a038
- Mack, J., & Bolton, J. R. (1999). Photochemistry of nitrite and nitrate in aqueous solution: A review. Journal of Photochemistry and Photobiology A, 128(1–3), 1–13. https://doi.org/10.1016/S1010-6030(99)00155-0
- Magbanua, J. B., Savant, S., & Truax, D. D. (2006). Combined ozone and ultraviolet inactivation of Escherichia coli. Journal of Environmental Science and Health, Part A, 41(6), 1043–1055. https://doi.org/10.1080/10934520600620279
- Mailhot, G., Sarakha, M., Lavedrine, B., Cáceres, J., & Malato, S. (2002). Fe(III)-solar light induced degradation of diethyl phthalate (DEP) in aqueous solutions. Chemosphere, 49(6), 525–532. https://doi.org/10.1016/s0045-6535(02)00418-6
- Mark, G., Korth, H. G., Schuchmann, H. P., & Von Sonntag, C. (1996). The photochemistry of aqueous nitrate ion revisited. Journal of Photochemistry and Photobiology A, 101(2–3), 89–103. https://doi.org/10.1016/S1010-6030(96)04391-2
- Mokhtar, N. M., Lau, W. J., Ismail, A. F., & Veerasamy, D. (2015). Membrane distillation technology for treatment of wastewater from rubber industry in Malaysia. Procedia CIRP, 26(1), 792–796. https://doi.org/10.1016/j.procir.2014.07.161
- Nur, M. M. A., Hadiyanto, H. (2015). Enhancement of chlorella vulgaris biomass cultivated in pome medium as biofuel feedstock under mixotrophic conditions (2015) Journal of Engineering and Technological Sciences, 47 (5),487-497. http://dx.doi.org/10.5614/j.eng.technol.sci.2015547487497
- Oh, B. S., Jung, Y. J., Oh, Y. J., Yoo, Y. S., & Kang, J. W. (2006). Application of ozone, UV and ozone/UV processes to reduce diethyl phthalate and its estrogenic activity. Science of the Total Environment, 367(2–3), 681–693. https://doi.org/10.1016/j.scitotenv.2006.02.051
- Oh, B. S., Park, S. J., Jung, Y. J., Park, S. Y., & Kang, J. W. (2007). Disinfection and oxidation of sewage effluent water using ozone and UV technologies. Water Science and Technology, 55(1–2), 299–306. https://doi.org/10.2166/wst.2007.036
- Oh, B. S., Park, S. J., Lee, H. G., Kim, K. S., Lee, K. H., & Kang, J. W. (2003). Application of ozone/UV process for the reclamation of sewage treatment plant effluent. Journal of Water and Environment Technology, 1(2), 141–153. https://doi.org/10.2965/jwet.2003.141
- Peyton, G. R., & Glaze, W. H. (1988). Destruction of pollutants in water with ozone in combination with ultraviolet radiation. 3. Photolysis of aqueous ozone. Environmental Science and Technology, 22(7), 761–767. https://doi.org/10.1021/es00172a003
- Rosenfeldt, E. J., Linden, K. G., Canonica, S., & Von Gunten, U. (2006). Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Research, 40(20), 3695–3704. https://doi.org/10.1016/j.watres.2006.09.008
- Rosman, N. H., Nor Anuar, A., Othman, I., Harun, H., Sulong Abdul Razak, M. Z., Ujang, Z., Mat Hassan, M. A. H., Chelliapan, S., & Ujang, Z. (2013). Cultivation of aerobic granular sludge for rubber wastewater treatment. Bioresource Technology, 129(1), 620–623. https://doi.org/10.1016/j.biortech.2012.12.113
- Sharrer, M. J., & Summerfelt, S. T. (2007). Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquacultural Engineering, 37(2), 180–191. https://doi.org/10.1016/j.aquaeng.2007.05.001
- Sjogren, J. C., & Sierka, R. A. (1994). Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Applied and Environmental Microbiology, 60(1), 344–347. https://doi.org/10.1128/AEM.60.1.344-347.1994
- Thomson, J., Roddick, F. A., & Drikas, M. (2004). Vacuum ultraviolet irradiation for natural organic matter removal. Journal of Water Supply: Research and Technology-Aqua, 53(4), 193–206. https://doi.org/10.2166/aqua.2004.0017
- Watari, T., Mai, T. C., Tanikawa, D., Hirakata, Y., Hatamoto, M., Syutsubo, K., Fukuda, M., Nguyen, N. B., & Yamaguchi, T. (2017). Performance evaluation of the pilot scale upflow anaerobic sludge blanket–Downflow hanging sponge system for natural rubber processing wastewater treatment in South Vietnam. Bioresource Technology, 237(2), 204–212. https://doi.org/10.1016/j.biortech.2017.02.058
- Zoschke, K., Börnick, H., & Worch, E. (2014). Vacuum-UV radiation at 185 nm in water treatment–a review. Water Research, 52(1), 131–145. https://doi.org/10.1016/j.watres.2013.12.034
- Zoschke, K., Dietrich, N., Börnick, H., & Worch, E. (2012). UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Research, 46(16), 5365–5373. https://doi.org/10.1016/j.watres.2012.07.012